Abstract
Little is known concerning the fine-scale diversity, population structure, and biogeography for Symbiodinium spp. populations inhabiting particular invertebrate species, including the gorgonian corals, which are prevalent members of reef communities in the Gulf of Mexico, the Caribbean, and the western Atlantic. This study examined the Symbiodinium sp. clade B symbionts hosted by the Caribbean gorgonian Pseudopterogorgia elisabethae (Bayer). A total of 575 colonies of P. elisabethae were sampled in 1995 and 1998–2000 from 12 populations lying along an ~450 km transect in the Bahamas and their Symbiodinium sp. clade B symbionts genotyped at two polymorphic dinucleotide microsatellite loci. Twenty-three unique, two-locus genotypes were identified in association with these P. elisabethae colonies. Most colonies hosted only a single Symbiodinium sp. clade B genotype; however, in some instances (n=25), two genotypes were harbored simultaneously. For 10 of the 12 populations, 66–100% of the P. elisabethae colonies hosted the same symbiont genotype. Added to this, in 9 of the 12 populations, a Symbiodinium sp. clade B genotype was either unique to a population or found infrequently in other populations. This distribution of Symbiodinium sp. clade B genotypes resulted in statistically significant (P<0.05 or <0.001) differentiation in 62 of 66 pairwise comparisons of P. elisabethae populations. Tests of linkage disequilibrium suggested that a combination of clonal propagation of the haploid phase and recombination is responsible for maintaining these distinct Symbiodinium sp. clade B populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mutualistic associations between invertebrates and dinoflagellates are common features of tropical marine communities. These algae, collectively known as zooxanthellae and predominately belonging to the genus Symbiodinium Freudenthal (Taylor 1974), play a vital role in their host's nutrition and physiology (reviewed in Davies 1993). It was once thought that all invertebrate–Symbiodinium associations involved a single species of dinoflagellate, Symbiodinium microadriaticum (Taylor 1974). However, infectivity, ultrastructural, behavioral, and molecular studies on cultured, as well as freshly isolated and in hospite, algae have demonstrated that zooxanthellae comprise a heterogeneous group of many species and strains (reviewed by Rowan 1998). To date, 10 species of Symbiodinium and 14 other species of symbiotic dinoflagellates have been described, either formally or informally (Trench and Blank 1987; Banaszak et al. 1993; Trench 1993, 2000).
Early studies quantifying Symbiodinium spp. diversity and phylogenetic relationships relied on cultured materials (Schoenberg and Trench 1980a, 1980b, 1980c; Blank and Huss 1989). Unfortunately, Symbiodinium spp. cultures can be difficult to establish (Schoenberg and Trench 1980a) and may not be representative of the host or population from which they were isolated (Santos et al. 2001; LaJeunesse 2002). The application of polymerase chain reaction (PCR)-based molecular techniques has circumvented the need for cultures, leading to an explosion of data regarding Symbiodinium spp. diversity and phylogenetics, particularly from natural populations. It is now known that the genus is highly diverse, on a level comparable to that observed among orders of free-living dinoflagellates (Rowan and Powers 1992). Members of Symbiodinium are typically identified as belonging to one of several large groups, or clades [i.e. Symbiodinium spp. clades A, B, C (Rowan and Powers 1991), D (Carlos et al. 1999), E (S. californium; LaJeunesse and Trench 2000; LaJeunesse 2001), F (LaJeunesse 2001), and the symbionts of foraminiferans (Pawlowski et al. 2001; Pochon et al. 2001)] based on analyses of nuclear ribosomal DNA (rDNA) genes. Phylogenies inferred from nuclear 5.8S- (LaJeunesse 2001) and large subunit (28S)-rDNA (Pawlowski et al. 2001; Pochon et al. 2001) group Symbiodinium sp. clade A into one assemblage, while Symbiodinium spp. clades B/C/D/E/F and the formaniniferan symbionts form a second, closely related group. These relationships are also supported by phylogenies based on organellar (i.e. chloroplast) large subunit (cp23S)-rDNA (Santos et al. 2002).
Although the diversity and phylogenetics of the entire complex of taxa is now well established, there are few data on the population structure and biogeography of Symbiodinium spp. over the geographic range of a host species. For hosts that associate with more than a single clade of Symbiodinium spp., population structure and biogeography of the dinoflagellate symbionts appear to depend on geography (LaJeunesse and Trench 2000; Rodriguez-Lanetty et al. 2001; Burnett 2002). In contrast, population structure and biogeography may or may not be apparent in hosts that harbor a single clade of Symbiodinium spp. For example, populations of Symbiodinium sp. clade C associated with the scleractinian corals Acropora longicyathus and Seriatopora hystrix exhibit significant genetic differentiation between geographical regions of the Indo-West Pacific (Loh et al. 2001). On the other hand, Symbiodinium sp. clade A of giant clams are nearly indistinguishable throughout the Indo-Pacific (Baillie et al. 2000). In all of these cases, host species were from the Pacific or Indian Oceans. For Atlantic hosts, data concerning Symbiodinium spp. population structure and biogeography over a geographic range are almost nonexistent. The gorgonian Plexaura kuna has been found to host Symbiodinium sp. clade B at various geographic sites and depths in the Caribbean (Goulet and Coffroth 2003). A study of the Symbiodinium spp. populations from two widely separated Caribbean reefs found that many "types" [defined as possessing a distinctive internal transcribed spacer 2 (ITS-2) sequence] occurred at both sites and were shared by individuals of the same host species (LaJeunesse 2002). To date, a comprehensive survey of the fine-scale diversity, population structure, and biogeography of Symbiodinium spp. from a single host species distributed in the Gulf of Mexico, Caribbean, or western Atlantic has not been conducted.
The present study addresses this deficiency by using microsatellites to characterize the Symbiodinium sp. clade B populations hosted by the gorgonian Pseudopterogorgia elisabethae in the Bahamas in the Caribbean. Microsatellites are segments of DNA in which a specific motif of one to six bases is repeated in an array. Length variability in a microsatellite array is typically generated by polymerase slippage during DNA replication and variants (i.e. alleles) detected by PCR and gel electrophoresis. Over the last decade, these single locus, multiallelic, codominant segments of DNA have proved to be versatile and accessible markers for population genetic studies (reviewed by Chambers and MacAvoy 2000). Previously, microsatellites have been used to assess the relatedness between Symbiodinium sp. clade B cultures and populations from which they were established (Santos et al. 2001) and demonstrate haploidy in the vegetative (i.e. coccoid) life stage of Symbiodinium spp. (Santos and Coffroth 2003).
The economically important Caribbean gorgonian P. elisabethae is the sole source of pseudopterosins, natural products with anti-inflammatory properties (Fenical 1987), which are used as a topical agent in cosmetics. This gorgonian is geographically widespread on coral reefs of the Caribbean (Bayer 1961; Kinzie 1970; Lasker and Coffroth 1983); however, little is known about the biology, ecology, and genetics of P. elisabethae or its algal symbionts. Sexual reproduction in P. elisabethae results in brooded asymbiotic planulae (Kinzie 1974) that take up Symbiodinium sp. clade B (Goulet 1999; Santos et al. 2001, 2002, 2003) from the immediate environment following settlement and metamorphosis (horizontal transmission of symbionts). Thus, Symbiodinium sp. clade B symbionts could be heterogeneous either within the same colony or among different colonies. Based on cp23S-rDNA genotyping, it has been demonstrated that P. elisabethae associates specifically with a single lineage of Symbiodinium sp. clade B (Symbiodinium sp. B184, cf. Santos et al. 2003). To characterize the fine-scale diversity, population structure, and biogeography of these Symbiodinium sp. clade B symbionts, 575 colonies of P. elisabethae were sampled from 12 sites along an ~450 km transect in the Bahamas, and their symbionts were genotyped at two polymorphic dinucleotide microsatellite loci.
Materials and methods
Biological materials and nucleic acid isolation
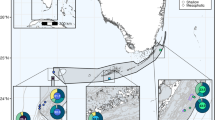
Pseudopterogorgia elisabethae (Bayer) colonies were sampled either in 1995 (Sweetings Cay) or between 1998 and 2000 (all other sites) using SCUBA. Collections were made at 12 sites along an ~450 km transect of the Bahamas: Sweetings Cay (SC; 21.6 m), Gorda Rock (GR; 16.7 m), Abaco Shallow (AS; 8.7 m), and Abaco Deep (AD; 16.7 m) at Abaco Island; South Hampton Reef (SH; 16 m) and East End Point (EE; 12.7 m) at Eleuthera Island; Little San Salvador (LS; 10.3 m), Cat Island (CI; 26 m), Hog Cay (HC; 12.3 m), Rum Cay (RC; 21.6 m), and Pillar Reef (PR; 11 m); and Riding Rock (RR; 17 m) at San Salvador (Fig. 1). For the sites at San Salvador, the two P. elisabethae populations were within ~1 km of each other, allowing for a within-reef comparison. Sampling was conducted in two ways. Where P. elisabethae was abundant, collections were performed along 20-m transects, collecting a branch of each individual found within 1 m of the transect line. Where P. elisabethae was less abundant (e.g. GR, EE, CI, and RC), all colonies encountered were sampled until the desired sample size was achieved. Tissue samples were preserved in either 95% ethanol or salt-saturated DMSO preservation buffer (Seutin et al. 1991) immediately following collection. Between 43 and 50 P. elisabethae colonies were selected haphazardly from each collection (total sample size=575 colonies) for the study. Total nucleic acids were extracted from approximately 3 cm of P. elisabethae tissue by first grinding the tissue in STE buffer [0.05 M Tris-HCl (pH 8.0), 0.1 M EDTA, 0.1 M NaCl, 0.2% SDS] followed by a modified extraction protocol using the Prep-A-Gene DNA extraction kit (Bio-Rad Laboratories, Hercules, Calif.).
Symbiodinium sp. clade B. Map of 12 Pseudopterogorgia elisabethae populations in the Bahamas [denoted by two-letter abbreviations as follows: SC Sweetings Cay (26°34′N, 77°48′W); GR Gorda Rock (26°07′N, 77°33′W); AS Abaco Shallow (26°02′N, 77°28′W); AD Abaco Deep (25°59′N, 77°25′W); SH South Hampton Reef (25°25′N, 76°48′W); EE East End Point of Eleuthera (24°37′N, 76°10′W); LS Little San Salvador (24°35′N, 75°58′W); CI Cat Island (24°09′N, 75°32′W); HC Hog Cay (23°37′N, 75°21′W); RC Rum Cay (23°37′N, 74°52′W); PR Pillar Reef; RR Riding Rock (24°04′N, 74°32.5′W)]
Isolation and screening of Symbiodinium sp. clade B microsatellite loci
Two Symbiodinium sp. clade B microsatellite loci, CA4.86 and CA6.38, were used in this study (Table 1). Details regarding the isolation of these microsatellite loci and screening of the PCR primers are presented in Santos and Coffroth (2003).
Microsatellite amplifications and allele detection from Symbiodinium sp. clade B populations
PCR reactions for each microsatellite locus were performed in 10 μl volumes containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 0.001% gelatin, 200 μM dNTPs, 1 U Taq polymerase, and 10 ng of template DNA. PCR primer and MgCl2 concentrations varied for each locus. For loci CA4.86 and CA6.38, MgCl2 concentrations were 2.5 mM and 1.5 mM, respectively. Primer concentrations for locus CA4.86 were 0.2 pmol CA4.86R, 0.18 pmol CA4.86L, and 0.02 pmol 5′-IRD800 M13 forward primer. For locus CA6.38, primer concentrations were 3 pmol CA6.38R, 2 pmol CA6.38L, and 0.2 pmol 5′-IRD800 M13 forward primer. Nineteen nucleotides (5′-CACGACGTTGTAAAACGAC-3′) were added to the 5′ end of primers CA4.86L and CA6.38L to allow incorporation of the 5′-IRD800 fluorescent-labeled M13 forward primer (LI-COR Biotechnology Division, Lincoln, Neb.) into PCR products. Thermocycling conditions were: initial denaturing step of 94°C for 2 min, 35 cycles of 94°C for 30 s, annealing temperature of 50°C or 56°C (see Table 1) for 30 s, 72°C for 30 s, and a final extension of 72°C for 3 min. Following amplification, reactions were diluted with 5 μl loading buffer [95% (v/v) formamide, 10 mM EDTA, 0.01% bromophenol blue (final pH 9.0)], denatured for 3 min at 95°C, and snap-cooled on ice immediately prior to polyacrylamide gel electrophoresis.
Electrophoresis was done in 25 cm long, 0.25 mm thick 6.5% Long Ranger (FMC Bioproducts, Rockland, Me.)/1× Tris-borate (TBE) polyacrylamide gels under denaturing (7 M urea) conditions and visualized using a LI-COR NEN Global IR2 DNA sequencer system (LI-COR Biotechnology Division). Conditions were 1,500 V, 40 W, 40 mA, 50°C, and the default scan speed. DNA ladders, spanning either 97–147 bp or 172–272 bp at 25 bp increments, were run in every fifth lane across the gel for size reference. A single gel was reloaded up to three times over a 5 h period. Sizing of alleles was done with the fragment analysis program Gene ImagIR v3.55 (Scanalytics, Fairfax, Va.), using the DNA ladders as size references. Alleles were scored according to "true" base pair (bp) number, excluding the nineteen 5′-nucleotides of the fluorescent-labeled M13 forward primer.
Data analysis
A variety of descriptive and statistical analyses were conducted on the data set. First, allelic frequencies were derived from the total number of alleles observed for a locus from all samples, while allelic diversities were estimated as the expected heterozygosity index (H; Nei 1978). Allelic frequencies and diversities were calculated separately for each population, as well as across all populations, using the program "Tools For Population Genetic Analysis" (TFPGA) v1.3 (Miller 1997). Estimates of population structure were made using both infinite-alleles (IAM; Kimura and Crow 1964) and stepwise-mutation (SMM; Kimura and Ohta 1978) evolutionary models. F ST, which assumes an IAM, was estimated using θ (Weir and Cockerham 1984). Single-locus estimates of θ were weighted as described by Weir and Cockerham (1984) to create a combined measure. R ST, an analog of θ that assumes a SMM (Slatkin 1995), was estimated using ρST (Rousset 1996). The standardization approach of Goodman (1997) was used to make a two-locus measure of R ST. Values of F ST and R ST were obtained with FSTAT v2.9.3.2 (Goudet 2001).
Second, the alleles detected at the loci were used to construct a two-locus genotype [i.e. haplotype, as recent work has demonstrated that the vegetative form of Symbiodinium spp. is haploid (Santos and Coffroth 2003)] for the Symbiodinium sp. clade B population of each P. elisabethae colony. To test for geographical differentiation, a categorical analysis consisting of a χ2 test of absolute genotype frequencies at each site (Hudson et al. 1992) was conducted. Due to bias with low expected values, the P value was obtained by simulating the null distribution of no geographic subdivision (10,000 permutations) using the algorithm of Roff and Bentzen (1989) implemented in CHIPERM v1.2 (written by D. Posada; available at http://bioag.byu.edu/zoology/crandall_lab/programs.htm). Pairwise tests for Symbiodinium sp. clade B population differentiation were also conducted by randomizing genotypes between pairs of populations using FSTAT v2.9.3.2. To graphically depict the relationship between Symbiodinium sp. clade B populations, an unweighted pair group method using arithmetic averages (UPGMA) dendrogram was constructed using Nei's minimum genetic distance (Nei 1972) in TFPGA v1.3. In addition, a Mantel test (Mantel 1967) was performed to test for a relationship between geographic distance and genetic distance. The Mantel test was conducted in TFPGA v1.3 using Nei's minimum genetic distance and 1,000 permutations.
Third, to determine if recombination was occurring among the Symbiodinium sp. clade B genotypes, the null hypothesis of random association among alleles at loci was tested with a permutation procedure. The program LIAN v3.1 (Haubold and Hudson 2000) was used to calculate the number of alleles shared (D) between all pairwise comparisons of genotypes and to measure the variance of this distance measurement (V D). To test if the observed data set differed from random expectations, the observed V D was compared to the distribution of V D values in 10,000 simulated data sets. Simulated data sets were generated by random reshuffling of alleles among Symbiodinium sp. clade B genotypes. Significant linkage disequilibrium (LD), or non-random association of alleles, was detected if the observed V D was >95% of the values generated in the reshuffled data sets. A "standardized" index of association (\( I_A^S \); Haubold and Hudson 2000) was used to measure the strength of LD. Testing for LD was conducted separately on two data sets. The first comprised the Symbiodinium sp. clade B genotypes of all P. elisabethae colonies in the study, while the second included only a single representative of each unique genotype.
For the descriptive and statistical analyses, we assumed that alleles identical in state (i.e. size) were identical by descent. Although microsatellite alleles may sometimes violate this assumption (e.g. Viard et al. 1998), the occurrence of size homoplasy (alleles identical in state, but not identical by descent) is not considered a significant problem for analyses of population genetic structure (Estoup et al. 2002).
Results
Symbiodinium sp. clade B microsatellites from Pseudopterogorgia elisabethae
Screening of the Symbiodinium sp. clade B populations of P. elisabethae at CA4.86 and CA6.38 revealed that both loci were polymorphic. Eight and ten distinct alleles were identified from CA4.86 and CA6.38, respectively (Table 2). Allele sizes for CA4.86 ranged between 185 and 207 bp, while alleles for CA6.38 ranged between 96 and 122 bp (Table 2). For most P. elisabethae colonies (n=533, 92.7% of the colonies), only a single Symbiodinium sp. clade B microsatellite allele was detected per locus. A single allele per microsatellite locus was also recovered from the majority of Symbiodinium sp. clade B populations of the Caribbean gorgonian Plexaura kuna and a series of Symbiodinium sp. clade B cultures (Santos and Coffroth 2003). This is consistent with Symbiodinium spp., like other dinoflagellates, possessing a haploid vegetative life stage (Blank 1987; Pfiester and Anderson 1987; Santos and Coffroth 2003). In some cases (n=25, 4.3% of the colonies), two alleles were recovered from one or both loci. The recovery of two alleles from a locus occurred in one to two P. elisabethae colonies at 8 of the 12 sites. For the P. elisabethae population at Little San Salvador, 12 of 50 colonies had Symbiodinium sp. clade B populations with two alleles at one or both loci. These 25 colonies are thought to host two genotypes of Symbiodinium sp. clade B simultaneously (Santos and Coffroth 2003). In addition, the Symbiodinium sp. clade B populations of some P. elisabethae colonies (n=17, 3.2% of the colonies) did not amplify at one or both loci despite multiple PCR attempts. For the purposes of this study, we assumed that the absence of PCR amplification at a locus represented a null allele for that locus. Details regarding samples with two alleles or null alleles are presented in Electronic Appendix 1.
Genetic diversity of Symbiodinium sp. clade B populations from P. elisabethae
In each of the Symbiodinium sp. clade B populations, one of three alleles at locus CA4.86 was most prevalent, and in 10 of the 12 populations, one of these three alleles was present in >70% of the P. elisabethae colonies (Table 2). For locus CA6.38, one of five alleles was most prevalent in a population, being present in >60% of the colonies (Table 2). At Abaco Deep and Little San Salvador, two alleles were numerically dominant at locus CA4.86 and were recovered at approximately equal frequencies (Table 2). There was a substantial range in genetic variation among the Symbiodinium sp. clade B populations. Allelic diversity (H) ranged from 0 to 0.611 for locus CA4.86 and from 0 to 0.461 for locus CA6.38 (Electronic Appendix 2). The average H over all populations for CA4.86 and CA6.38 was 0.611 and 0.760, respectively. The H for both loci and over all populations was 0.685, suggesting a high level of genetic diversity among the Symbiodinium sp. clade B populations of P. elisabethae.
Estimates of population structure were indicative of strong subdivision in the Symbiodinium sp. clade B populations of P. elisabethae. F ST estimates were 0.579 and 0.701 for CA4.86 and CA6.38, respectively, with an F ST for both loci of 0.647. Estimates of R ST were similar to those for F ST; 0.479 for CA4.86 and 0.794 for CA6.38, with an R ST for both loci of 0.639. Prior to constructing Symbiodinium sp. clade B genotypes, the 42 P. elisabethae colonies for which two alleles and/or null alleles were detected at a locus were excluded since a genotype could not be determined unambiguously. From the remaining 533 colonies, 23 unique Symbiodinium sp. clade B genotypes were found to associate with P. elisabethae in the Bahamas (Table 3).
Population structure and geographical differentiation of Symbiodinium sp. clade B populations from P. elisabethae
Striking differences in the distribution of Symbiodinium sp. clade B genotypes were evident across the Bahamas (Table 3). In 10 of the 12 P. elisabethae populations, a single Symbiodinium sp. clade B genotype was recovered from ~66% to 100% of the colonies at a site. In six of these populations (SC, CI, HC, RC, PR, and RR), the numerically dominant Symbiodinium sp. clade B genotype was either unique to that population or found infrequently in other populations. In three populations (GR, AS, and SH), the same numerically dominant Symbiodinium sp. clade B genotype was shared by 66.8–95.6% of the P. elisabethae colonies. This symbiont genotype was predominately confined to these sites. Many (45.6%) of the P. elisabethae colonies at Abaco Deep harbored the numerically dominant Symbiodinium sp. clade B genotype found at Gorda Rock, Abaco Shallow, and South Hampton Reef, but also associated with a genotype found almost exclusively in this population (23.9% of the colonies). The remaining P. elisabethae populations (EE and LS) also shared a common Symbiodinium sp. clade B genotype in 36.1% and 65.9%, respectively, of the colonies. However, colonies at Little San Salvador also hosted a Symbiodinium sp. clade B genotype (41.6% of the colonies) that was not found at East End Point and only found infrequently in other populations. For all populations other than Riding Rock, in addition to the numerically dominant Symbiodinium sp. clade B genotype, between one and seven additional genotypes were observed in a population, but typically at low frequencies (Table 3). The distribution of Symbiodinium sp. clade B genotypes followed no patterns related to depth; distinct genotypes were found at the same depth (e.g. SC and RC), while the same Symbiodinium sp. clade B genotype could be found at different depths (e.g. PR and RR; Table 3).
The categorical analysis of Symbiodinium sp. clade B genotype frequencies by site revealed highly significant differentiation (χ2=3,072.74; P<10−4) between populations. Pairwise tests of Symbiodinium sp. clade B population differentiation gave similar results. Of the 66 pairwise comparisons, 62 were significant at P values of from <0.05 to <0.001 (Table 4). For the within-reef comparison, the Symbiodinium sp. clade B populations at Riding Rock and Pillar Reef were not significantly different. The other three pairwise comparisons that did not differ significantly involved the Symbiodinium sp. clade B populations at Gorda Rock, Abaco Shallow, and South Hampton Reef, all of which shared a common genotype.
Comparisons of the Symbiodinium sp. clade B populations from P. elisabethae based on genetic distance detected some correspondence to geographic location in the Bahamas (Electronic Appendix 3). Although the correlation was low (r=0.268), the Mantel test detected a significant positive correlation between genetic and geographic distance (P=0.017). Furthermore, the UPGMA dendrogram identified several clusters that also corresponded to the sites' geography (Fig. 2). In general, similar Symbiodinium sp. clade B populations were recovered from sites that were very close geographically. Thus, Riding Rock and Pillar Reef, which are located within 1 km on the same reef, formed a cluster. In the northwestern Bahamas, Gorda Rock, Abaco Shallow, Abaco Deep, and South Hampton Reef also formed a cluster. The first three of these populations are geographically close, but South Hampton Reef is ~90 km from the nearest other site. In the middle Bahamas, Symbiodinium sp. clade B populations from the geographically close sites of East End Point and Little San Salvador also grouped together. On the other hand, Cat Island and Hog Cay, which like East End Point and Little San Salvador, are within Exuma Sound (Fig. 1), showed significant differentiation (Fig. 2; Table 4). The Symbiodinium sp. clade B populations of Sweetings Cay, at the northwestern end of the Bahamas, clustered more closely with populations from Hog Cay than Hog Cay did with Cat Island Symbiodinium sp. clade B populations. The relationships are driven in large part by the high similarity of populations separated by tens of kilometers. At greater distances, there was no clear relationship between genetic and geographic distance.
Multilocus linkage disequilibrium of Symbiodinium sp. clade B populations from P. elisabethae
Analysis of the complete Symbiodinium sp. clade B population data set identified a highly significant deviation (\( I_A^S = 0.139 \); P<10−4) from random association among alleles. However, 17 (73.9%) of the 23 Symbiodinium sp. clade B genotypes were represented in the data set more than once. Among clonal organisms, \( I_A^S \) is often >0 when one or a few genotypes are widespread and/or abundant (Maynard-Smith et al. 1993). In order to test for true LD, Maynard-Smith et al. (1993) suggest analysis of a data set comprising only one representative of each unique genotype. The curtailed data set of 23 unique Symbiodinium sp. clade B genotypes revealed no significant deviation (\( I_A^S = - 0.114 \); P=0.999) from random associations.
Discussion
This study is the first to explore the fine-scale diversity, population structure, and biogeography of Symbiodinium sp. clade B populations from a single, geographically widespread Caribbean host species using microsatellites. Using only two microsatellites, we have identified 23 unique Symbiodinium sp. clade B genotypes in Pseudopterogorgia elisabethae, all belonging to a single lineage, Symbiodinium sp. B184 (cf. Santos et al. 2003, unpublished data). In addition, we have documented significant population differentiation among the Symbiodinium sp. clade B from different Bahamian reefs. These microsatellite loci also appear to be stable over time. For example, Symbiodinium sp. clade B culture SSPe, which was established from a P. elisabethae colony collected at Riding Rock in January 1999 (Santos et al. 2001), had the same alleles as those in the symbiont populations of Riding Rock P. elisabethae colonies in the present study. The SSPe culture has been transferred into fresh medium every 3–4 weeks since its isolation so this represents allelic stability over ~36 transfers in 3 years. Taken together, these properties make microsatellites powerful tools for exploring questions of Symbiodinium spp. biology.
Mechanisms driving population differentiation of Symbiodinium sp. clade B across the Bahamas
Our documentation of striking population differentiation in Symbiodinium sp. clade B across the Bahamas is surprising given the recovery of Symbiodinium spp. isolates with identical rDNA sequences, such as cp23S-rDNA (Santos et al. 2002) and the less conserved ITS-rDNA (Baillie et al. 2000; LaJeunesse 2001, 2002; Santos et al. 2001), from Pacific and Atlantic hosts. This implies widespread dispersal of these symbiotic algae. Furthermore, the recovery of Symbiodinium spp. from environmental water samples (Loeblich and Sherley 1979; Chang 1983; Carlos et al. 1999) lends support for the existence of planktonic or benthic populations that are capable of dispersing. We suggest the following hypotheses to explain the discrepancy between the data presented here and these previous studies. Firstly, we are capable of identifying significant levels of differentiation among these populations of Symbiodinium sp. clade B since microsatellites reveal substantially more within-lineage variation than other molecular markers (e.g. Blackston et al. 2001). Secondly, the symbiotic algae of P. elisabethae may have greater levels of genetic variation due to limited intrinsic dispersal abilities. Symbiodinium spp. isolates display characteristic motility patterns (Fitt et al. 1981; Fitt and Trench 1983; Crafts and Tuliszewski 1995), and differences in this trait may have important implications in the dispersal of some Symbiodinium spp., such as those associated with P. elisabethae. A third hypothesis is that the Symbiodinium sp. clade B populations at each site have been/are subjected to selection greater than the effects of migration between habitats. Thus, it is possible that all the symbiont genotypes identified in this study are found throughout the Bahamas. However, particular Symbiodinium sp. clade B genotypes may be selected differentially in each P. elisabethae population since they have a selective advantage, ultimately leading to the prevalence of a single genotype within a population. Of the possible forms that a selective advantage between symbiont genotypes may take, the most likely involves differences in physiological and/or biochemical characteristics. Variation in photobiology (Chang et al. 1983; Iglesias-Prieto and Trench 1994, 1997; Perez et al. 2001), in water-soluble peridinin–chl a protein (sPCP) synthesis and form (Govind et al 1990; Iglesias-Prieto and Trench 1997), and in isozyme profiles (Colley 1984; Baillie et al. 1998), as well as temperature tolerance (Kinzie et al. 2001; Perez et al. 2001), have been documented between different Symbiodinium spp. isolates. However, these data are limited, making extrapolation to other isolates or in hospite populations difficult. Establishing cultures of the Symbiodinium sp. clade B genotypes associated with P. elisabethae, followed by detailed characterization, would provide a way to test this hypothesis and to add to the information on Symbiodinium spp. physiology and biochemistry. These hypotheses are also not mutually exclusive, and, if we accept that the first hypothesis is correct, then some mechanism such as drift and/or selection is still required to generate the differences among populations that we have identified using microsatellites.
Oceanic currents and Symbiodinium sp. clade B biogeography
In a few cases, Symbiodinium sp. clade B populations at different sites in the Bahamas shared the same numerically dominant genotype. We hypothesize that oceanic currents, driven by trade winds as well as tidal flows, produce the observed patterns by aiding in the transport of Symbiodinium sp. clade B genotypes between these populations. For example, of the two numerically dominant genotypes at Little San Salvador, one is unique to the population, while the other is identical to the numerically dominant Symbiodinium sp. clade B genotype at East End Point (Table 4). Although separated by deep water, the two populations are relatively close (22.5 km) and transport of a symbiont genotype from East End Point to Little San Salvador via oceanic currents is consistent with this distribution pattern. Similarly, a common symbiont genotype was found at Gorda Rock, Abaco Shallow, and South Hampton Reef, which span a distance of ~89 km. This implies transport of this Symbiodinium sp. clade B genotype across the Northeast Providence Channel, a deep (~3,600 m) channel that separates Abaco and Eleuthera Islands (Fig. 1). These observations also suggest that distance alone is not a good predictor of whether a symbiont genotype will be unique or shared between host populations. Thus, most Symbiodinium sp. clade B genotypes appear to be retained within local populations, similar to model simulations (Cowen et al. 2000) and empirical data (Taylor and Hellberg 2003) from larval dispersing organisms in the Caribbean; however, dispersal by oceanic currents may also influence the population structure of Symbiodinium spp.
Recombination in Symbiodinium sp. clade B populations
Tests for multilocus linkage disequilibrium have been used to estimate levels of recombination among protozoan and fungal populations (Maynard-Smith et al. 1993; Anderson et al. 2000; de Meeus et al. 2002; Fisher et al. 2002). Analyses of complete, as well as curtailed, data sets distinguish if an organism is panmictic (random association among alleles due to recombination), "epidemic" (LD resulting from temporal expansion of particular genotypes in an otherwise recombining population), or truly clonal (levels of recombination insufficient to break down lineages) (Maynard-Smith et al. 1993). We found strong LD for the complete data set of Symbiodinium sp. clade B genotypes from P. elisabethae; on the other hand, linkage equilibrium was restored when a data set comprised solely of unique genotypes was reanalyzed. These results suggest that the symbiont populations of P. elisabethae are "epidemic", being maintained by a combination of clonal propagation of the haploid phase and recombination. Our study is the first to document this form of population structure in Symbiodinium spp., which was hypothesized by LaJeunesse (2001). Furthermore, although recombination has been rarely observed for dinoflagellates (Pfiester and Anderson 1987) and inconsistent descriptions of recombination have been reported for Symbiodinium spp. (Freudenthal 1962; Taylor 1974), our study contributes to the growing evidence that these dinoflagellates undergo recombination during their life cycle (reviewed by Santos and Coffroth 2003). However, the frequency of recombination, where it occurs (e.g. within an invertebrate host or in the environment) and the circumstances that induce it remain unknown and may prove difficult to establish. Thus, molecular data, such as those presented here, may prove to be the only method of documenting recombination in these enigmatic dinoflagellates.
References
Anderson TJC, Haubold B, Williams JT, Estrada-Franco JG, Richardson L, Mollinedo R, Bockarie M, Mokili J, Mharakura S, French N, Whitworth J, Velez ID, Brockman AH, Nosten F, Ferreira MU, Day KP (2000) Microsatellite markers reveal a spectrum of population structures in the malaria parasite Plasmodium falciparum. Mol Biol Evol 17:1467–1482
Baillie BK, Monje VA, Silvestre V, Sison M, Belda-Baillie CA (1998) Allozyme electrophoresis as a tool for distinguishing different zooxanthellae symbiotic with giant clams. Proc R Soc Lond B Biol Sci 256:1949–1956
Baillie BK, Belda-Baillie CA, Maruyama T (2000) Conspecificity and Indo-Pacific distribution of Symbiodinium genotypes (Dinophyceae) from giant clams. J Phycol 36:1153–1161
Banaszak AT, Iglesias-Prieto R, Trench RK (1993) Scrippsiella velellae sp. nov. (Peridiniales) and Gloeodinium viscum sp. nov. (Phytodiniales), dinoflagellate symbionts of two hydrozoans (Cnidaria). J Phycol 29:517–528
Bayer FM (1961) The shallow water Octocorallia of the East Indian region. Martinus Nijoff, The Hague
Blackston CR, Dubey JP, Dotson E, Su C, Thulliez P, Sibley D, Lehmann T (2001) High-resolution typing of Toxoplasma gondii using microsatellite loci. J Parasitol 87:1472–1475
Blank RJ (1987) Cell architecture of the dinoflagellate Symbiodinium sp. inhabiting the Hawaiian stony coral Montipora verrucosa. Mar Biol 94:143–155
Blank RJ, Huss VAR (1989) DNA divergency and speciation in Symbiodinium (Dinophyceae). Plant Syst Evol 163:153–163
Burnett WJ (2002) Longitudinal variation in algal symbionts (zooxanthellae) from the Indian Ocean zoanthid Palythoa caesia. Mar Ecol Prog Ser 234:105–109
Carlos AA, Baillie BK, Kawachi M, Maruyama T (1999) Phylogenetic position of Symbiodinium (Dinophyceae) isolates from tridacnids (Bivalvia), cardiids (Bivalvia), a sponge (Porifera), a soft coral (Anthozoa), and a free-living strain. J Phycol 35:1054–1062
Chambers GK, MacAvoy ES (2000) Microsatellites: consensus and controversy. Comp Biochem Physiol 126:455–476
Chang FH (1983) Winter phytoplankton and microzooplankton populations off the coast of Westland, New Zealand, 1979. NZ J Mar Freshw Res 17:279–304
Chang SS, Prezelin BB, Trench RK (1983) Mechanisms of photoadaptation in three strains of the symbiotic dinoflagellate Symbiodinium microadriaticum. Mar Biol 76:219–229
Colley NJ (1984) The cell biology of dinoflagellate symbiosis in a coelenterate. PhD dissertation, University of California, Santa Barbara
Cowen RK, Lwiza KMM, Sponaugle S, Paris CB, Olson DB (2000) Connectivity of marine populations: open or closed? Science 287:857–859
Crafts CB, Tuliszewski JR (1995) Motility rhythms in cultured zooxanthellae isolated from the scyphomedusa Linuche unguiculata. Bull Mar Sci 56:822–825
Davies PS (1993) Endosymbiosis in marine cnidarians. In: John DM, Hawkins SJ, Price JH (eds) Plant–Animal interactions in the marine benthos. Clarendon, Oxford, pp 511–540
de Meeus T, Renaud F, Mouveroux E, Reynes J, Galeazzi G, Mallie M, Bastide JM (2002) Genetic structure of Candida glabrata populations in AIDS and non-AIDS patients. J Clin Microbiol 40:2199–2206
Estoup A, Jarne P, Cornuet JM (2002) Homoplasy and mutation model at microsatellite loci and their consequences for population genetic analysis. Mol Ecol 11:1591–1604
Fenical W (1987) Marine soft corals of the genus Pseudopterogorgia: a resource for novel anti-flamatory diterpenoids. J Nat Prod 6:1001–1008
Fisher MC, Rannala B, Chaturvedi V, Taylor JW (2002) Disease surveillance in recombining pathogens: multilocus genotypes identify sources of human Coccidioides infections. Proc Natl Acad Sci USA 99:9067–9071
Fitt WK, Trench RK (1983) The relation of diel patterns of cell division to diel patterns of motility in the symbiotic dinoflagellate Symbiodinium microadriaticum Freudenthal in culture. New Phytol 94:421–432
Fitt WK, Chang SS, Trench RK (1981) Motility patterns of different strains of the symbiotic dinoflagellate Symbiodinium (=Gymnodinium) microadriaticum (Freudenthal) in culture. Bull Mar Sci 31:436–443
Freudenthal HD (1962) Symbiodinium gen. nov. and Symbiodinium microadriaticum sp. nov., a zooxanthella: taxonomy, life cycle and morphology. J Protozool 9:45–52
Goodman SJ (1997) Rst Calc: a collection of computer programs for calculating estimates of genetic differentiation from microsatellite data and determining their significance. Mol Ecol 6:881–885
Goudet J (2001) FSTAT, a program to estimate and test gene diversities and fixation indices (version 2.9.3.2). Available from http://www.unil.ch/izea/softwares/fstat.html
Goulet TL (1999) Temporal and spatial stability of zooxanthellae in octocorals. PhD dissertation, State University of New York at Buffalo, Buffalo
Goulet TL, Coffroth MA (2003) Genetic composition of zooxanthellae between and within colonies of the octocoral Plexaura kuna, based on small subunit rDNA and multilocus DNA fingerprinting. Mar Biol 142:233–239
Govind NS, Roman SJ, Iglesias-Prieto R, Trench RK, Triplett EL, Prezelin BB (1990) An analysis of the light-harvesting peridinin-chlorophyll a-proteins from dinoflagellates by immunoblotting techniques. Proc R Soc Lond B Biol Sci 240:187–195
Haubold B, Hudson RR (2000) LIAN version 3: detecting linkage disequilibrium in multilocus data. Bioinformatics 16:847–848
Hudson RR, Boos DD, Kaplan NL (1992) A statistical test for detecting geographic subdivision. Mol Biol Evol 9:138–151
Iglesias-Prieto R, Trench RK (1994) Acclimation and adaptation to irradiance in symbiotic dinoflagellates. I. Responses of the photosynthethic unit to changes in photon flux density. Mar Ecol Prog Ser 113:163–175
Iglesias-Prieto R, Trench RK (1997) Acclimation and adaption to irradiance in symbiotic dinoflagellates. II. Response of chlorophyll-protein complexes to different photon-flux densities. Mar Biol 130:23–33
Kimura M, Crow JF (1964) The number of alleles that can be maintained in a finite population. Genetics 49:725–738
Kimura M, Ohta T (1978) Stepwise mutation model and distribution of allelic frequencies in a finite population. Proc Natl Acad Sci USA 75:2868–2872
Kinzie III RA (1970) The ecology of the gorgonians (Cnidaria: Octocorallia) of Discovery Bay, Jamaica. PhD dissertation, Yale University, New Haven, Conn.
Kinzie III RA (1974) Experimental infection of aposymbiotic gorgonian polyps with zooxanthellae. J Exp Mar Biol Ecol 15:335–345
Kinzie III RA, Takayama M, Santos SR, Coffroth MA (2001) The adaptive bleaching hypothesis: experimental tests of critical assumptions. Biol Bull (Woods Hole) 200:51–58
LaJeunesse TC (2001) Investigating the biodiversity, ecology, and phylogeny of endosymbiotic dinoflagellates in the genus Symbiodinium using the internal transcribed spacer region: in search of a "species" level marker. J Phycol 37:866–880
LaJeunesse TC (2002) Diversity and community structure of symbiotic dinoflagellates from Caribbean coral reefs. Mar Biol 141:387–400
LaJeunesse TC, Trench RK (2000) Biogeography of two species of Symbiodinium (Freudenthal) inhabiting the intertidal sea anemone Anthopleura elegantissima (Brandt). Biol Bull (Woods Hole) 199:126–134
Lasker HR, Coffroth MA (1983) Octocoral distributions at Carrie Bow Cay, Belize. Mar Ecol Prog Ser 13:21–28
Loeblich III AR, Sherley JL (1979) Observations on the theca of the mobile phase of free-living and symbiotic isolates of Zooxanthella microadriaticum (Freudenthal) comb. nov. J Mar Biol Assoc UK 59:195–205
Loh WKW, Loi T, Carter D, Hoegh-Guldberg O (2001) Genetic variability of the symbiotic dinoflagellates from the wide ranging coral species Seriatopora hystrix and Acropora longicyathus in the Indo-West Pacific. Mar Ecol Prog Ser 222:97–107
Mantel NA (1967) The detection of disease clustering and a generalised regression approach. Cancer Res 27:209–220
Maynard-Smith J, Smith NH, O'Rourke M, Spratt BG (1993) How clonal are bacteria? Proc Natl Acad Sci USA 90:4384–4388
Miller MP (1997) Tools for population genetic analysis (TFPGA) 1.3: a Windows program for the analysis of allozyme and molecular population genetic data. Computer software distributed by author. Available from http://bioweb.usu.edu/mpmbio/index.htm
Nei M (1972) Genetic distance between populations. Am Nat 106:283–292
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of organisms. Genetics 89:583–590
Pawlowski J, Holzmann M, Fahrni JF, Pochon X, Lee JJ (2001) Molecular identification of algal endosymbionts in large miliolid foraminifera: dinoflagellates. J Eukaryot Microbiol 48:368–373
Perez SF, Cook CB, Brooks WR (2001) The role of symbiotic dinoflagellates in the temperature-induced bleaching response of the subtropical sea anemone Aiptasia pallida. J Exp Mar Biol Ecol 256:1–14
Pfiester LA, Anderson DM (1987) Dinoflagellate reproduction. In: Taylor FJR (ed) The biology of dinoflagellates. Blackwell, London, pp 611–648
Pochon X, Pawlowski J, Zaninetti L, Rowan R (2001) High genetic diversity and relative specificity among Symbiodinium-like endosymbiotic dinoflagellates in soritid foraminiferans. Mar Biol 139:1069–1078
Rodriguez-Lanetty M, Loh W, Carter D, Hoegh-Guldberg O (2001) Latitudinal variability in symbiont specificity within the widespread scleractinian coral Plesiastrea versipora. Mar Biol 138:1175–1181
Roff DA, Bentzen P (1989) The statistical analysis of mitochondrial DNA polymorphisms: chi-square and the problem of small samples. Mol Biol Evol 6:539–545
Rousset F (1996) Equilibrium values of measures of population subdivision for stepwise mutation processes. Genetics 142:1357–1362
Rowan R (1998) Diversity and ecology of zooxanthellae on coral reefs. J Phycol 34:407–417
Rowan R, Powers DA (1991) A molecular genetic identification of zooxanthellae and the evolution of animal–algal symbioses. Science 251:1348–1351
Rowan R, Powers DA (1992) Ribosomal RNA sequences and the diversity of symbiotic dinoflagellates (zooxanthellae). Proc Natl Acad Sci USA 89:3639–4363
Santos SR, Coffroth MA (2003) Molecular genetic evidence that dinoflagellates belonging to the genus Symbiodinium Freudenthal are haploid. Biol Bull (Woods Hole) 241:10–20
Santos SR, Taylor DJ, Coffroth MA (2001) Genetic comparisons of freshly isolated vs. cultured symbiotic dinoflagellates: implications for extrapolating to the intact symbiosis. J Phycol 37:900–912
Santos SR, Taylor DJ, Kinzie III RA, Hidaka M, Sakai K, Coffroth MA (2002) Molecular phylogeny of symbiotic dinoflagellates inferred from partial chloroplast large subunit (23S)-rDNA sequences. Mol Phylogenet Evol 23:97–111
Santos SR, Gutierrez-Rodriguez C, Coffroth MA (2003) Phylogenetic identification of symbiotic dinoflagellates via length heteroplasmy in domain V of chloroplast large subunit (cp23S)-rDNA sequences. Mar Biotechnol (in press)
Schoenberg DA, Trench RK (1980a) Genetic variation in Symbiodinium (=Gymnodinium) microadriaticum Freudenthal, and specificity in its symbiosis with marine invertebrates. I. Isozyme and soluble protein patterns of axenic cultures of Symbiodinium microadriaticum. Proc R Soc Lond B Biol Sci 207:405–427
Schoenberg DA, Trench RK (1980b) Genetic variation in Symbiodinium (=Gymnodinium) microadriaticum Freudenthal, and specificity in its symbiosis with marine invertebrates. II. Morphological variation in S. microadriaticum. Proc R Soc Lond B Biol Sci 207:429–444
Schoenberg DA, Trench RK (1980c) Genetic variation in Symbiodinium (=Gymnodinium) microadriaticum Freudenthal, and specificity in its symbiosis with marine invertebrates. III. Specificity and infectivity of S. microadriaticum. Proc R Soc Lond B Biol Sci 207:445–460
Seutin G, White BN, Boag PT (1991) Preservation of avian blood and tissue samples for DNA analyses. Can J Zool 69:82–92
Slatkin M (1995) A measure of population subdivision based on microsatellite allele frequencies. Genetics 139:457–462
Taylor DL (1974) Symbiotic marine algae: taxonomy and biological fitness. In: Vernberg WB (ed) Symbiosis in the sea. University of South Carolina Press, Coulmbia, pp 245–262
Taylor MS, Hellberg ME (2003) Genetic evidence for local retention of pelagic larvae in a Caribbean reef fish. Science 299:107–109
Trench RK (1993) Microalgal–invertebrate symbioses: a review. Endocyto Cell Res 9:135–175
Trench RK (2000) Validation of some currently used invalid names of dinoflagellates. J Phycol 36:972
Trench RK, Blank RJ (1987) Symbiodinium microadriaticum Freudenthal, S. goreauii sp. nov., S. kawagutii sp. nov. and S. pilosum sp. nov.: gymnodinioid dinoflagellate symbionts of marine invertebrates. J Phycol 23:469–481
Viard F, Franck P, Dubois M-P, Estoup A, Jarne P (1998) Variation of microsatellite size homoplasy across electromorphs, loci, and populations in three invertebrate species. J Mol Evol 47:42–51
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
Acknowledgements
We are grateful to the Bahamas Department of Fisheries for permission to collect and export samples from the Bahamas. The technical and logistical support of the staff and scientists of the Don Gerace Research Center is also greatly appreciated. We would also like to thank the crew of the R.V. "Sea Ray" for logistical support during Pseudopterogorgia elisabethae collections. Sherwood SCUBA is gratefully acknowledged for donating SCUBA equipment used in this study. We thank Dr. D. Posada (Variagenics, Inc.) for advice regarding statistical analyses. This research was supported by a National Science Foundation (NSF) Minority Graduate Fellowship (S.R.S.), IIE/Conacyt/Fulbright-Garcia Robles Fellowship (C.G.R.), NSF grants OCE-95-30057 and OCE-99-07319 (M.A.C.) and grants from the New York State Sea Grant Program (no. R/XG-9) and the National Undersea Research Center at the Caribbean Marine Research Center (no. CMRC-99-3301 and 99-NRHL-01-01C) (M.A.C. and H.R.L.). P. elisabethae samples were collected under the legal authorization of the Bahamian government.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.P. Grassle, New Brunswick
Rights and permissions
About this article
Cite this article
Santos, S.R., Gutiérrez-Rodríguez, C., Lasker, H.R. et al. Symbiodinium sp. associations in the gorgonian Pseudopterogorgia elisabethae in the Bahamas: high levels of genetic variability and population structure in symbiotic dinoflagellates. Marine Biology 143, 111–120 (2003). https://doi.org/10.1007/s00227-003-1065-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-003-1065-0