Abstract
Environmental variations can have major consequences on photosynthesis and in turn impact the settlement, productivity, or survival of plants in the intertidal zones. Low tides in Hawaii fluctuate <1 m yet expose intertidal macroalgae to high tropical temperatures and irradiances, which are predicted to have negative physiological consequences. To better understand environmental variations, temperature, exposure duration, and irradiance were measured in two intertidal zones on O‘ahu over 8 days with negative tidal heights. Then to test whether these conditions were stressful, the photosynthetic response (via pulse amplitude modulated fluorometry) and relative water content of a common intertidal macroalgae, Padina sanctae-crucis, was measured over varying tidal heights. We found that P. sanctae-crucis was exposed to air from 0 to 6 h on days with tropical irradiance (237–2000 µmol photon m−2 s−1) and a range of temperatures (20.4–32.3 °C), yet plants were able to maintain relatively high water content. Photosynthetic parameters for P. sanctae-crucis derived from light response curves were found to vary with irradiance. High irradiances were associated with lowered maximum rates of electron transport (rETRmax) and effective quantum yield (Φ PSII). It was also determined that macroalgae exhibited different curves with tidal state. P. sanctae-crucis had relatively increased saturation irradiances (E k) and increased rETRmax but lowered effective quantum yield (Φ PSII) during negative tidal heights when aerial exposure is more common. P. sanctae-crucis was then exposed to air for up to 40 min in a laboratory experiment to determine the effect on fluorescence parameters. The 40-min aerial exposure, a duration smaller than which occurs at low tide, resulted in reduced rETRmax, α, and Φ PSII. This manipulation combined with field observations indicate that aggregations of thalli combined with water motion and minimal tidal fluctuations help to limit water loss and maintain photosynthetic rates. From these results, we can conclude, for this system, high irradiances are a major factor that likely limits production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anthropogenic activities have lead to changes in ocean carbon chemistry and increased sea surface temperatures (Ciais et al. 2013). Changes in the environment can have major consequences on photosynthesis and in turn impact the settlement, productivity, or survival of marine plants. To better understand the impact of changing oceans on biota, it is becoming necessary to understand how organism physiology adapts and acclimates to small-scale fluctuations in the environment (Helmuth 2009; Tomanek and Helmuth 2002).
Intertidal habitats are a model system for investigating how organisms adjust to rapid fluctuations (Tomanek and Helmuth 2002). Intertidal habitats are usually considered one of the most stressful environments for marine primary producers because of heterogeneity of habitat and rapidly changing physical and biotic conditions (Doty 1946; Connell 1961; Paine 1974; Schonbek and Norton 1978). Intertidal macroalgae that are anchored to the substratum rely on water flow to acquire limiting nutrients and CO2. These algae must also tolerate diurnally and rapidly fluctuating irradiances and temperatures and cope with periods of desiccation that accompany tidal changes (Davison and Pearson 1996). Such physiological stresses are known to alter distribution patterns (Doty 1946; Connell 1961; Paine 1974; Schonbek and Norton 1978) and impact primary production (Silva et al. 2005; Williams and Dethier 2005).
Our understanding of stress for rocky intertidal macroalgae comes primarily from laboratory studies (Johnson et al. 1974; Dring and Brown 1982; Smith and Berry 1986; Bell 1995; Dudgeon et al. 1995; Matta and Chapman 1995; Hunt and Denny 2008) and, to a lesser extent, from field studies conducted at temperate latitudes (Foster 1982; Oates and Murray 1983; Davison et al. 1993; Ganzon-Fortes 1997; Wright et al. 2004; Dethier et al. 2005; Williams and Dethier 2005; Dethier and Williams 2009). Species vary in their ability to withstand and recover from stress (Smith and Berry 1986; Matta and Chapman 1995; Davison and Pearson 1996; Beach and Smith 1997). Intertidal algae are often more tolerant of harsh emersion conditions than subtidal species (Davison and Pearson 1996). However, even the most robust intertidal brown algae (e.g., fucoids) exhibit higher rates of photosynthesis when submersed (Chapman 1995).
Temperate algae experience extended exposure to air (Williams and Dethier 2005), and photosynthesis becomes inhibited as cells overheat and dry (Davison and Pearson 1996). Therefore, desiccation is a key factor controlling production in intertidal habitats (Schonbek and Norton 1978; Davison et al. 1993). Desiccation rates can vary with solar irradiance, air temperature, wind speed, algal morphology, humidity, and varying tidal heights (Bell 1995; Dudgeon et al. 1995; Matta and Chapman 1995; Beach and Smith 1997; Schaffelke and Deane 2005; Hunt and Denny 2008). For example, turf algae which are diminutive in size and aggregated tend to be more resistant to desiccation stress than larger macrophytes (Hay 1981).
While effects of irradiance, temperature, and aerial exposure on algal production have been well studied in the laboratory for temperate and some tropical species, less is known about the actual stress intertidal algae experience in natural settings (Bell 1995; Davison and Pearson 1996; Williams and Dethier 2005). Because it is difficult to mimic natural conditions, comparisons between field and laboratory measurements of production can reveal discrepancies in photosynthetic rates (Tait and Schiel 2010). For instance, in the laboratory, investigators often isolate macroalgae from natural surroundings for prolonged durations that may facilitate stress reduction (Bertness et al. 1999; Molina-Montenegro et al. 2005). Although more recent fluorescence technologies have some limitations, they also allow researchers to measure aspects of photosynthesis relatively rapidly and in situ (or near to in situ, as in this study) (White and Critchley 1999; Ralph and Gademann 2005).
It is expected that stress experienced by reef algae in Hawaii’s tropical intertidal environment differs from the models provided by temperate systems. Tropical reef algae experience some of the highest temperatures and irradiances worldwide (Beach and Smith 1996a) with ultraviolet radiation 2× higher than in temperate regions (Beach and Smith 1996b) and the diurnal timing of spring low tides coincides with daylight hours during warm months (April–July) (www.tidesandcurrents.noaa.gov, Fig. 1). However, tides in the islands fluctuate <1 m, and seasonal wave heights can swamp exposed shores (Gosline 1965; Kay 1979; Abbott 1999). Past studies focusing on one Hawaiian intertidal alga growing high on basaltic shores, Ahnfeltiopsis concinna, found that physiological stress and response varied over microscales from the canopy to understory (Beach and Smith 1996a). For instance, A. concinna was able to adjust the ratio of pigments over a few centimeters from the plant canopy to understory to better photo-protect the exposed portion of the thalli, a tactic to cope with the excessive irradiance. In other Hawaiian macroalgal taxa, species distribution varies over shores (Cox et al. 2013) and functional forms are known to coincide with temperature zones (Bird et al. 2013) suggesting that physiological or biological variability exists among intertidal algae, even in tropical settings.
The timing and magnitude of low tides for Honolulu, Hawaii, 2008. Negative tidal heights, <−0.03 m, occur in daylight in April–July (top) when average air temperatures (°C, above bars) are warm. Low-spring low tides occur in morning (before 1000) and evening (after 1800) hours (bottom) and often do not coincide with mid-day and afternoon conditions (bar above graph shows approximate hours of daylight for the island of Oahu). Data compiled from www.noaa.gov. Months are abbreviated by first letter
These few studies discussed above provide relevant data for stress coping mechanisms of marine algae in tropical intertidal zones (Beach and Smith 1996a, Bird et al. 2013). Coping mechanisms include preferential settlement, photo-protection, and morphological variation. These studies also provide the opportunity to ask additional fundamental questions about how tropical conditions combined with micro-tidal regimes (<1 m) impact photosynthesis of a common macroalgae, Padina sanctae-crucis, found on narrow shores. Because environmental variations can have major consequences on photosynthesis and in turn impact the settlement, productivity, or survival of plants in the intertidal zone, we asked the following questions: (1) What is the range of conditions (temperature, duration of exposure to air, and irradiance) experienced by P. sanctae-crucis over days with low tides of negative tidal height? (2) Will the alga be able to retain water under these tropical conditions? (3) Will photosynthesis be reduced during low tides (periods with negative tidal height)? To address these questions, this study quantified the variation in environmental conditions in the intertidal zone and the photosynthetic activity of the common reef alga P. sanctae-crucis before, during, and after peak low tides at two sites on the south shore of O‘ahu, Hawaii. The rapid light response curve (RLC) function in the diving-PAM (pulse amplitude modulated fluorometer) was used to determine photosynthetic activity. Parameters derived from RLCs have been used successfully to reflect the relative condition of marine plants during diel and tidal cycles (Beer et al. 1998; Gévaert et al. 2003; Ralph and Gademann 2005). Algae with decreased photosynthesis may be expected to have relatively lowered effective quantum yield (Φ PSII), photosynthetic efficiency (α, the initial slope of the RLC curve), electron transport rates, and less ability to cope with irradiances above saturation (β, the negative slope of the curve at high irradiances). To examine variation with tidal phasing and changes in irradiance, measurements were collected over several consecutive days experiencing varying low tide tidal heights at different times of the day. P. sanctae-crucis was sampled extensively to determine the extent to which photosynthesis and relative water content (RWC) varied on narrow (~15–30 m wide) tidal benches. Finally, an experiment was conducted in the laboratory to isolate the effect of exposure on P. sanctae-crucis fluorescence.
Methods
Site description
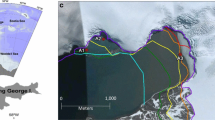
Reef algal physiology and environmental measurements were sampled at two sites on the south shore of O‘ahu, the intertidal reefs of Diamond Head (21º17′45.18, 158º06′13.42) and Kalaeloa (21º15′19.89, 157º48′37.23). Both sites possess limestone solution benches/platforms with similar diversity and abundance of reef algal species (Cox et al. 2013). Diamond Head has an intertidal region which is ~15 m wide at mean low, low water and contains a nearshore depression that allows water to pool during low tides with little to no tidal fluctuation. The tidal reef at Kalaeloa is a ~30-m-wide bench with an area mid-shore that is slightly elevated with respect to sea level.
Padina sanctae-crucis
On both shores, P. sanctae-crucis (a tropical cosmopolitan species) forms monospecific stands near the high tide line and extends into the mid-intertidal area and, although less abundant, can continue into the subtidal habitat (Cox et al. 2013; Abbott and Huisman 2004). P. sanctae-crucis has a thin, fan-shaped morphology, and its surface is lightly calcified.
Tidal selection and timing
Environmental and fluorescence measurements were collected, at sites, on May 4–11, 2008, between 0700 and 1530 hours during typical low tides for summer months and also during some of the lowest, low tides of the year (www.tidesandcurrents.noaa.gov) (Fig. 1; Table 1). Stress events could influence fitness, and thus, it was necessary to capture environmental conditions and responses to typical and more “extreme” low, low tides that occur every year.
Reef algal physiology and environmental measurements were sampled at each site for 4 days (Diamond Head = May 4, 7, 8, 11; Kalaeloa = May 5, 6, 9, 10) for a total of eight consecutive days of sampling (Table 1). An effort was made to sample reef algae and the environment continuously before, during, and after the peak low tide. We took this continuous, sampling approach instead of measuring two conditions, submersed or emersed, in order to account for changes in irradiance that may be unrelated to tides. When low tides occurred in early morning hours (on May 4 at 0834 and May 5 at 0907), the measurements were sampled near to low tide and sampling continued periodically until the shore was fully submersed.
Environmental variables
The environmental variables temperature, irradiance, wind speed, and aerial exposure duration were measured on the same days (May 4–11, 2008) at the two sites simultaneously with the collection of fluorescence measures (between 0700 and 1530 hours). One Hobo© Water Temp ProV2 temperature logger was placed in each region of the intertidal reef (near the high tide mark, mid-shore, and near the subtidal zone), and temperatures were logged every 60 s. Irradiances were measured with two, Li-Cor 4 pi sensors and LI-1400 data loggers. One Li-Cor sensor was placed near the intertidal to measure irradiances in air. The second sensor was placed in a tidepool with ~12 cm of water above it. Irradiance was collected every second, and the integration recorded every 15 min by the data recorder. Landscape photographs were taken every 15 min from the same location to estimate aerial exposure for different areas of the shore. In these images, habitat near, mid, and far from the subtidal zone was visually assessed. For each 15-min photograph, if water covered an area of habitat, it was considered submersed, and if water did not cover the habitat, it was considered exposed. From this serial image assessment, we could roughly estimate the aerial exposure duration by habitat. With the exception of irradiance, which is inherently linked to energy harvesting, these measurements were collected at the landscape scale and were used solely to describe the habitat. Therefore, habitat measurements were not included in analyses as explanatory variables for photosynthesis.
Method used to measure photosynthetic activity
Padina sanctae-crucis was sampled with the pre-programmed rapid light curve routine in the diving-PAM (Walz Co) immediately after collection from the shore. It was not possible to use the portable PAM directly on attached individuals or to permanently attach the instrument because of the rugged, slippery rock surfaces, and constant wave forces that occur in these habitats.
Upon arrival at each site, three to nine individuals of P. sanctae-crucis were collected in haphazard order at varying distances from the low tide line. Samples were immediately placed into separate open containers. These containers were actually a series of shallow (3 cm deep) wells or depressions in white Styrofoam material. Containers were either (1) filled with seawater, collected with the alga, if the alga was submersed or (2) left dry but damp if the alga was emersed. Then, containers were brought to shore and fluorescence measurements taken in a haphazard order and within 30 min from collection. Samples were submersed in freshly collected seawater during the measurement of fluorescence (a RLC can be produced in ~3 min). While individual measurements were made, the remaining samples were maintained briefly in shaded areas to prevent drying or overheating. A pilot experiment (discussed in detail below) showed that this procedure had a limited handling effect on fluorescence measures. Then, new, fresh samples were collected, and the protocol repeated until the shore was submersed for at least 1 h.
This protocol resulted in 3–9 different individual thalli being measured every 30–45 min from 0700 to 1530 hours on 8 days during periods with positive and negative tidal heights. The resulting dataset consisted of 357 fluorescence measures. We compared 156 measures collected on positive tidal heights to 201 measures collected on negative tidal heights.
Rapid light response curves (RLCs)
The RLC routine in the diving-PAM was used to determine the photosynthetic activity of light-adapted reef alga (White and Critchley 1999; Ralph and Gademann 2005). The PAM was equipped with a red light-emitting diode and an internal halogen lamp to provide actinic light. Algal samples were placed one layer thick into the dark leaf clip attached to the dark adapter (both accessories of Walz diving-PAM), which held the fiber optic cable at a 90° to the sample and maintained a distance of 3 mm from the algal surface. The PAM settings (measuring intensity = 3, gain = 2, damp = 2) were adjusted to optimize signal (~400 U) and dampen background noise. Eight increasing irradiances were delivered to the alga over 90 s. Irradiances ranged between 0 and 1625 µmol photon m−2 s−1 at the light curve intensity setting of 4. After sampling, irradiance values were adjusted for battery decline using the standard function in the WinControl software. Thus, irradiance ranges for each increasing step were as follows: 0, 36–86, 58–133, 80–192, 122–288, 170–426, 273–681, 421–1042, 652–1625 µmol photon m−2 s−1. The PAR readings were measured with the microfiber quantum sensor of the diving-PAM that was calibrated to a Li-Cor quantum sensor for measurement in water. The actinic light was applied for 10 s followed by a saturating pulse (0.8 s) to compare the minimum (F) and maximum quantum yield (\(F_{\text{m}}^{{\prime }}\)). These values were then used to calculate the effective quantum yield (Φ PSII) as the indication of the amount of energy used in the photochemistry of PSII (Genty et al. 1989).
From Φ PSII, an approximation of the rate of electrons (rETR) pumped through the photosynthetic chain (Beer et al. 2001) was calculated using the following equation:
where the factor 0.5 accounts for the assumption that half the photon energy absorbed by photosynthetic pigments was diverted into photosystem II. The actual factor is not known; however, 0.5 value is commonly used in the literature for a variety of marine and terrestrial plants (see Walz Manual for the diving-PAM, Genty et al. 1989; White and Critchley 1999; Beer and Bjork 2000; Maxwell and Johnson 2000; Ihnken et al. 2010) and was a constant value in calculations. AF = 0.75 is the fraction of incident light that is absorbed by P. sanctae-crucis. We determined AF following the methods described in Beer and Bjork (2000) on a low tide on June 11, 2010. Measurements were conducted for four individuals and averaged (0.75 ± SE 0.08). The same AF value of 0.75 for P. sanctae-crucis was found using in vivo absorbance method by Beach et al. (2006).
Although the intensity range of actinic light steps varied among samples with battery decline, rETR values plotted against irradiance had points within the initial slope, ramped to an asymptote, and leveled off at higher irradiances. Curves were fitted (R > 98 %, p < 0.001) with the exponential model proposed by Platt et al. (1980) using the following Levenberg–Marquardt regression algorithm:
where P s is a scaling parameter defined as the maximum potential rETR; α is the photosynthetic efficiency measured by the initial slope of the RLC before the onset of saturation; E is the photon flux density; β is the negative slope of the RLC for high irradiances. In the absence of dynamic photo-inhibition (β = 0), the function becomes a standard rectangular hyperbola, with an asymptotic maximum rETR value (Harrison and Platt 1986), and the equation loses exp(−βE/P s). rETRmax (relative electron transport maximum) and E k (minimum saturation irradiance) were estimated using the following equations:
The light-adapted effective quantum yield (Φ PSII) was taken from the first measure of the RLC when irradiance was 0 µmol photon m−2 s−1, prior to the increasing intensity of the actinic light steps.
RLCs, as a measure of light harvesting ability, reflect the short-term history of plants to environmental conditions, influenced by long-term conditioning (Ralph and Gademann 2005). For this reason, there is a concern that our sampling protocol which removed plants from their habitat and maintained them for 15–30 min before measurements may have altered the short-term environmental conditions and biased results. However, we took great care to collect our data in the best manner possible, and we took several steps to investigate the quality and accuracy of the data. First, we tried to limit the amount of time between collection and fluorescence measures. All samples were measured within approximately 30 min, and most were taken within 15 min from time of collection. Secondly, we tried to mimic conditions and limit the introduction of new stresses (extreme temperatures and irradiance) on the shore during the holding period. Furthermore, all samples were handled similarly, and thus, any directional bias should be consistent across samples. Also, analyses from the same sampling interval found that the first RLC produced had similar parameters to the last RLC produced, suggesting that values did not change substantially over the 15- to 30-min time period. Lastly, we conducted a pilot experiment where values of fluorescence were measured initially, plants held as described, and then, fluorescence measured again every 10 min for a 40-min time interval. Light curve parameters did not significantly change from initial values with the exception of one variable (the minimum saturation irradiance E k) (Online Resource 1). This variable initially decreased over the first 10 min, in direct opposition to our findings.
Statistical analyses of photosynthetic activity
Environmental variables were either summarized by site (temperature, aerial exposure, wind speed, and irradiance), shore location (temperature and aerial exposure), and in air, water, or both (irradiance). Prior to computation, temperature and irradiance were smoothed with a polynomial regression and weights computed from a Gaussian density function.
An analysis of covariance (ANCOVA) was used to test whether low tides impacted fluorescence while accounting for changes in irradiance. Measures by site were pooled into one data set and transformed (exponentiate log10 or rank) when necessary to meet ANCOVA requirements of equal variance and normality. For each model, homogeneity of slopes was also tested.
Variables used in the ANCOVA models include: (1) tidal state and (2) irradiance as a co-variable and (3) interaction of tidal state and irradiance. Tidal state was used to specifically test whether tides impacted plant physiology. This term was used because a low tide can be defined as the duration of the day when the tidal height is negative, and this is a measurement for when water level drops below mean sea level. Thus, in the analyses, photosynthetic parameters of algae measured during positive tidal height intervals (n = 156) were coded as 1 and measurements from negative tidal height intervals (n = 201) were coded as 2. The irradiance which was logged every 15 min was averaged by hour. Then, the hourly irradiance that corresponded in time with the sampling interval for photosynthetic measures was used in analyses. For these measures, we used irradiance measured in air when the algae were emersed and we used the irradiance measured in water to correspond to the intervals when algae were submersed. With this approach, each Φ PSII, α, E k, rETRmax, and β value for each P. sanctae-crucis individual had a corresponding and integrated irradiance measure. Due to sensor failure or loss (losses occurred on May 4 and 11), algal performance measures with missing irradiance were excluded from analyses. This approach resulted in 357 photosynthetic-irradiance measures over 8 days (117 measures from Diamond Head and 240 measures from Kalaeloa).
Heterogeneity of slopes, a violation of ANCOVA assumptions, was found for the response variable rETRmax. Thus, values were grouped by defined irradiances (700–1199 and 1200–1700 μmol photon m−2 s−1), and differences between tidal states at two irradiances were tested using a two-way ANOVA. Data from irradiances below 700 and above 1700 μmol photon m−2 s−1 did not occur for both positive and negative tidal heights and thus were not included in the analysis. An interaction of irradiance and tidal state was included in the model.
Investigations into the effect of aerial exposure
Relative water content of Padina with tides
To examine the effect of low tide on RWC, the RWC of P. sanctae-crucis was measured on June 22–25, 2009, at 0800, 1100, and 1400 hours at Diamond Head using the methods of Smith and Berry (1986). These sampling times occurred before, during, and after exposure from low tides. The mean low, low water ranged from −0.03 to −0.15 m and occurred from 0841 to 1115 hours. For each sampling, six to seven individuals were collected far and near the subtidal habitat and the fresh weight obtained. Samples were placed overnight in seawater-filled containers and allowed to rehydrate. The difference between fresh and rehydrated wet weight was standardized to dry weight [RWC = (fresh weight − dry weight)/(hydrated weight − dry weight)] * 100) and reported.
For each day, a two-way ANOVA was used to determine whether RWC of P. sanctae-crucis varied with sampling interval and position on shore (far or near the subtidal zone). An interaction of sampling interval and shore location was tested. Prior to analyses, data were also screened for homogeneity of variance and normality. Tukey’s multiple comparison tests were used to determine differences among groups after statistically significant main effects were observed.
Experimental manipulation to test exposure response
On June 11, 2010, an experiment was conducted to examine the response of P. sanctae-crucis to aerial exposure. Because exposure could potentially be experienced differently for isolated versus tightly clustered populations, 12 individuals and 12, 7 cm aggregations of three or more individuals of P. sanctae-crucis were collected at 0830 hours. Aggregations of P. sanctae-crucis were connected via holdfasts and possessed the Vaughniella stage growing loosely around the bases. Samples were divided into six control “isolated” and “aggregated” samples and six experimental “isolated” and “aggregated” samples, placed into 24 separate shallow containers of water (5 cm depth) and covered with a shade cloth. Samples were exposed to natural outdoor temperatures and irradiances, which ranged from 26.7 to 29.0 °C and reached 250 µmol photon m−2 s−1 under the shade cloth, respectively.
Prior to exposure, initial RLC measurements were collected with the PAM from six experimental and control samples. Experimental samples were then placed into an empty container and exposed to air for 40 min, about the length of time or shorter than would occur during a −0.1 m low tide. After 40 min, experimental samples were placed back into the seawater-filled containers for 10 min to gauge whether they could quickly recover to rates measured before any exposure. Fluorescence measurements were collected from experimental and control samples at 10- and 40-min intervals of exposure and after 10-min recovery period. All fluorescence measurements were taken in water. Then, the light curve parameters were derived from fluorescence measurements as described earlier.
The initial measurements and exposure to air were haphazardly staggered in time so that two aggregated and one isolated or two isolated and one aggregated experimental and control samples were conducted within the same 10-min period using two PAM fluorometers, until all 24 samples had been measured over ~3 h. Control and experimental samples were sampled simultaneously, and fresh seawater was periodically (~every 5–10 min) added to submersed samples to maintain ample ambient levels of carbon and nutrients and to maintain ambient temperatures.
For each RLC parameter, a repeated-measures two-way ANOVA (sampling interval nested within treatment) was used to determine whether performance changed for experimental isolated or aggregated samples of P. sanctae-crucis after 10- and 40-min durations of air exposure. We further evaluated whether RLC parameters were able to recover to initial and control values upon submersion. After each RM-ANOVA, a Tukey’s multiple comparison test was used to determine differences among groups after statistically significant main effects were observed. Prior to analyses, data were screened for normality and homogeneity of variance and transformed (exponential, log10, or rank) when necessary.
Results
Environmental variables
The environmental conditions during the sampling period were typical for tropical shores (Table 1, Online Resource #2). The mean irradiance during the sampling period was 1415 µmol photon m−2 s−1 in air and 1351 µmol photon m−2 s−1 in water and ranged from 237 to 2000 µmol photon m−2 s−1 from early morning to late afternoon. Irradiances varied with lowered irradiance in the hours of 0700 and 1500 and higher irradiance at 1200–1400 (Online Resource 2). The mean difference in irradiance between air and water was minimal, a median difference of 10 µmol photon m−2 s−1. However, irradiance between air and water measurements differed as much as 868 µmol photon m−2 s−1 at one sampling hour. The average temperature in the intertidal zone was 25.9 °C and ranged from 20.4 to 32.3 °C (Online Resource #2). Exposure to air occurred from 0–6 h depending on low tide magnitude and also differed across regions of the shore.
Physiological response with tides
The Φ PSII for P. sanctae-crucis ranged from 0.200 to 0.759 with an overall mean of 0.594 ± SE 0.006. The rank Φ PSII values were negatively (−0.119) related to increasing irradiances (Fig. 2, Panel a, ANCOVA, df = 1, F = 130.8, p < 0.001, Online Resource 3). In addition, the Φ PSII for P. sanctae-crucis were significantly higher in samples that were measured on a positive tidal height (ANCOVA, df = 1, F = 54.8, p < 0.001) when compared to those measured during the negative tidal height. The ANCOVA adjusted Φ PSII mean for P. sanctae-crucis measured on a positive tidal height was 0.650, while the adjusted mean measured on a negative tidal height was 0.594.
a–f The fluorescence measures for P. sanctae-crucis plotted with irradiance as measured on positive (gray circles or bars) and negative (black circles or bars) tidal heights. In a–d the solid lines represent the results of an ANCOVA model. The values of Φ PSII (a) and α (b) decreased with increasing irradiance. However, Φ PSII differed with tidal state (negative tidal height = black line, positive tidal height = gray line) and α did not (black line for both tidal states). Log E k c increased with increasing irradiance, and algae had higher log E k on negative tidal heights. The dynamic photo-inhibition term (β d) was not found to differ with irradiance nor tidal state. e, f The maximum relative electron transport rate (rETRmax) for P. sanctae-crucis at negative and positive tidal heights plotted by irradiance. Because of the heterogeneity of slopes, the mean (SE) rETRmax for algae are summarized by two irradiance levels: 700–1199, 1200–1700 (f) and a two-way ANOVA used. *above bars represent the differences (p < 0.05) found between irradiance categories, while lower case letters represent statistical differences between tidal states
Alpha was negatively related to irradiance (ANCOVA, df = 1, F = 79.7, p < 0.001) and did not differ with tidal state (Fig. 2, Panel b, ANCOVA, df = 1, F = 0.6, p = 0.43, Online Resource #3). The mean α was 0.239 ± SE 0.003 with a minimum of 0.057 and maximum of 0.383.
The E k of P. sanctae-crucis differed significantly with tidal conditions and irradiance (Fig. 2, Panel c, Online Resource #3). The mean E k of 149.9 ± SE 3.5 μmol photon m−2 s−1 (range 36.0 to 458.5 μmol photon m−2 s−1) was well below the maximum daily irradiance (950–2000 μmol photon m−2 s−1). For each day, the measured irradiance was above the mean E k by 8 h and was still well above the minimum saturation when sampling ended at 1200–1530 h. The E k of algae increased with increasing irradiance (ANCOVA, df = 1, F = 48.4, p < 0.001) and was higher for individuals measured during negative tidal heights (Fig. 2, Panel c, ANCOVA, df = 1, F = 28.4, p = 0.003) when aerial exposure would be more common. The ANCOVA adjusted E k mean for algae during negative tidal heights (back transformed) was 146.6 ± SE 1.0 compared to 118.6 ± SE 1.0 μmol photon m−2 s−1 for positive tidal heights.
The dynamic photo-inhibition parameter (β, the negative slope of the curve at high irradiances) of algae did not differ with irradiance or tidal state (Fig. 2, Panel d, Online Resource #3). Algal photo-inhibition varied from 0 to 22, but the median was 0.030 (n = 357). No patterns were detected visually or statistically.
The overall mean rETRmax for P. sanctae-crucis was 33.3 ± SE 0.56 μmol electrons m−2 s−1 (Fig. 2, Panels e, f). In contrast to the increasing E k, the rETRmax of P. sanctae-crucis was significantly higher at lower irradiances (700–1199 μmol photon m−2 s−1) than irradiances that ranged from 1200 to 1700 μmol photon m−2 s−1 (Fig. 2, Panel f, two-way ANOVA, df = 1, F = 7.9, p = 0.005). However, for each of these irradiance categories, P. sanctae-crucis had a significantly higher rETRmax during the negative tidal heights (means of 38.8 ± SE 1.4 and 32.4 ± SE 1.1 μmol electrons m−2 s−1) than during positive tidal heights (means of 30.9 ± SE 2.3 and 27.9 ± SE 1.5 μmol electrons m−2 s−1, two-way ANOVA, df = 1, F = 13.8, p < 0.001, Online Resource #3).
Investigations into the effect of exposure
Relative water content of Padina with tides
The RWC of P. sanctae-crucis sampled repeatedly over a 4-day interval with low tides ranged from 65 to 120 %. Values >100 % are due to the error in wet weight measures for this thin lightweight species. Even though the magnitude of low tide varied by day, the water content of P. sanctae-crucis at Diamond Head changed little among the 0800, 1100, 1400 h with varying tidal heights on days 2, 3, and 4 (Fig. 3). On the day with the lowest low tide (−0.15 m, day 1), P. sanctae-crucis sampled from the zone near the subtidal habitat, where the slope is slightly higher with more aerial exposure, had lost significantly more water during a negative tidal height than individuals sampled further up the shore (two-way ANOVA, position, df = 1, F = 15.9, p < 0.001). These individuals located closer to the subtidal habitat continued to have significantly lower water content at the 1400-h sampling when the shore was submersed. For this day, the 14-h sampling interval was 5 h after the lowest tidal height of the day.
Relative water content of P. sanctae-crucis reported as the mean (SE) of far (F) and near (N) samples in reference to their distance from the subtidal habitat at different sampling hours on 4 days with different tidal heights. Black bars represent samplings that occurred on negative tidal heights. Letters represent groups that are not statistically different (statistical alpha = 0.05) and NS = no statistical differences
Experimental manipulation to test exposure response
In an outdoor laboratory, aerial exposure for 10 and 40 min impacted several measured RLC parameters. For example, the Φ PSII and α for P. sanctae-crucis were negatively impacted by exposure to air, and effects were different for isolated versus aggregated individuals as indicated by significant main effects in statistical testing (Fig. 4a, b; Φ PSII: treatment, df = 3, F = 4.08, p = 0.02, sampling interval, df = 3, F = 7.14, p < 0.001, treatment x sampling interval, df = 9, F = 3.37, p = 0.001; α: treatment, df = 3, F = 4.2, p = 0.02, sampling interval, df = 3, F = 2.9, p = 0.04, treatment x sampling interval, df = 9, F = 2.8, p = 0.007, Online Resource #4). After 40 min of exposure to air, isolated and aggregated algal samples had lowered values, but submersed (control) algae maintained initial rates (Fig. 4a, b). Furthermore, aggregated thalli were able to recover to initial status with submersion, but isolated thalli did not recover in the experimental time frame. Post hoc Tukey’s pairwise comparisons between sampling intervals (initial, 10, 40 min, and recovery) within each treatment confirm this observed pattern.
Results of exposure duration experiment for isolated (Iso) and aggregated (Aggreg) samples of P. sanctae-crucis. Mean Φ PSII (a), α (b), rETRmax (c), β (d), E k (e), (SE) at initial, 10, 40 min, and recovery intervals are shown (see key). Control samples (C) remained submersed in seawater, while treatment samples (T) were exposed to air and then submersed for 10 min to recover. Letters above bars denote statistically different (p < 0.05) sampling intervals (lower case letters) within a treatment and among the different treatment groups (capital letters) as determined by Tukey’s multiple comparison when significant (p < 0.05) main effects were observed (repeated-measures two-way ANOVA found significances). Note pairwise differences when interactions of the main effects were significant are not shown (see Online Resource #4). Solid bars represent groups that did not differ statistically among the sampling intervals
The rETRmax values of algae maintained in isolated and aggregated treatments were also negatively impacted by exposure to air (Fig. 4c; treatment, df = 3, F = 8.1, p < 0.001, sampling interval, df = 3, F = 6.8, p < 0.001, Online Resource #4). The reduction in rETRmax with exposure to air was dramatic at the 40-min interval for both isolated and aggregated Padina. The rETRmax for algae in the aggregated treatment were able to increase their rates upon re-submersion in water, while the rates continued to decrease for isolated algae. Despite the slight signs of recovery in the aggregated treatment, rates were still much reduced in contrast to samples not exposed to air.
The β varied in an unpredictable manner and was not statistically found to be impacted by exposure duration (Fig. 4d, Online Resource #4). Similarly, E k values of algae did not vary with exposure to air, nor did values differ among algae maintained in isolated or aggregated conditions (Fig. 4e, Online Resource #4). The nonsignificant p value for treatment in the two-way RM-ANOVA model indicated a lack of exposure effect. Yet, statistically significant differences were detected by the model for sampling interval (df = 3, F = 5.4, p = 0.002) and an interaction of sampling interval with treatment (df = 9, F = 2.5, p = 0.02). The isolated algae in the control treatment had E k values at the initial sampling interval that were highly variable and statistically more elevated than the similar E k that occurred for algae at the 10- and 40-min recovery intervals.
Discussion
Our results confirm that a common Hawaiian alga growing on a relatively flat shore can experience up to 6 h of aerial exposure on days with daily fluctuations in tropical irradiance and temperature. Our in situ survey of fluorescence shows that photo-physiology of P. sanctae-crucis is not only acclimated to this high light environment, but it is also dynamic. The RLCs varied with diurnal changes in irradiance and more quickly with tidal state. Together, measures of water content and the manipulative laboratory experiment suggest that aggregations of P. sanctae-crucis combined with micro-tidal fluctuations and constant water motion may help to avoid water loss that could cause severe reductions in Φ PSII, α, and rETRmax. It is clear from this research that high irradiance in intertidal zones can cause stress in the form of inhibition, and this factor may be relatively more important to limiting photosynthesis for algae in tropical intertidal zones. Further testing with a variety of techniques to gauge photosynthesis and respiration (not just PSII) and environmental measurements, at the scale of the individual, is needed.
As expected, for P. sanctae-crucis, there is a relationship with the photo-physiology and irradiance. Species have various abilities to adjust photosynthetic characteristics to optimize performance in different light environments (Boardman 1977). P. sanctae-crucis exhibits RLC parameters that typify sun-adapted species from shallow waters, with relatively high E k (149.9 ± SE 3.5 μmol photon m−2 s−1) and rETRmax (33.3 ± SE 0.562 μmol electrons m−2 s−1). This is within range (E k 131.8–659.7 μmol m−2 s−1 and rETRmax 12.9–112.2 μmol electrons m−2 s−1) recorded for Hawaiian shallow water macroalgae (summarized in Cox, 2011). P. sanctae-crucis also exhibited effective quantum yield in the morning hours that is common for brown algae (~0.75, Büchel & Wilhelm 1993; Dring et al. 1996; Gévaert et al. 2003).
In addition, P. sanctae-crucis RLC parameters exhibit daily adjustments to irradiance. Φ PSII is effectively a relative measure of the number of reaction centers actively involved in photosynthesis, while the α represents an estimate of the amount of photosynthesis per incident photon (Henley 1999; Ralph and Gademann 2005). At low irradiance, P. sanctae-crucis had more elevated Φ PSII and α values that decreased with increasing irradiances. An irradiance-driven pattern was also documented in situ for shallow water Laminaria and periphyton community (Gévaert et al. 2003; Laviale et al. 2009; Delebecq et al. 2011). The correlation with irradiance levels can be explained by diurnal patterns of photosynthesis and light availability (Henley 1999). In the morning, after a long period of darkness, photosynthesis is light-limited and reaction centers are available for light harvesting. This is when PSII is very active in the transfer of energy for utilization by photosynthesis. Then, as irradiance levels increase, the macroalgae should become less light-limited and non-photochemical quenching is likely to increase.
Irradiance in shallow tropical waters is typically thought of as a stressor and not a limiting factor, and thus, it was predicted that exposure to tropical irradiances could be stressful (Beach and Smith, 1996a). For all 8 days of measurement by ~8 h, irradiances were above the minimum saturation irradiance (E k) and irradiances remained well above the minimum saturation when sampling ended at 12–1530 hours. Furthermore, rETRmax was reduced at irradiances above >1200 μmol m−2 s−1. The correlative and decreasing relationship between irradiance and Φ PSII, combined with the reduced rETRmax at high irradiances, indicates a downregulation of PSII. When light energy input exceeds the capacity for energy utilization, this results in thermal dissipation of excess excitation energy in the pigment antenna of PSII (referred to as dynamic photo-inhibition) (Henley 1999; Ralph and Gademann 2005). This regulatory process limits the accumulation of reactive oxygen species acting to protect the algae against photooxidative damage (chronic photo-inhibition) (Henley 1999; Ralph and Gademann 2005). With our methodology, we are unable to measure the recovery of Φ PSII to determine whether this downregulation was dynamic or chronic. However, the β parameter can indicate a plant’s dynamic ability to cope with light stress (Ralph and Gademann 2005). In our in situ assessments, β did not differ with light intensity. Because we had to correct RLC actinic irradiance for battery decline, the algal samples did not always receive the same upper intensity actinic light range. Thus, β results could be influenced by our methodology and may not be a reliable indicator of algal abilities.
The fluorescence-based values from reef algae on Hawaiian shores may not support the traditional temperate paradigm that macroalgae experience stress and lower photosynthesis during low tides (Davison and Pearson 1996). Even for the hardiest intertidal genera, Fucus, field measurements from 14 shores in Washington had net photosynthesis two orders of magnitude lower when exposed to air during low tides (Williams and Dethier 2005). The results for the effect on photosynthesis, as measured in this study, for P. sanctae-crucis were mixed. P. sanctae-crucis had lowered Φ PSII during negative tidal heights indicating a smaller number of reaction centers actively engaged in photosynthesis. However, rETRmax was relatively higher during tides of negative tidal height when exposure is more common. In this situation, oxygen measures which integrate carbon fixation and respiration would be beneficial along with the fluorescence measures to clarify the impact on photosynthesis.
If macroalgae experience greater light availability during negative tidal heights and possess a photo-protective mechanism, this could allow for increased or maintained photosynthetic electron transport rate despite the reduced photochemical activity (Gévaert et al. 2003). Light attenuates with water depth. Given that during negative tidal heights, water depth is lower, aerial exposure is more common, and light availability should be greater for intertidal algae during low tides. Our simultaneously collected irradiance measures in air and water show that even in this high light environment with relatively clear water irradiances in general tended to be slightly more elevated in air than in water. Excess light availability would result in reduced Φ PSII via an increase in non-photochemical quenching. Furthermore, elevated irradiances correlated to increased E k and rETRmax for all algae. Other algae are known to have photo-protective mechanisms, such as the xanthophyll cycle that increase the amount of energy dissipated as heat to prevent damage to PSII (Demmig-Adams and Adams 1996; Hanelt et al. 1993; Gavert et al. 2003). PSII activity is also influenced by recent previous light history (Ralph and Gademann 2005). In this context, it is interesting to note that even at low irradiances that typify morning hours, the Φ PSII of plants measured during negative tidal height were lower than those measured during a positive tidal height. Macroalgae may be experiencing chronic photo-inhibition during negative tidal heights that translates to a reduced number of reaction centers actively involved in photosynthesis into the next day. Here, previous light history effects can also explain the apparent disaccord between our interpretation that greater light availability can attribute to the observed differences in photo-physiology and the outcomes of the ANCOVAs. The ANCOVAs compare rates at the same given irradiance and do not factor in previous light exposure.
Another possibility that could increase the rETRmax and E k is temperature changes. As the tide retreats from the shore, the buffering capacity of water is removed. Exposed plants may be heated differently. Temperature is known to vary over fine spatial scales on shores (Cox and Smith 2011; Helmuth and Hoffmann 2001), under canopies of algae (Molina-Montenegro et al. 2005), and even from holdfast to canopy within a species (Beach and Smith 1997). Temperature was not measured at the scale of the algae in this study, but it is known to alter enzymatic processes. Thus, temperature changes can alter photosynthetic rates and directly impact the function of photo-protective mechanisms (Henley 1999; Colvard et al. 2014). Most plants increase photosynthesis with increasing temperature until an optimum is reached. Temperature above the optimum causes rates to decline. We speculate that thallus temperature could increase with reduced water depth from the tidal ebb and flow and thus account for increased rETRmax and E k during low tide exposure. Hot temperatures (above optimum) are also likely to act together with high light stress to cause a decline in rETRmax.
Aerial exposure can cause cells to dry and decrease photosynthetic activity. However, there is some evidence that intertidal species which remain hydrated could absorb carbon into a wetted cell wall, if not fix carbon via photosynthesis in air. Select temperate intertidal species increased photosynthesis 6x in air when compared to submersed individuals when measured via gas exchange in a laboratory; this may be a case where carbon is absorbed into the cell wall, but there was no isotopic labeling to confirm fixation (Johnson et al. 1974). Additionally, rapid air–water CO2 diffusion, as compared to slow diffusion in seawater, is hypothesized to result in an immediate increase in photosynthesis in air (Silva et al. 2005). Gao et al. (1999) found increases in photosynthesis for macroalgae after a certain extent of dehydration when the diffusion boundary layer of water around the thallus reduces enough that aerial CO2 update becomes easier. Indeed, P. sanctae-crucis had relatively increased electron transport rates during negative tidal heights, and measures of RWC showed that macroalgae in natural settings remained wetted. In the laboratory, macroalgae exposed to air for 40 min (much shorter than the aerial exposure duration in natural settings) had lowered Φ PSII, α, and rETRmax. This leads us to conclude that the removal of water and exposure to carbon in air was not beneficial for photosynthesis. An alternate hypothesis could be that the re-wetting action of the waves maintained higher RWC in situ than what occurred after 40 min in air in the laboratory. RWC and photosynthesis was not measured concurrently to confirm the relationship.
Padina sanctae-crucis in Hawaii did not experience substantial water loss during emersion. The RWC of P. sanctae-crucis measured over three different tides at three different times of day showed little variation on moderate low tides (>−0.12 m). In comparison, temperate species in the green algal genus Ulva are known to dry 3x faster at noon than at other sampling intervals (Beer and Eshel 1983). RWCs in algae studied here were fairly high and ranged from 65 to 120 %, and mean values ranged from 81 to 99 %. RWC during negative tidal heights ranged from 65 to 120 %. Values higher than 100 % occurred when the 24-h hydrated weight was lower than the fresh weight. This indicates to us that there was not much water loss for algae in situ, and differences were small and subject to weight errors. Despite the slight inflation, using the same methodology, the Hawaiian red alga A. concinna isolated on basaltic shores had substantially lower RWC [canopy: mean 66 %, lowest 18 %, understory: mean 76 %, lowest 36 % (Beach and Smith 1997)]. Furthermore, Porphyra desiccates to 15 % RWC in 3 h (Smith and Berry 1986). This is also a value substantially lower than those recorded in this study. Fucus was measured to have a water content from 40 to 100 % (Williams and Dethier 2005). This could be explained by exposure duration and/or morphology. Fucus typically experiences much longer tidal exposure than intertidal seaweeds in Hawaii where microtides result in a maximum exposure of 6 h. Also, these intertidal benches are relatively flat, and waves often push water upon the shore and over the narrow bench. This constant re-wetting action may allow algae to retain water. Padina, Ulva, and Porphyra are relatively thin species. The calcium carbonate impregnating the blade surface of P. sanctae-crucis could reflect excessive irradiances (Beach et al. 2006) and lower thermal heating (Cox and Smith 2011) which could limit water loss.
Aggregations of seaweeds are known to reduce desiccation stress (Hay 1981; Bell 1992; Bertness et al. 1999; Molina-Montenegro et al. 2005), and we clearly show that P. sanctae-crucis growing in aggregations had a different response to aerial exposure than isolated individuals. Aggregations of loosely woven P. sanctae-crucis blades with the Vaughniella stage at their bases may allow this species to retain water and thus avoid desiccation. We caution that we have only indirectly evaluated potential desiccation stress with measurements of RWC and exposure duration. However, the fact that in the laboratory, (1) algae had reduced Φ PSII, rETRmax, and E k within 40 min of exposure to air and aggregated individuals had a different recovery response than isolated individuals, and (2) P. sanctae-crucis maintained relatively high RWC which provides evidence that algae are able to avoid severe desiccation stress in their natural settings.
One of the main limits to our study is the sole reliance on fluorescence technology to gauge photosynthesis. The advantage of fluorescence measures over other techniques is that it can rapidly provide information about photosynthesis and be deployed in situ. However, fluorescence does not provide information about the rates of the carbon fixation and respiration. There is extensive literature discussing the advantages and disadvantages of different photosynthetic methodologies (Longstaff et al. 2002; Beer and Axelsson 2004). One of the greatest difficulties for fluorescence methods is to relate values to carbon gain, particularly when used in high light environments (Henley et al. 1991). At least, for P. sanctae-crucis, laboratory measures of rETR correspond to oxygen measures of productivity at irradiances of 250 µmol photon m−2 s−1 (Cox and Smith 2011, dissertation). Despite any limitations, PAM fluorometry enabled the collection of an extensive quantity of photosynthesis measures showing the rapid acclimation of plants to changing irradiance and tidal states.
Conclusions
This study evaluated the light-adapted fluorescence measurements of 357 algal thalli in a “natural experiment” with changing irradiances, temperature, and tidal conditions. With our methods, it was possible to identify clear patterns in algal physiology. We determined that P. sanctae-crucis physiology is dynamic. It adjusts to daily changes in irradiance and tidal state. Furthermore, macroalgae appear to downregulate PSII, but have increased electron transport rate during periods with negative tidal heights. This response suggests that they rely on a photo-protective mechanism for dissipation of excess energy, such as the xanthophyll cycle. Lastly, we identified that the habit of growing in aggregations limits any negative aspects of aerial exposure on fluorescence. Furthermore, we expect species level differences in response to the changing environment. For example, A. concinna occurs higher and on basaltic shores where it may experience comparatively more water loss and thus respond differently with tidal ebb and flow (Beach and Smith, 1997). Because of the heterogeneity in habitat and species differences, it would be useful to repeat the study with multiple species while taking measures of the environment at the scale of the organism and not the habitat (as in this study). We suggest using a variety of photosynthetic measures. Measures at the individual scale that additionally include carbonate chemistry could increase our knowledge on the actual interaction of warming, pH fluctuations, and aerial exposure, at different irradiances on photosynthesis. From these results, it seems likely that high irradiances and associated warming temperatures are key factors that control production in tropical systems.
References
Abbott IA (1999) Marine red algae of the Hawaiian Islands. Bishop Museum Press, Honolulu
Abbott IA, Huisman JM (2004) Marine green and brown algae of the Hawaiian Islands. Bishop Museum Press, Honolulu
Beach KS, Smith CM (1996a) Ecophysiology of tropical rhodophytes. II. Microscale acclimation in photosynthesis. J Phycol 32:710–718
Beach KS, Smith CM (1996b) Ecophysiology of tropical Rhodophytes. I. Microscale acclimation in pigmentation. J Phycol 32:701–710
Beach KS, Smith CM (1997) Ecophysiology of a tropical rhodophyte III. Recovery from emersion stresses in Ahnfeltiopsis concinna (J. Ag.) Silva et DeCew. J Exp Mar Biol Ecol 221:151–167
Beach KS, Borgeas HB, Smith CM (2006) Ecophysiological implications of the measurement of transmittance and reflectance of tropical seaweeds. Phycologia 45:450–457
Beer S, Axelsson L (2004) Limitations in the use of PAM fluorometry for measuring photosynthetic rates of macroalgae at high irradiances. Eur J Phycol 39:1–7
Beer S, Bjork M (2000) Measuring rates of photosynthesis of two tropical seagrasses by pulse amplitude modulated (PAM) fluorometry. Aquat Bot 66:69–76
Beer S, Eshel A (1983) Photosynthesis of Ulva sp. I. Effects of desiccation when exposed to air. J Exp Mar Biol Ecol 70:91–97
Beer SB, Vilenkin A, Weil M, Veste L, Eshel S (1998) Measuring photosynthetic rates in seagrasses by pulse amplitude modulated (PAM) fluorometry. Mar Ecol Prog Ser 174:293–300
Beer S, Bjork M, Gademann R, Ralph PJ (2001) Measurements of photosynthesis in seagrasses. In: Short FT, Coles R (eds) Global seagrass research methods. Elsevier Publishers, The Netherlands, pp 183–198
Bell EC (1992) Consequences of morphological variation in an intertidal macroalga: physical constraints on growth and survival of Mastocarpus papillatus Kutzing. Dissertation, Standford University
Bell EC (1995) Environmental and morphological influences on thallus temperature and desiccation of the intertidal alga Mastocarpus papillatus Kutzing. J Exp Mar Biol Ecol 191:29–55
Bertness MD, Leonard GH, Levine JM, Schmidt PR, Ingraham AO (1999) Testing the relative contribution of positive and negative interactions in rocky intertidal communities. Ecology 80:2711–2726
Bird CE, Franklin EC, Smith CM, Toonen RJ (2013) Between tide and wave marks: a unifying model of physical zonation on littoral shores. PeerJ 1:e154
Boardman K (1977) Comparative photosynthesis of sun and shade plants. Ann Rev Plant Physiol 28:355–377
Büchel C, Wilhelm C (1993) In vivo analysis of slow chlorophyll fluorescence induction kinetics in algae: progress, problems and perspectives. Photochem Photobiol 58:137–148
Chapman ARO (1995) Functional ecology of fucoid seaweeds: twenty-three years of progress. J Phycol 32:197–211
Ciais P, Sabine C, Bala G, Bopp L, Brovkin V, Canadell J, Chhabra A, DeFries R, Galloway J, Heimann M, Jones C, Le Quéré C, Myneni RB, Piao S, Thornton P (2013) Carbon and Other biogeochemical cycles. In: Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Climate change 2013: the physical science basis contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA
Colvard NB, Carrington E, Helmuth B (2014) Temperature-dependent photosynthesis in the intertidal alga Fucus gardneri and sensitivity to ongoing climate change. J Exp Mar Biol Ecol 458:6–12
Connell JH (1961) The influence of intra-specific competition and other factors on the distribution of the barnacle Chthamalus stellatus. Ecology 42:710–723
Cox TE, (2011) Aspects of ecology and algal physiology in Hawaii’s rocky intertidal zones. Dissertation University of Hawaii, Department of Botany, Honolulu, Hawaii. p 233
Cox TE, Smith CM (2011) Thermal ecology on an exposed algal reef: infrared imagery a rapid tool to survey temperature at local spatial scales. Coral Reefs 30:1109–1120
Cox TE, Philippoff J, Baumgartner E, Zabin CJ, Smith CM (2013) Spatial and temporal variation in rocky intertidal communities along the Main Hawaiian Islands. Pac Sci 67:23–45
Davison IR, Pearson GA (1996) Stress tolerance in intertidal seaweeds. J Phycol 32:197–211
Davison IR, Johnson LE, Brawley SH (1993) Sublethal stress in the intertidal zone: tidal emersion inhibits photosynthesis and retards development in embryos of the brown alga Pelvetia fastigiata. Oecologia 96:483–492
Delebecq G, Davoult D, Menu DE, Joaquin M-A, Migné A, Dauvin J-C, Gevaert F (2011) In situ photosynthetic performance of Laminaria digitata (Phaeophyceae) during spring tides in Northern Brittany. Cah Mar Biol 52:1–10
Demmig-Adams B, Adams WWI (1996) The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci 1:21–26
Dethier MN, Williams SL (2009) Seasonal stresses shift optimal intertidal algal habitats. Mar Biol 156:555–567
Dethier MN, Williams SL, Freeman A (2005) Seaweeds under stress: manipulated stress and herbivory affect critical life-history functions. Ecol Monogr 75:403–418
Doty MS (1946) Critical tide factors that are correlated with the vertical distribution of marine seaweeds and other organisms along the Pacific Coast. Ecology 27:315–328
Dring MJ, Brown FA (1982) Photosynthesis of intertidal brown seaweeds during and after periods of emersion: a renewed search for physiological causes of zonation. Mar Ecol Prog Ser 8:301–308
Dring MJ, Makarov V, Schoschina E, Lorenz M, Lüning K (1996) Influence of ultraviolet-radiation on chlorophyll fluorescence and growth in different life-history stages of three species of Laminaria (Phaeophyta). Mar Biol 126:183–191
Dudgeon SR, Kubler JE, Vadas RL, Davison IR (1995) Physiological responses to environmental variation in intertidal red seaweeds: does thallus morphology matter? Mar Ecol Prog Ser 117:193–206
Foster MS (1982) Factors controlling the intertidal zonation of Iridaea flaccida (Rhodophyta). J Phycol 18:285–294
Ganzon-Fortes ET (1997) Influence of tidal location on morphology, photosynthesis and pigments of the agarophyte, Gelidiella acerosa, from Northern Philippines. J Appl Phycol 9:525–532
Gao K, Ji Y, Aruga Y (1999) Relationship of CO2 concentrations to photosynthesis of intertidal macroalgae during emersion. Hydrobiologia 398(399):355–359
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Gévaert F, Créach A, Davoult D, Migné A, Levavasseur G, Arzel P, Holl A-C, Lemoine Y (2003) Laminaria saccharina photosynthesis measured in situ: photoinhibition and xanthophyll cycle during a tidal cycle. Mar Ecol Prog Ser 247:43–50
Gosline WA (1965) Vertical zonation of inshore fishes in the upper water layers of the Hawaiian Islands. Ecology 46:823–831
Hanelt D, Huppertz K, Nultsch W (1993) Daily course of photosynthesis and photoinhibition in marine macroalgae investigated in the laboratory and field. Mar Ecol Prog Ser 97:31–37
Harrison WG, Platt T (1986) Photosynthesis-irradiance relationships in polar and temperate phytoplankton populations. Polar Biol 5:153–164
Hay ME (1981) The functional morphology of turf-forming seaweeds: persistence in stressful marine habitats. Ecology 62:739–750
Helmuth BS (2009) From cells to coastlines: how can we use physiology to forecast the impacts of climate change? J Exp Biol 212:753–760
Helmuth BS, Hoffmann GE (2001) Microhabitats, thermal heterogeneity, and patterns of physiological stress in the rocky intertidal zone. Biol Bull 210:374–384
Henley WJ (1999) Measurement and interpretation of photosynthetic light response curves in algae in the context of photoinhibition and diel changes. J Phycol 29:729–739
Henley WJ, Levavasseur G, Franklin LA, Lindley ST, Ramus J, Osmond CB (1991) Diurnal responses of photosynthesis and fluorescence in Ulva rotundata acclimated to sun and shade in outdoor culture. Mar Ecol Prog Ser 75:19–28
Hunt LJH, Denny MW (2008) Desiccation protection and disruption: a trade-off for an intertidal marine alga. J Phycol 44:1164–1170
Ihnken S, Eggert A, Beardall J (2010) Exposure times in rapid light curves affect photosynthetic parameters in algae. Aquat Bot 93:185–194
Johnson WS, Gigon A, Gulmon SL, Mooney HA (1974) Comparative photosynthetic capacities of intertidal seaweeds under exposed and submerged conditions. Ecology 55:450–453
Kay AE (1979) Hawaiian marine shells, reef and shore fauna of Hawaii. Bishop Museum Press, Honolulu
Laviale M, Prygiel J, Lemoine Y, Courseaux A, Cre´ach A (2009) Stream periphyton photoacclimation response in field conditions: effect of community development and seasonal changes. J Phycol 45:1072–1082
Longstaff B, Kildea T, Runcie J, Cheshire A, Dennison WC, Hurd C, Kana T, Raven JA, Larkum AWD (2002) An in situ study of photosynthetic oxygen exchange and electron transport rate in the marine macroalgae Ulva lactuca (Chlorophyta). Photosynth Res 74:281–293
Matta JL, Chapman DJ (1995) Effects of light, temperature, and desiccation on the net emersed productivity of the intertidal macroalga Colpomenia peregrina Sauv. (Hamel). J Exp Mar Biol Ecol 189:13–27
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence-a practical guide. J Exp Bot 51:659–668
Molina-Montenegro MA, Munoz AA, Badano EI, Morales BV, Fuentes KM, Cavieres LA (2005) Positive associations between macroalgal species in a rocky intertidal zone and their effects on the physiological performance of Ulva lactuca. Mar Ecol Prog Ser 292:173–180
Oates BR, Murray SN (1983) Photosynthesis, dark respiration and desiccation resistance of the intertidal seaweeds Hesperophycus harveyanus and Pelvetia fastigiata F. gracilis. J Phycol 19:371–380
Paine RT (1974) Intertidal community structure: experimental studies on the relationship between a dominant competitor and its principal predator. Oecologia 15:93–120
Platt T, Gallegos CL, Harrison WG (1980) Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J Mar Res 38:687–701
Ralph PJ, Gademann R (2005) Rapid light curves: a powerful tool to assess photosynthetic activity. Aquat Bot 82:222–237
Schaffelke B, Deane D (2005) Desiccation tolerance of the introduced marine green alga Codium fragile ssp. tomentosoides—clues for likely transport vectors. Biol Invasions 7:557–565
Schonbek M, Norton TA (1978) Factors controlling the upper limits of fucoid seaweed on the shore. J Exp Biol Ecol 31:303–313
Silva J, Santos R, Calleja ML, Duarte CM (2005) Submerged versus air-exposed intertidal macrophyte productivity: from physiological to community-level assessments. J Exp Mar Biol Ecol 317:87–95
Smith CM, Berry JA (1986) Recovery of photosynthesis after exposure of intertidal seaweeds to osmotic and temperature stresses: comparative studies of species with differing distributional limits. Oecologia 70:6–12
Tait LW, Schiel DR (2010) Primary productivity of intertidal macroalgal assemblages: comparison of laboratory and in situ photorespirometry. Mar Ecol Prog Ser 416:115–125
Tomanek L, Helmuth BS (2002) Physiological ecology of rocky intertidal organisms: a synergy of concepts. Integr Comp Biol 42:771–775
White AJ, Critchley C (1999) Rapid light curves: a new fluorescence method to assess the state of the photosynthetic apparatus. Photosynth Res 59:63–72
Williams SL, Dethier MN (2005) High and dry: variation in net photosynthesis of the intertidal seaweeds Fucus gardneri. Ecology 86:2373–2379
Wright JT, Williams SL, Dethier MN (2004) No zone is always greener: variation in the performance of Fucus gardneri embryos, juveniles and adults across tidal zone and season. Mar Biol 145:1061–1073
Acknowledgments
We would like to thank Mailie Rechirei, Drs. Chris Bird and Kelly Boyle, and members of UH’s 2010 Botany 180 class for their assistance with data collection. Further, we thank Drs. Alison Sherwood, Peter Vroom, Dave Carlon, Brian Popp, and multiple reviewers for their edits and suggestions for this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Hill.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cox, T.E., Smith, C.M. Photosynthetic rapid light curves for Padina sanctae-crucis vary with irradiance, aerial exposure, and tides in Hawaii’s micro-intertidal zones. Mar Biol 162, 1061–1076 (2015). https://doi.org/10.1007/s00227-015-2649-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-015-2649-1