Abstract
The threatened seagrass Halophila johnsonii Eiseman coexists subtidally with H. decipiens Ostenfeld in southeastern Florida, but only H. johnsonii also occurs intertidally. Pulse amplitude modulated fluorometry and fiber-optic spectrometry were used to investigate the photobiology of two populations of H. johnsonii and H. decipiens in an attempt to explain these distribution patterns. Maximum photosynthetic quantum yields (F v/F m) were measured in situ as a function of depth distribution within, and between, these two species at two sites (Jupiter Sound, 26°57′N; 80°04′W, and northern Biscayne Bay, 25°55′N; 80°07′W) along the east coast of Florida, USA, during 6–10 March 2001. Reciprocal transplants at the northern site were used to evaluate the plasticity of photosynthetic patterns and pigment absorption spectra and to gain insights into the mechanisms responsible for variations in the observed depth-distribution patterns. Subtidal-population F v/F m values were generally higher for H. johnsonii than for H. decipiens, at both sites. At the northern site, intertidal H. johnsonii had significantly lower F v/F m (0.494±0.138) than both subtidal H. johnsonii (0.696±0.045) and subtidal H. decipiens (0.668±0.048). In contrast, at the southern site intertidal H. johnsonii had the highest F v/F m (0.663±0.047) and were the largest plants. F v/F m values of subtidal plants of both species decreased when they were transplanted into shallow, intertidal beds. Correspondingly, F v/F m increased for intertidal H. johnsonii transplanted into the subtidal, 2 m deep beds. Rapid light curves indicated that H. decipiens had lower maximum relative electron transport rates (RETRmax) than did H. johnsonii. In addition, the onset of photoinhibition occurred at lower irradiances for H. decipiens (537–820 μmol photons m−2 s−1) compared to H. johnsonii (1141–2670 μmol photons m−2 s−1). RETRmax values decreased for intertidal H. johnsonii transplanted into subtidal beds, but they increased for both species when transplanted from subtidal to intertidal beds. Absorption spectra for the acetone-soluble leaf pigments of intertidal H. johnsonii exhibited a dominant peak near 345 nm; this UV peak was 30% lower for subtidal plants. Pigment absorption spectra for H. decipiens lacked the 345 nm peak and absorbances, normalized to leaf pairs, were lower across the spectrum. Our results indicate that photosynthetic tolerance to higher irradiances and presence of UV-absorbing pigments (UVP) in H. johnsonii may allow this species to exploit the shallowest waters without competition from the closely related, but UVP-lacking H. decipiens.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The 12 recognized species in the genus Halophila are distributed in warm-temperate and tropical waters worldwide; the only pan-tropical species is H. decipiens (den Hartog 1970). Species of Halophila have the widest depth distribution of all seagrasses, occurring from the intertidal to 85 m depths (den Hartog 1970). The genus Halophila is morphologically distinguished from the other seagrass genera by having a pair of small leaves or a pseudowhorl of leaves at each rhizome node, being shallow rooted, and having two to three orders of magnitude less standing crop (leaf canopy) per unit area.
Halophila johnsonii has only been found along approximately 200 km of coastline in southeastern Florida, between Sebastian Inlet (27°51′N; 80°27′W) and north Biscayne Bay (25°45′N; 80°07′W). This narrow range and apparent endemism indicates that H. johnsonii has the most limited geographic distribution of any seagrass in the world. Within its limited distribution range, H. johnsonii is also the least abundant species (Virnstein et al. 1997). Unlike most Halophila spp., which can survive perturbations by using sexual reproduction to disperse and maximize offspring survival (Kenworthy 2000), H. johnsonii appears to reproduce only through asexual branching or apomixis, as no male flowers have been observed (Eiseman and McMillan 1980). Thus, H. johnsonii may be at a competitive disadvantage after periods of unfavorable conditions compared to the highly fecund H. decipiens. Based on these attributes, H. johnsonii was recently listed as threatened under the Endangered Species Act (Federal Register 1998), the only marine plant so listed.
H. johnsonii grows in non-contiguous patches, at depths extending from the intertidal down to 3 m (Kenworthy 1993; Virnstein et al. 1997). Intertidal populations may be completely exposed at low tides, suggesting tolerance to desiccation, to wide temperature ranges, and to high solar and UV radiation levels. However, Björk et al. (1999) reported that shallow intertidal species, such as Halophila ovalis and Halodule wrightii, were generally more sensitive to desiccation than subtidal species. They suggested that tolerance to high irradiances allows these species to occupy the uppermost intertidal zones. Although it is more commonly found in monotypic patches, H. johnsonii can also occur mixed with H. decipiens at depths of 1–3 m. However, H. decipiens is more commonly a deep-water species, and it does not occur intertidally with H. johnsonii. Distribution patterns and experimental observations suggest that H. johnsonii has a wider tolerance of salinity, temperature, and optical water quality conditions than does H. decipiens (Dawes et al. 1989). In laboratory incubations, H. johnsonii did not exhibit photoinhibition at high light intensities, as did H. decipiens (Dawes et al. 1989).
The present study compared the photobiology of H. johnsonii and H. decipiens in an attempt to explain their depth distributions. Maximum photosynthetic quantum yields (F v/F m) and maximum relative electron transport rates (RETRmax) were measured in situ using a pulse-amplitude modulated (PAM) fluorometer as a function of depth distribution within, and between, these two species at two locations. We also conducted reciprocal transplants at a northern site, to evaluate plasticity of photosynthetic patterns and pigment absorption spectra, and to gain mechanistic insights into observed constraints in the distribution patterns of these two seagrasses.
Materials and methods
Sample sites
The in situ photosynthetic characteristics and leaf pigment absorption spectra of Halophila johnsonii Eiseman (Hjon) and H. decipiens Ostenfeld (Hdec) were measured at the south end of Jupiter Sound near Jupiter Inlet (JI; 26°57′N; 80°04′W) and at Haulover Park in northern Biscayne Bay (BB; 25°55′N; 80°07′W) during the period 6–10 March 2001. At the JI site, intertidal (S, shallow) patches of monospecific H. johnsonii (HjonS) occurred on a sandbar among sparse patches of Halodule wrightii. The sandbar was approximately 25 m from shore and adjacent to the Intracoastal Waterway (ICCW). Water depths increased rapidly (intertidal to >3 m) over a short horizontal distance (<5 m). The subtidal (D, deep) H. johnsonii (HjonD) and H. decipiens (HdecD) populations occurred at approximately 2 m depths along the slope between the sand bar and the ICCW channel. The sandbar was subjected to relatively frequent boat wake waves and semi-diurnal tidal currents, due to its close proximity to the ICCW and Jupiter Inlet (about 2 km to the south). At BB, the intertidal population of H. johnsonii occurred shoreward of sparse Halodule wrightii patches adjacent to a red mangrove–lined shoreline (Rhizophora mangle). Many of the plants were actually under the seaward edge of the red mangrove canopy. The subtidal H. johnsonii (HjonD) and H. decipiens (HdecD) populations occurred at approximately 2 m depths, 50 m offshore. The BB site had a more gentle slope and broader grassbed than JI, and was adjacent to a no-wake (idle speed) zone of the ICCW, but it was also near an inlet (about 2 km north of Haulover Inlet), so like JI, was well flushed.
Fluorescence measurements
PAM chlorophyll fluorescence was measured with a Diving-PAM (Walz, Germany), using SCUBA. To minimize the effects of diurnal variations in fluorescence parameters, measurements were obtained between 1000 and 1600 hours at both sites and were randomized between species and among treatments. The tip of the instrument's optical fiber was placed 2–5 mm from, and perpendicular to, the middle of the adaxial surface of leaves using dark leaf clips. Maximum quantum yield of electron transfer in photosystem II (PSII) was determined by the saturating-light method on 5-min, dark-adapted leaves as: (F m−F o)/F m=F v/F m, where F o is the minimal fluorescence of a dark-adapted leaf in which all PSII reaction centers are open and F m is the corresponding maximum fluorescence measured with all PSII reaction centers closed following a short (0.8 s), saturating light period (e.g. Van Kooten and Snel 1990). Preliminary measurements comparing 0-, 5-, 10-, and 15-min dark-acclimation periods indicated no significant change in F v/F m after 5 min.
Rapid light curves (RLC) were produced by the Diving-PAM under the control of an internal program using artificial photosynthetic photon flux density (PPFD) from an internal halogen lamp. Eight discrete irradiance steps were used at 10-s intervals, following initial yield measurements of 115, 238, 378, 537, 820, 1141, 1785, and 2670 μmol photons m−2 s−1. The leaf was shaded from ambient light during the RLC by the dark leaf clip. The RLCs used a relative measure (because leaf absorbance was not directly measured) of electron transport rates (RETR, sensu Schwarz et al. 2000; Beer et al. 2001), calculated from the following equation: RETR=Y×PPFD×0.5×AF, where Y is quantum yield in ambient light, PPFD is the programmed level of photosynthetically active radiation (PAR, 400–700 nm) delivered by the halogen lamp (values were validated in the laboratory using a calibrated cosine-corrected LiCor quantum sensor), 0.5 assumes half of the photons absorbed are absorbed by PSII, and AF is the fraction of the PPFD absorbed by the leaf. The instrument default value for AF is 0.84, which is a representative value for terrestrial plant leaves. Reported absorption factors (AF) for seagrasses range from 0.44 for Zostera marina (Beer et al. 1998) to 0.84 for Thalassia testudinum (Major and Dunton 2002). Because we were interested in comparative differences in photosynthetic characteristics and changes in responses with depth, we used the instrument default AF value to calculate RETR, as recommended by Beer et al. (2001). The units of RETR are micromoles electrons per square meter per second. Data from the RLCs were fitted to a hyperbolic tangent function (Jassby and Platt 1976) using a non-linear, curve-fitting procedure in SigmaStat (Jandel Scientific, San Rafael, Calif, USA). The equation yielded values for the initial light-limited slope of RETR versus PPFD (α) and light-saturated RETR (RETRmax). These values were then used to calculate the irradiance at the onset of saturation (E k) as described by Schwarz et al. (2000).
Pigment extraction and analysis
Leaf material was ground in low light, using a chilled (in ice) mortar and pestle in 90% acetone. To minimize age-related, ramet-to-ramet variation in leaf pigment content, sampled leaf material was standardized as the second leaf pair back from the rhizome apical. Acetone extracts (4–6 ml) were poured into graduated centrifuge tubes wrapped in aluminum foil and stored at 4°C in the dark to allow suspended material to settle. Absorbance spectra (280–750 nm) were measured in 1 cm quartz cuvettes illuminated by a halogen/deuterium light source using an Ocean Optics (Dunedin, Fla., USA) S2000 fiber optic spectrometer. Absorbance spectra for each leaf pair were normalized by volume of acetone in the extracts. Chlorophyll a and b contents were calculated using the equations of Jeffrey and Humphrey (1975). Ultraviolet pigment peaks were detected using the automatic peak location function in GRAMS/32 spectroscopic analysis software (Galactic Industries, Salem, N.H., USA).
Reciprocal transplants
To gain insight into the role of photobiology in determining intra- and inter-specific distribution patterns, reciprocal transplants were established at the northern JI site. Control and reciprocal transplants were established by removing 7.5 cm×7.5 cm plugs with the sediment and rhizomes intact from established beds using a sod plugger and placing the plugs into 7.5 cm×7.5 cm peat pots (Heidelbaugh et al. 1999). Ten replicate planting units (PU) were transplanted for each treatment: H. johnsonii intertidal to subtidal (HjonS2D), H. johnsonii intertidal to intertidal (HjonS2S, intertidal control), H. johnsonii subtidal to intertidal (HjonD2S), H. johnsonii subtidal to subtidal (HjonD2D, subtidal control), H. decipiens subtidal to intertidal (HdecD2S), and H. decipiens subtidal to subtidal (HdecD2D, subtidal control). Transplants and transplant plots were established within the same beds where initial PAM-fluorometric and pigment measurements were made. After 4 days, photosynthetic characteristics of the transplants were determined, and leaf material was sampled for pigment extraction and analysis.
Statistics
Fluorescence and pigment data were compared in a pairwise manner between depths, sites, reciprocal transplant treatments, and species. All data were tested for normality and homogeneity of variances by the Kolmogorov–Smirnov test and the Levene median test, respectively. If assumptions were satisfied, t-tests were performed. If data were non-normal or variances non-homogeneous then non-parametric analyses (Mann–Whitney signed rank test) were used. All statistics were calculated using SigmaStat 2.0 (Jandel Scientific, San Rafael, Calif., USA) with significance determined at the 95% probability level (P<0.05). All values are reported as means±1 SD.
Results
Fluorescence
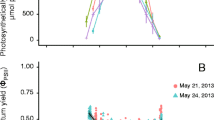
Maximum quantum yields (F v/F m) for Halophila johnsonii and H. decipiens at the northern and southern sites ranged from 0.250 to 0.740 (Fig. 1a). Within both species, the subtidal JI population had significantly higher F v/F m values than the subtidal BB population. Within sites, F v/F m values for subtidal H. johnsonii were generally higher than those for H. decipiens, although these differences were not significant. Intertidal H. johnsonii at BB had F v/F m values that were significantly higher than those of intertidal JI plants. The intertidal BB plants were also the largest and most robust plants we sampled. Values of F v/F m for the intertidal BB plants were not significantly different than those of the subtidal BB population.
Halophila johnsonii (Hjon) and Halophila decipiens (Hdec). Comparison of maximum quantum yields (F v/F m) for: a intertidal (S shallow) and subtidal (D deep) populations at Jupiter Inlet (JI) and Biscayne Bay (BB) and b reciprocal transplants from intertidal-to-subtidal (S2D) and subtidal-to-intertidal (D2S) locations at Jupiter Inlet, 4 days after transplanting (S2S shallow, intertidal control; D2D deep, subtidal control; dotted line mean; solid horizontal line median; box 25th and 75th percentiles; error bars 10th and 90th percentiles, n=6, except HdecD2S where n=1)
Maximum quantum yields of the reciprocal transplants of H. johnsonii indicated rapid, depth-based adjustments in photosynthetic characteristics (Fig. 1b). F v/F m increased for intertidal plants when moved to greater depths, which is consistent with the higher F v/F m of subtidal plants (compare Fig. 1a and b). Accordingly, F v/F m decreased for subtidal plants of H. johnsonii when moved to the shallower intertidal zone. Intertidal control transplants (HjonS2S) exhibited higher yields than ambient intertidal plants (HjonS—JI). Only one planting unit of subtidal H. decipiens survived being transplanted into the intertidal zone. F v/F m values for the subtidal treatment control, HdecD2D and the sole surviving HdecD2S were generally lower than those of any of the H. johnsonii transplants and ambient subtidal plants (HdecD—JI).
RLC data exhibited typical saturation kinetics and also revealed depth-, site-, and species-based differences in the photobiology of H. johnsonii and H. decipiens (Fig. 2). Photoinhibition was evident for both species. Intertidal H. johnsonii exhibited photoinhibition only at the highest PPFD applied (1785–2670 μmol photons m−2 s−1, Fig. 2a), while subtidal plants exhibited initial photoinhibition at a lower PPFD (>1141 μmol photons m−2 s−1, Fig. 2b). Subtidal H. decipiens exhibited photoinhibition at even lower PPFD than subtidal H. johnsonii (537 μmol photons m−2 s−1 for HdecD—JI and 820 μmol photons m−2 s−1 for HdecD—BB, Fig. 2c). At the JI site, values for the light-limited increase in RETR with PPFD, α, were indicative of typical sun/shade plant patterns (Fig. 3a), being significantly higher for subtidal H. johnsonii and H. decipiens compared to intertidal H. johnsonii. In contrast, α for intertidal H. johnsonii at BB was significantly greater than for subtidal plants of both species and significantly greater than for intertidal H. johnsonii at JI. Within both species, subtidal JI plants had significantly higher α than subtidal BB plants. Average RETRmax was lower for subtidal H. johnsonii than for intertidal plants at both sites (Fig. 3b), although the difference was significant only for the BB population. RETRmax was significantly higher for H. johnsonii compared to H. decipiens at JI, and, for the latter species, the BB population had significantly higher RETRmax compared to the JI population. The irradiance at the onset of saturation, E k, was highest for intertidal H. johnsonii and lowest for subtidal H. decipiens at JI (Fig. 3c). There was little depth- or species-based variation in E k at BB, possibly reflecting less variability in PPFD between the intertidal (S) and subtidal (D) locations at BB during our PAM measurements (BB—S=380±180 μmol photons m−2 s−1, BB—D=410±100, JI—S=695±209, JI—D=267±116, irradiances measured by the cosine-corrected quantum sensor of the Diving-PAM).
Halophila johnsonii and Halophila decipiens. Rapid light curves for: a intertidal H. johnsonii, b subtidal H. johnsonii, and c subtidal H. decipiens populations at Jupiter Inlet and Biscayne Bay. Abbreviations as in Fig. 1 (PPFD photosynthetic photon flux density; RETR relative electron transport rates; symbols mean; error bars SD, JI n=12, BB n=6)
Halophila johnsonii and Halophila decipiens. Mean (±SD) rapid light curve coefficients for: a slope of RETR versus PPFD (α), b maximum relative electron transport rate (RETRmax), and c irradiance at the onset of saturation (E k) for intertidal and subtidal H. johnsonii and subtidal H. decipiens populations at Jupiter Inlet and Biscayne Bay. Abbreviations as in Fig. 1. Significant differences (t-test, P<0.05) are indicated with asterisks; JI n=12, BB n=6
Changes were evident in RLC characteristics for the reciprocal transplants after 4 days (Fig. 4). Intertidal-to-subtidal transplants of H. johnsonii had generally lower RETR at all PPFDs (Fig. 4a). Subtidal-to-intertidal transplants of both H. johnsonii (Fig. 4b) and H. decipiens (Fig. 4c) exhibited less photoinhibition and did not initiate photoinhibition until higher PPFD than their corresponding subtidal-to-subtidal controls. In addition, subtidal-to-intertidal transplants had lower α, higher RETRmax, and lower E k than their controls (Fig. 5). Intertidal-to-subtidal transplants of H. johnsonii exhibited little change in α, but they exhibited a significant decline in RETRmax and an increase in E k.
Halophila johnsonii and Halophila decipiens. Rapid light curves for reciprocal transplants after 4 days: a intertidal-to-subtidal (S2S, n=5) H. johnsonii, b subtidal-to-intertidal (D2S, n=4) H. johnsonii, and c subtidal-to-intertidal (D2S, n=2) H. decipiens populations at Jupiter Inlet [S2S shallow control (Hjon n= 5); D2D deep control (Hjon n=4, Hdec n=4)]. Other abbreviations as in Figs. 1, 2 (symbols mean; error bars SD)
Halophila johnsonii and Halophila decipiens. Mean (±SD) rapid light curve coefficients for: a slope of RETR versus PPFD (α), b maximum relative electron transport rate (RETRmax), and c irradiance at the onset of saturation (E k) for intertidal-to-subtidal (S2D) H. johnsonii and subtidal-to-intertidal (D2S) reciprocal transplants of H. johnsonii and H. decipiens at Jupiter Inlet [S2S shallow control (Hjon n= 5); D2D deep control (Hjon n=4, Hdec n=4)]. Other abbreviations as in Figs. 1, 2. Significant differences (t-test, P<0.05) indicated with asterisks
Absorption spectra and pigments
The UV-visible light absorption spectra for 90% acetone extracts of H. johnsonii leaves showed strongest absorption in the UV-A region, between 320 and 380 nm (Fig. 6a, b). In contrast, the absorption spectra for H. decipiens leaves lacked this UV-A peak, although there was a shoulder in the spectra between 320–400 nm (Fig. 6c). Both populations and species exhibited peaks characteristic of chlorophylls. Absorption spectra for both H. johnsonii and H. decipiens indicated that the southern BB populations had generally higher pigment content per leaf pair. For intertidal H. johnsonii, chlorophyll a and b levels were significantly higher in the BB than the JI populations (Fig. 7a, b). Peak UV absorbances were also significantly higher for the BB intertidal and subtidal H. johnsonii populations compared to the JI populations (Fig. 7d). Chlorophyll a/b ratios for subtidal H. johnsonii were significantly greater than chlorophyll a/b ratios for subtidal H. decipiens at both sites (JI: 2.29±0.17 vs. 2.01±0.13, P=0.03 and BB: 2.17±0.04 vs. 1.96±0.05, P<0.001 for H. johnsonii vs. H. decipiens, respectively; Fig. 7c).
Halophila johnsonii and Halophila decipiens. Mean absorption spectra of 90% acetone leaf extracts for: a intertidal Hjon, b subtidal Hjon, and c subtidal Hdec populations at Jupiter Inlet (n=8) and Biscayne Bay (n=4). Abbreviations as in Fig. 1
Halophila johnsonii and Halophila decipiens. Mean (±SD) leaf: a chlorophyll a, b chlorophyll b, c chlorophyll a/b ratios, and d peak UV absorbance for intertidal and subtidal H. johnsonii and subtidal H. decipiens populations at Jupiter Inlet and Biscayne Bay. Abbreviations as in Fig. 1. Significant site differences (t-test, P<0.05) are indicated with asterisks; JI n=8, BB n=4
The UV-visible absorption spectra for the reciprocal transplants of H. johnsonii (Fig. 8) showed a decrease in the UV-A absorption for intertidal plants transferred to the subtidal plot and an increase in this peak for subtidal plants transplanted into the intertidal zone. This increase was significant for the HjonD2S treatment (Fig. 9d). No significant changes in chlorophyll contents were observed (Fig. 9a–c). There was also little change in the absorption spectra for subtidal-to-intertidal transplants of H. decipiens, although there was a slight increase in the UV peak for subtidal-to-intertidal transplants (Fig. 9d).
Halophila johnsonii and Halophila decipiens. Mean absorption spectra of 90% acetone leaf extracts of: a intertidal-to-subtidal Hjon (S2D, n=4), b subtidal-to-intertidal Hjon (D2S, n=4), and c subtidal-to-intertidal Hdec (D2S, n=3) reciprocal transplants after 4 days at Jupiter Inlet [S2S shallow control (Hjon n=3); D2D deep control (Hjon n=3, Hdec n=3)]. Other abbreviations as in Fig. 1
Halophila johnsonii and Halophila decipiens. Mean (±SD) leaf: a chlorophyll a, b chlorophyll b, c chlorophyll a/b ratios, and d peak UV absorbance for intertidal-to-subtidal (S2D, Hjon n=4) and subtidal-to-intertidal (D2S, Hjon n=4, Hdec n=3) reciprocal transplants of H. johnsonii and H. decipiens at Jupiter Inlet. Other abbreviations as in Fig. 1. Significant transplant differences (t-test, P<0.05) indicated with asterisk
Discussion
Photosynthetic characteristics, measured by pulse amplitude modulated chlorophyll a fluorescence in situ and pigment compositions of Halophila johnsonii and H. decipiens exhibited significant differences that may, at least partially, explain the different depth distributions of these two species. Absorption spectra of leaf pigments revealed that the intertidally distributed species H. johnsonii contains high levels of ultraviolet-absorbing pigments (Yakoleva and Titlyanov 2001), which are characteristic of high-light-adapted species (Franklin et al. 1996; Hader et al. 1998a). UVPs were largely absent in H. decipiens. Photoinhibition was evident for both species; however, the irradiance levels for the onset of photoinhibition were much lower for the deeper, subtidally distributed species (537–830 μmol photons m−2 s−1 for H. decipiens vs. 1785–2670 μmol photons m−2 s−1 for H. johnsonii). Within H. johnsonii, the deeper growing subtidal plants exhibited greater photoinhibition than intertidal individuals. Depth-related increases in photoinhibition, as measured by changes in F v/F m, have been observed for macroalgae (Franklin et al. 1996; Yakoleva and Titlyanov 2001) and seagrasses (Ralph et al. 1998). High-irradiance-induced decreases in F v/F m are thought to reflect dissipation of excess energy within the light-harvesting antennae (i.e. photoprotection, Krause 1991), photodamage to PSII (Franklin et al. 1996), or both processes (Ralph and Burchett 1995). The responses exhibited by H. johnsonii probably reflect primarily photoprotection, or possibly changes in leaf optical properties, as evidenced by both the increase in UVPs and reduction in F v/F m under increased PAR conditions (Franklin et al. 1996; Gorbunov et al. 2001; Major and Dunton 2002; present paper, see below).
Dawes et al. (1989) observed strong photoinhibition at irradiances >300 μE m−2 s−1 in H. decipiens collected from 20 m depths, and they suggested that deep-water populations of H. decipiens are intolerant of irradiances >1000 μE m−2 s−1. Maximum photosynthetic rates (P max) and compensation irradiances (I c) of H. decipiens, measured using oxygen fluxes, were lower, while α values were more than double those of H. johnsonii (Dawes et al. 1989). The relatively lower RETRmax and E k values and generally higher α that we measured in situ for H. decipiens are in agreement with the laboratory-based oxygen-flux measurements. Both the oxygen and fluorescence results indicate greater photosynthetic light-efficiency, adaptation to lower irradiances, and inhibition by high irradiances for the more deeply distributed H. decipiens, compared to the shallow-water H. johnsonii.
The lowest quantum yields (F v/F m) were measured in the shallow-intertidal H. johnsonii population at the northern Jupiter Inlet site. This population existed on an offshore sand bar that was partially exposed at low tides. Prolonged exposures to high irradiance are known to induce declines in F v/F m in marine plants (Ralph and Burchett 1995; Hader et al. 1998a, 1998b; Yakoleva and Titlyanov 2001; Durako and Kunzelman 2002). It has recently been suggested that this represents a trade-off between photosynthetic efficiency and photoprotection (Major and Dunton 2002). Accordingly, F v/F m values were generally higher for deeper plants of both species at both sites, consistent with past reports of increases in leaf fluorescence ratios in response to decreased irradiance in seagrasses (Dawson and Dennison 1996; Major and Dunton 2002). The relatively high F v/F m values for the intertidal H. johnsonii at Biscayne Bay at first seemed anomalous. However, it may simply reflect the location of the sampled population, immediately adjacent to a red mangrove–lined shoreline (the landward edge of the bed was actually under the shadow of the mangrove canopy). Because the mangrove canopy is located east of the shallow plants, they are shaded during most of the morning and do not receive direct sunlight until late morning, thus significantly reducing the daily quantum flux to these plants. The intertidal plants at the BB site had the highest pigment levels and were the largest and most robust plants of the populations we sampled.
The decrease in F v/F m in macroalgae and seagrasses in response to high irradiances has also been associated with an increase in UV-blocking or UV-absorbing pigment (UVP) levels (Dawson and Dennison 1996; Franklin et al. 1996; Hader et al. 1998a, 1998b; Yakoleva and Titlyanov 2001). Ralph et al. (1998) observed that seagrasses in shallow water have a higher capacity for light protection and non-photochemical quenching compared to deep-water seagrasses. In our reciprocal transplants, H. johnsonii exhibited a significant reduction in F v/F m and a significant increase in UVPs, within 4 days of being transplanted from 2 m depths to the intertidal zone (i.e. to higher PAR and UV conditions). The decreases in F v/F m in H. johnsonii were due to decreasing F m rather than increases in F o (data not shown). These changes are indicative of non-photochemical quenching rather than destruction of PSII reaction centers, and they are consistent with the observed increase in UVPs in the subtidal-to-intertidal transplants (Dawson and Dennison 1996; Franklin et al. 1996). H. decipiens had generally lower F v/F m values compared to H. johnsonii, and very low UVP absorbance. The lack of UVPs may have contributed to this species' high mortality when transplanted to the shallow site. Maximum quantum yields for the transplanted H. decipiens were even lower when compared to the H. johnsonii transplants and the undisturbed subtidal H. decipiens. The reduction in photosynthesis, particularly for the subtidal transplant controls (D2D), may also reflect greater stress or injury, for these morphologically more-delicate plants, in response to transplanting.
Photosynthetic organisms exposed to high levels of visible and UV radiation have developed several pigment-based mechanisms to counteract the damaging effects of the additional radiation (Sinha et al. 1998). Seagrasses rely on production of UV-absorbing pigments for protection against high irradiances and UV radiation (Trocine et al. 1981; Dawson and Dennison 1996; Hader et al. 1998a). UV-blocking pigment absorbances increase in response to increases in both PAR and UV (Dawson and Dennison 1996; Detras et al. 2001). In this study, chlorophyll and UVP contents were normalized to relative ramet age (second leaf pair behind the apical). We did this because of our observations of significant age-related variability in leaf photosynthetic and pigment characteristics in the relatively short-lived leaves (15–20 days, authors' unpublished data) in these two species. However, the significantly higher leaf chlorophyll contents and levels of the UVPs for the southern population may partially have been due to the larger leaf sizes of the Biscayne Bay plants, rather than differing irradiance levels between the two sites (they are only ~100 km apart). The chlorophyll a/b ratios (~2) that we calculated for both H. johnsonii and H. decipiens were slightly lower than those recently reported for Thalassia testudinum exposed to different UV treatments (2.46–2.82, Detras et al. 2001) and irradiances (3.1–3.4, Major and Dunton 2002). The lack of significant changes in chlorophyll a/b ratios to varying irradiance treatments in T. testudinum and the relative constancy of chlorophyll a/b ratios in H. johnsonii and H. decipiens across sites and depths indicate little role for these pigments in photoprotection of seagrasses. In contrast, UVP levels significantly increased within 4 days for the subtidal-to-intertidal reciprocal transplants, indicating that photoadaptation to higher UV radiation occurs rapidly in H. johnsonii. Our in situ results were similar to the significant changes observed after 1 week of exposure to experimental manipulations of PAR and UV in aquarium-grown seagrasses (Dawson and Dennison 1996). The UVP levels in our reciprocal transplants also responded to decreasing irradiances in a manner similar to the patterns exhibited by other subtropical seagrasses, decreasing in response to reductions in PAR and UV (Dawson and Dennison 1996; Detras et al. 2001).
The cyanobacterium Anabaena sp. is able to increase its UVP content in response to UV stress within 24 h, and thus may be able to adapt to day-by-day fluctuations in UV radiation (Sinha et al. 1999). The absorption spectra of methanol extracts of Anabaena sp. exposed to elevated PAR and UV exhibited a prominent peak at 334 nm, which was attributed to a mycosporine-like amino acid (MAA, Sinha et al. 1999). MAAs are water-soluble substances having absorption maxima ranging from 310 to 360 nm (Sinha et al. 1998). In terrestrial plants, UV radiation induces the synthesis of flavonoids. McMillan et al. (1980) isolated two sulfated flavones and a single sulfated phenolic acid from T. testudinum. Flavones are a type of flavonoid accumulated in herbaceous plants. In seagrasses, they have peak absorbances of 310–350 nm (Abal et al. 1994). The absorption spectra for acetone extracts of H. johnsonii leaves exhibited a peak of 343–348 nm, which could be indicative of MAA or flavonoid absorption. However, flavonoids are known to protect vascular plants from UV radiation, and MAAs are thought to serve this same function in lower organisms (Sinha et al. 1998). MAAs have not been isolated from any vascular plant.
As first suggested by Dawson and Dennison (1996), changes in F v/F m and UV-blocking pigments in response to elevated levels of PAR and UV may be used as an indicator of radiation sensitivity. Tolerant species may photoadapt to high PAR and UV by large increases in UVPs and associated decreases in F v/F m. However, sensitive species lack these photoprotective mechanisms, thus constraining their upper depth distribution. The presence of UVPs in H. johnsonii may allow this species to exploit the shallowest waters without competition from the closely related, but UVP-lacking H. decipiens. However, survival of the shallowest H. johnsonii populations may be threatened by other perturbations associated with intertidal fringe areas such as exposure to breaking waves, desiccation at low tides (Björk et al. 1999), and shoreline development activities. While H. decipiens may not be able to survive intertidally, its depth range extends much deeper than the 3 m maximum depth of occurrence observed for the threatened H. johnsonii (Kenworthy 2000). At the lower depth limits, turbidity is a more critical factor. Thus, degradation of water quality due to human impacts, which would result in a more narrow depth range, may pose a more significant threat to H. johnsonii than continued increases in UV radiation.
References
Abal EG, Lorenagan N, Bowen P, Perry CJ, Udy JW, Dennison WC (1994) Physiological and morphological responses of the seagrass Zostera capricorni Aschers, to light intensity. J Exp Mar Biol Ecol 178:113–129
Beer S, Vilenkin B, Weil A, Veste M, Susel L, Eshel A (1998) Measuring photosynthetic rates in seagrasses by pulse amplitude modulated (PAM) fluorometry. Mar Ecol Prog Ser 174:293–300
Beer S, Björk M, Gademann R, Ralph P (2001) Measurements of photosynthetic rates in seagrasses. In: Short FT, Coles RG (eds) Global seagrass research methods. Elsevier, Amsterdam, pp 183–198
Björk M, Uku J, Weil A, Beer S (1999) Photosynthetic tolerances to dessication of tropical intertidal seagrasses. Mar Ecol Prog Ser 191:121–126
Dawes CJ, Lobban CS, Tomasko DA (1989) A comparison of the physiological ecology of the seagrasses Halophila decipiens Ostenfeld and H. johnsonii Eiseman from Florida. Aquat Bot 33:149–154
Dawson SP, Dennison WC (1996) Effects of ultraviolet and photosynthetically active radiation on five seagrasses. Mar Biol 125:629–638
den Hartog C (1970) The seagrasses of the world. North-Holland, Amsterdam
Detras Y, Armstrong RA, Connelly XM (2001) Ultraviolet-induced responses in two species of climax tropical marine macrophytes. J Photochem Photobiol B Biol 62:55–66
Durako MJ, Kunzelman JI (2002) Photosynthetic characteristics of Thalassia testudinum measured in situ by pulse-amplitude modulated (PAM) fluorometry: methodological and scale-based considerations. Aquat Bot 73:173–185
Eiseman NJ, McMillan C (1980) A new species of seagrass, Halophila johnsonii, from the Atlantic coast of Florida. Aquat Bot 9:15–19
Federal Register (1998) Endangered and threatened species: threatened status for Johnson's seagrass. 63:49035–49041
Franklin LA, Seaton GGR, Lovelock CE, Larkum AWD (1996) Photoinhibition of photosynthesis on a coral reef. Plant Cell Environ 19:825–836
Gorbunov MY, Kolber ZS, Lesser MP, Falkowski PG (2001) Photosynthesis and photoprotection in symbiotic corals. Limnol Oceanogr 46:75–85
Hader DP, Kumar HD, Smith RC, Worrest RC (1998a) Effects on aquatic ecosystems. J Photochem Photobiol B Biol 46:53–68
Hader DP, Lebert M, Figueroa FL, Jimanez C, Viaegla B, Perez-Rodriguez E (1998b) Photoinhibition in Mediterranean macroalgae by solar radiation measured on site by PAM fluorescence. Aquat Bot 61:225–236
Heidelbaugh WS, Hall LM, Kenworthy WJ, Whitfield P, Virnstien RW, Morris LJ, Hannisak MD (1999) Reciprocal transplanting of the threatened seagrass, Halophila johnsonii (Johnson's seagrass) in the Indian River lagoon, Florida. In: Bortone SA (ed) Seagrasses: monitoring, ecology, physiology, and management. CRC Press, Boca Raton, Fla., pp 197–210
Jassby AD, Platt T (1976) Mathematical formulations of the relationship between photosynthesis and light for phytoplankton. Limnol Oceanogr 21:540–547
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c 1, and c 2 in higher plants, algae, and natural phytoplankton. Biochem Physiol Pflanz 167:191–194
Kenworthy WJ (1993) The distribution, abundance and ecology of Halophila johnsonii Eiseman in the lower Indian River, Florida. Final Rept Office of Protected Species, National Marine Fisheries Service, Silver Spring, Md.
Kenworthy WJ (2000) The role of sexual reproduction in maintaining populations of Halophila decipiens: implications for the biodiversity and conservation of tropical seagrass ecosystems. Pac Conserv Biol 5:260–268
Krause GH (1991) Photoinhibition of photosynthesis. An evaluation of damaging and protective mechanisms. Physiol Plant 74:566–574
Major KM, Dunton KH (2002) Variations in light-harvesting characteristics of the seagrass, Thalassia testudinum: evidence for photoacclimation. J Exp Mar Biol Ecol 275:173–189
McMillan C, Zapata O, Escobar L (1980) Sulphated phenolic compounds in seagrasses. Aquat Bot 8:267–278
Ralph PJ, Burchett MD (1995) Photosynthetic responses of the seagrass Halophila ovalis (R. Br.) Hook. f. to high irradiance stress, using chlorophyll a fluorescence. Aquat Bot 51:55–66
Ralph PJ, Gademann R, Dennison WC (1998) In situ seagrass photosynthesis measured using a submersible, pulse-amplitude modulated fluorometer. Mar Biol 132:367–373
Schwarz AM, Bjark M, Buluda T, Mtolera M, Beer S (2000) Photosynthetic utilisation of carbon and light by two tropical seagrass species measured in situ. Mar Biol 137:755–761
Sinha RP, Klisch M, Graniger A, Hader DP (1998) Ultraviolet-absorbing/screening substances in cyanobacteria, phytoplankton and macroalgae. J Photochem Photobiol B Biol 47:83–94
Sinha RP, Klisch M, Hader DP (1999) Induction of a mycosporine-like amino acid (MAA) in the rice-field cyanobacterium Anabaena sp. by UV radiation. J Photochem Photobiol B Biol 52:59–64
Trocine RP, Rice JD, Wells GN (1981) Inhibition of seagrass photosynthesis by ultraviolet-B radiation. Plant Physiol (Rockv) 68:74–81
Van Kooten O, Snel JFH (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosyn Res 25:147–150
Virnstein RW, Morris LJ, Miller JD, Miller-Meyers R (1997) Distribution and abundance of Halophila johnsonii in the Indian River lagoon. District technical memo no. 24, St. Johns River Water Managment District
Yakoleva IM, Titlyanov EA (2001) Effect of high visible and UV irradiance on subtidal Chondrus crispus: stress, photoinhibition and protective mechanisms. Aquat Bot 71:47–61
Acknowledgements
This project was supported by the National Oceanic and Atmospheric Administration, Recover Protected Species Program, Center for Coastal Fisheries and Habitat Research, National Ocean Service, Beaufort, N.C.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.P. Grassle, New Brunswick
Rights and permissions
About this article
Cite this article
Durako, M.J., Kunzelman, J.I., Kenworthy, W.J. et al. Depth-related variability in the photobiology of two populations of Halophila johnsonii and Halophila decipiens . Marine Biology 142, 1219–1228 (2003). https://doi.org/10.1007/s00227-003-1038-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-003-1038-3