Abstract
Sucrose-based non-isocyanate polyurethanes (S-NIPU) were synthesized by the already codified reaction of sucrose, dimethyl carbonate and hexamethylene diamine and used for the first time as adhesives for bonding particleboard. To decrease the NIPU adhesive curing temperature, a silane coupling agent was used as a crosslinking promoter. The performance of particleboards prepared at a press temperature of 230 °C, 200 °C and 180 °C and a press time of 8 min, 10 min and 12 min was tested and evaluated. The structure of the oligomers obtained was detected by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Thermomechanical analysis and differential scanning calorimetry were also used to analyze their behavior. The particleboards bonded with the S-NIPU adhesive at 230 °C showed excellent properties, which, however, decreased as the press time was reduced. The silane coupling agent used as a crosslinking promoter significantly reduced the curing temperature of the adhesive and allowed to obtain good bonding at a lower press temperature. The results obtained confirmed that coupling the S-NIPU adhesive with a silane used as a crosslinking promoter can yield sufficiently improved results to function as a suitable adhesive for particleboard, medium density fiberboard and other types of particulate wood panels. It was also the first time that a NIPU adhesive was used for wood bonding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polyurethanes (PU) are widely used in many fields, because of their excellent durability, impact resistance, bond performance, corrosion resistance and so on. PUs are also used as high-performance adhesives in the wood industry (Stoeckel et al. 2013; Pizzi and Mittal 2011; Cornille et al. 2017), for example, in the production of oriented strand board (OSB) (Ayrilmis et al. 2009), straw particle board (Zhang and Hu 2014; Mo et al. 2003; Fiorelli et al. 2012) and laminated timber/glulam (Properzi et al. 2003; George et al. 2003). The traditional synthesis route of PU is based on the reaction of polyols with polyisocyanates. Because polyisocyanates (such as TDI and HDI) have strong volatility and toxicity, thus being both harmful and not environment friendly, non-isocyanate polyurethanes (NIPU) have been synthesized and the research on them is now in full swing. Studies on reaction routes to prepare NIPU do already exist. In general, a five-membered cyclic carbonate is reacted with diamines to form polyhydroxyurethanes. Numerous research works on this topic do exist in the literature (Rokicki and Piotrowska 2002; Tomita et al. 2001a, b; Kihara and Endo 1993; Kihara et al. 1996; Figovsky and Shapovalov 2002; Birukov et al. 2009; Boyer et al. 2010; Kathalewar et al. 2013a, b; Camara et al. 2014; Cornille et al. 2016).
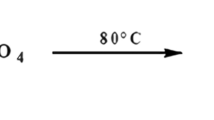
Some research works report the preparation of NIPU based on biomass materials, such as by chemical transformation of epoxidized vegetable oils, by the synthesis of biobased cyclic carbonates then reacted with diamines (Haniffa et al. 2017; Kathalewar et al. 2013a, b; Poussard et al. 2016). Recently, research on the use of biomass to prepare NIPUs has intensified with works on their preparation based on tannins or lignin, which showed some interesting performance as wood or steel surface coatings (Thébault et al. 2014, 2015; Santiago-Medina et al. 2018). Furthermore, non-isocyanate polyurethanes from monosaccharides (glucose) and disaccharides (sucrose) were prepared, and their suitability was proved as both wood and steel surface coatings, as simple thermosetting wood adhesives (Xi et al. 2018a), and even with the initial encouraging results as NIPU foams (Xi et al. 2018b). These NIPU resins, however, needed a relatively high temperature to cure to ensure a decent performance. To overcome such a problem and to be able to use NIPUs as wood panel adhesives at the usual temperatures for this application, a silane coupling agent (HK560) having the following structure:

was used as a crosslinking promoter in this paper. Particleboards were prepared and tested to check the adhesive performance.
Experimental
Materials
Sucrose by Acros Organics (Geel, Belgium), dimethyl carbonate by Sigma-Aldrich (Saint Louis, France), hexamethylenediamine 98% by Sigma-Aldrich (Saint Louis, France) and 98% silane coupling agent KH560 from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) were used.
Preparation of S-NIPU resin
The S-NIPU resin was prepared according to the procedure defined in a previous work (Xi et al. 2018a), namely: 20 g of sucrose were mixed with 13.5 g of dimethyl carbonate and 16.67 g of water, heated to 50 °C for 40 min and then cooled to room temperature. A total of 27.16 g hexamethylenediamine was then added to the mixture and heated to 90 °C for 120 min and then cooled to room temperature. A part of this mixture was dried at 103 °C overnight for the MALDI-TOF analysis.
Thermomechanical analysis (TMA)
The resins were tested by thermomechanical analysis. The samples were prepared by applying each adhesive between two beech wood plies, with dimensions of 21 mm × 6 mm × 1.1 mm. These beech–resin–beech sandwiches were tested in non-isothermal mode between 25 and 250 °C at a heating rate of 10 °C/minute with a Mettler Toledo 40 TMA equipment (Mettler Toledo, Zurich, Switzerland). They were tested in three-point bending on a span of 18 mm exercising a force cycle of 0.1/0.5 N on the specimens, with each force cycle of 12 s (6 s/6 s). The classical mechanics relationship between force and deflection
allows the calculation of the Young’s modulus E for each case tested. Such a measuring system has been introduced and is used to follow the progressive hardening of the adhesive with the increase in temperature and to indicate comparatively if an adhesive system is faster or slower hardening and if it gives stronger joints than another one.
Differential scanning calorimetry (DSC)
The test was performed on a Perkin–Elmer DSC calorimeter type DSC 204F1 (Perkin-Elmer, Rodgau, Germany). The freeze-dried specimens were heated from 35 to 240 °C at a heating rate of 15 K/min, and the thermal changes were recorded. The software used for data analysis was PYRISTM Version 4.0.
MALDI-TOF analysis
Samples for matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) analysis were prepared by first dissolving 5 mg of sample powder in 1 mL of a 50:50 v/v acetone/water solution. Then, 10 mg of this solution was added to 10 µL of a 2,5-dihydroxy benzoic acid (DHB) matrix. The locations dedicated to the samples on the analysis plaque were first covered with 2 µL of NaCl solution 0.1 M in 2:1 v/v methanol/water and pre-dried. Then, 1 µL of the sample solution was placed on its dedicated location, and the plaque was dried again. Red phosphorous was used to standardize the MALDI equipment. MALDI-TOF spectra were obtained by means of an Axima Performance mass spectrometer from Shimadzu Biotech (Kratos Analytical Shimadzu Europe Ltd., Manchester, UK) using a linear polarity-positive tuning mode. The measurements were taken by making 1000 profiles per sample with two shots accumulated per profile. The spectrum precision is of +1 Da.
Particleboard preparation and testing
Three identical monolayer particleboards of 350 × 310 × 10 mm3 size were prepared. The adhesive solids load was 10% on bone-dry wood. The panels were pressed with a three-stage hot pressing cycle, with a pressure cycle of 33 kg/cm2, 15 kg/cm2 and 5 kg/cm2 for 8 min (2 min–4 min–2 min), 10 min (3 min–4 min–3 min) and 12 min (4 min–5 min–3 min). Three different hot pressing temperatures were used: 180 °C, 200 °C and 230 °C. After cooling and sanding the panel surface, the boards were cut to 50 mm × 50 mm for the tests of dry internal bond (IB) strength, elastic modulus and water absorption thickness expansion ratio and a size of 250 mm × 50 mm for bending strength test. At least three samples were tested for each test according to China National Standard GB/T 17657-1999. The density profile of the boards was also tested by their thickness scan at a rate of 0.4 mm/s using a DAX 6000 profile densitometer (GreCon, Alfeld, Germany).
Results and discussion
Table S1 and Figs. S1–S7 in Supplementary Material report the MALDI-ToF results obtained for the S-NIPU resin adhesive. The reaction product of sucrose and dimethyl carbonate (DMC) is found as the species at 422.5 Da.

In addition, the reaction of one molecule of sucrose with two or more molecules of dimethyl carbonates was observed, for instance at 480 Da.

This type of species reacted with the hexamethylenediamine (HMD) to form a urethane link, such as the species at 568 Da are also found.

Oligomers with multiple urethane links formed as a linear molecule were also found, as for instance at 1137 Da

It is worth to clearly point out that a multitude of possible branched structures at the same molecular weight can occur, such as for example as follows:

The existence of branched species indicates that these resins do crosslink and do form tridimensional polymer networks during the curing process, which allows to obtain a good bonding performance as shown by the results found and discussed later for particleboard.
An unreacted sucrose unreacted with dimethyl carbonate is also observed in the reaction mixture as 365 Da (Table S1, Supplementary Material):

Oligomers obtained by reaction between two sucroses by elimination of a water molecule to form an ether bond have also been observed, such as the species at 690 Da:

The cured S-NIPU with KH560 adhesive was also tested by MALDI-ToF (Fig. S7, Supplementary Material), but the cured adhesive was practically insoluble in the acetone/water solution, so that the MALDI-ToF results obtained were rather limited. Only two small peaks of the initial reaction of the silane coupling agent with a low molecular weight NIPU could be identified, one of which at 933.5 Da (Fig. S7, Supplementary Material) having the following structure:

and one at 1108.8 Da (Fig. S7, Supplementary Material) with the following structure:

This is reported as indication that the reaction between the S-NIPU and the silane coupling agent does indeed occur (Fotea and D’Silva 2004; Serier et al.1991).
Performance of the S-NIPU resin and of the particleboard bonded with it
The performance of the laboratory monolayer particleboards bonded with the S-NIPU resin pressed at 230 °C for 12 min is shown in Table 1. The boards show encouraging results for internal bond (IB) strength, being as high as 1.02 MPa, thus far above the standard requirement (≥ 0.35 MPa) (China National Standard GB/T 4897.5-2003; EN 312 2003). The 24-h water thickness swelling is only 12%, without any water repellent like emulsified paraffin being added, showing that the board appears to have good water resistance. In particular, the boards presented a residual internal bond strength of 0.32 MPa even after immersion in boiling water for 1 h. These effects are due to the urethane link in the cured S-NIPU adhesive, the structure of which gives good stability and hydrolysis resistance. However, the wood degraded when kept at high temperature for an excessively long time, and then, the board color became brown, which is a disadvantage, and will reduce the performance and service life of the board. Thus, reducing the curing temperature of the adhesive or shortening the hot pressing time becomes a necessity when using the S-NIPU resin. The density profile characteristic of the particleboard prepared in Table 1 is shown in Fig. 1.
Effect of hot press time on particleboard properties
The test results for particleboards prepared at 230 °C at different pressing times are shown in Table 2. The hot press time has clearly a great influence on the performance of the boards (Table 2). When the curing time is 8 min, the particleboard internal bond strength is only 0.38 MPa. Although this is higher than the standard requirements of 0.35 MPa, it is still the lowest IB strength result when compared with the other two boards. All the panel properties improve as the press time increases. This is mainly due to the high curing activation energy of the S-NIPU adhesive (Xi et al. 2018a, b), as it is not completely cured at short press times. Consequently, this indicated that a reduction in the curing temperature of the adhesive is needed, possibly by adding a crosslinking promoter.
Differential scanning calorimetry (DSC)
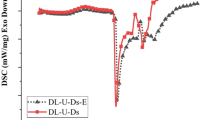
To reduce the curing temperature of the S-NIPU resin, 15% of a silane coupling agent (KH560) based on the resin’s solid content was used as crosslinking promoter. DSC was used to monitor the curing behavior of the S-NIPU adhesive and of the S-NIPU mixed with the crosslinker (called “S-NIPU + KH560”). The results are shown in Fig. 2. First of all, the DSC curves of both the two samples increase rapidly at the beginning of the test, this being due to the endothermic dissolution of the samples. Secondly, while the curing temperature peak of the S-NIPU is at 138.5 °C, the “NIPU+KH560” adhesive has this peak at just 126 °C, indicating that “NIPU+KH560” is more reactive and has a lower curing activation energy than S-NIPU. Thus, the silane coupling agent (KH560) can reduce the curing temperature of the S-NIPU resin to achieve more complete curing and a better bonding performance under the same pressing conditions.
TMA
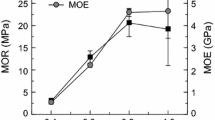
The thermomechanical analysis trace of S-NIPU + KH560 shown in Fig. 3 indicates that curing and crosslinking appear to start at a much lower temperature, namely at around 180 °C. Conversely, the S-NIPU resin alone appears to start curing at the much higher temperature of 205 °C. These results then lead to the same conclusion arrived at by DSC analysis. Thus, the silane coupling agent (KH560) can reduce the curing temperature of the S-NIPU resin by lowering its activation energy of curing. Furthermore, after curing, the Young’s modulus of the S-NIPU + KH560 adhesive is always higher than that of the S-NIPU alone. This means that the S-NIPU + KH560 adhesive performs better than the S-NIPU resin when cured under the same conditions, which is confirmed by the particleboard results shown in Table 3.
Influences of the KH560 silane on the performance of particleboards
The results for particleboards bonded with S-NIPU and S-NIPU + KH560 at 180 °C and 200 °C are shown in Table 3. The KH560 silane clearly appears to have a positive effect on the S-NIPU resin. By comparing the performance of two boards produced at 180 °C, KH560 improved the IB strength of the particleboard from 0.23 MPa of S-NIPU alone to 0.44 MPa of the S-NIPU + KH560. The other properties such as water resistance and bending strength are also improved. The same occurred at the 200 °C press temperature, where the effect of the KH560 on S-NIPU is even more evident. It is especially notable that at 200 °C, the performance of the board bonded with S-NIPU + KH560 is even better than for the board pressed at 230 °C with S-NIPU alone. This confirms that KH560 can decrease the curing temperature of S-NIPU and improve its performance. The schematic reaction between KH560 and S-NIPU is as follows:

Epoxy functional groups react with amino groups to form C-N linkages, and this reaction easily occurs even at lower temperature as has been reported by Rozenberg (1986), Riccardi et al. (1984) and Serier et al. (1991). This is why KH560 can reduce the curing temperature of the S-NIPU resin. Furthermore, based on this reaction, the tridimensional network structure is better, enough to improve the bonding performance of the S-NIPU adhesive.
The surface color of the particleboards prepared at 180 °C and 200 °C is closer to that of wood and not dark brown as the boards prepared at 230 °C, thus without wood degradation under these hot press conditions. The results of the S-NIPU + KH560 shown in Table 3 indicate that a satisfactory particleboard can be prepared at a hot press temperature between 180 and 200 °C. The density profiles characteristic of the particleboard prepared at 180 °C and 200 °C (Table 3) is shown in Fig. 4 and shows a fairly regular shape for all of them.
Conclusion
Sucrose as a bio-sourced material can yield a non-isocyanate polyurethanes (S-NIPU) resin that can be used to bond particleboards. The boards` testing performance confirms that the S-NIPU resin has a definite potential for application as a wood adhesive. It has excellent bonding properties, water resistance and heat resistance. The silane coupling agent (KH560) can significantly reduce the curing temperature of the S-NIPU resin, so as to achieve a more complete cure at a lower temperature. Incidentally, the IB strength of the particleboard prepared at 200 °C is as high as 1.06 MPa. This makes the S-NIPU resin an interesting possibility as a high-performance and environmentally friendly adhesive for wood panels.
References
Ayrilmis N, Buyuksari U, Avci E (2009) Utilization of waste tire rubber in manufacture of oriented strand board. Waste Manag 29(9):2553–2557
Birukov O, Potashnikova R, Leykin A, Figovsky O, Shapovalov L (2009) Advantages in chemistry and technology of non-isocyanate polyurethane. J Sci Israel-Technol Adv 11:160–167
Boyer A, Cloutet E, Tassaing T, Gadenne B, Alfos C, Cramail H (2010) Solubility in CO2 and carbonation studies of epoxidized fatty acid diesters: towards novel precursors for polyurethane synthesis. Green Chem 12:2205–2213
Camara F, Benyahya S, Besse V, Boutevin G, Auvergne R, Boutevin B, Caillol S (2014) Reactivity of secondary amines for the synthesis of nonisocyanate polyurethanes. Eur Polym J 55:17–26
China National Standard GB/T 17657 (1999) Test Methods for Evaluating the Properties of Wood-Based Panels and Surface Decorated Wood-Based Panels. The Standardization Administration of the People’s Republic of China, Beijing
China National Standard GB/T 4897.5-2003 (2003) Requirements for load-bearing boards for use in humid conditions. The Standardization Administration of the People’s Republic of China, Beijing
Cornille A, Michaud G, Simon F, Fouquay S, Auvergne R, Boutevin B, Caillol S (2016) Promising mechanical and adhesive properties of isocyanate-free poly (hydroxyurethane). Eur Polym J 84:404–420
Cornille A, Auvergne R, Figovsky O, Boutevin B, Caillol S (2017) A perspective approach to sustainable routes for non-isocyanate polyurethanes. Eur Polym J 87:535–552
EN312 2003 (2003) Particleboards -specifications European Committee for Standardisation
Figovsky O, Shapovalov L (2002) Features of reaction amino-cyclocarbonate for production of new type polyurethanes. Macromol Symp 187:325–332
Fiorelli J, Curtolo DD, Barrero NG, Savastano H Jr, Pallone EMDJA, Johnson R (2012) Particulate composite based on coconut fiber and castor oil polyurethane adhesive: an eco-efficient product. Ind Crops Prod 40:69–75
Fotea C, D’Silva C (2004) The use of silane reagents as primers to enhance the adhesion of chromium tanned heavy-duty leather (Salz leather). Int J Adhes Adhes 24(1):1–7
George B, Simon C, Properzi M, Pizzi A, Elbez G (2003) Comparative creep characteristics of structural glulam wood adhesives. Eur J Wood Prod 61(1):79–80
Haniffa MACM, Ching YC, Chuah CH, Kuan YC, Liu DS, Liou NS (2017) Synthesis, characterization and the solvent effects on interfacial phenomena of jatropha curcas oil based non-isocyanate polyurethane. Polymers 9(5):162
Kathalewar MS, Joshi PB, Sabnis AS, Malshe VC (2013a) Non-isocyanate polyurethanes: from chemistry to applications. Rsc Adv 3(13):4110–4129
Kathalewar M, Sabnis A, Waghoo G (2013b) Effect of incorporation of surface treated zinc oxide on non-isocyanate polyurethane based nano-composite coatings[J]. Progr Org Coat 76(9):1215–1229
Kihara N, Endo T (1993) Synthesis and properties of poly(hydroxyurethane)s. J Polym Sci Part A Polym Chem 31:2765–2773
Kihara N, Kushida Y, Endo T (1996) Optically active poly(hydroxyurethane)s derived from cyclic carbonate and l-lysine derivatives. J Polym Sci Part A Polym Chem 34:2173–2179
Mo X, Cheng E, Wang D, Sun XS (2003) Physical properties of medium-density wheat straw particleboard using different adhesives. Ind Crops Prod 18(1):47–53
Pizzi A, Mittal KL (eds) (2011) Wood adhesives. CRC Press, Boca Raton
Poussard L, Mariage J, Grignard B, Detrembleur C, Jérôme C, Calberg C, Heinrichs B, De Winter J, Gerbaux P, Raquez MJ, Bonnaud L, Dubois P (2016) Non-isocyanate polyurethanes from carbonated soybean oil using monomeric or oligomeric diamines to achieve thermosets or thermoplastics. Macromolecules 49(6):2162–2171
Properzi M, Pizzi A, Uzielli L (2003) Comparative wet wood glueing performance of different types of Glulam wood adhesives. Eur J Wood Prod 61(1):77–78
Riccardi CC, Adabbo HE, Williams RJJ (1984) Curing reaction of epoxy resins with diamines. J Appl Polym Sci 29(8):2481–2492
Rokicki G, Piotrowska A (2002) A new route to polyurethanes from ethylene carbonate, diamines and diols. Polymer 43:2927–2935
Rozenberg BA (1986) Kinetics, thermodynamics and mechanism of reactions of epoxy oligomers with amines. In: Dusek K (ed) Epoxy resins and composites II. Springer, Berlin, pp 113–165
Santiago-Medina FJ, Basso MC, Pizzi A, Delmotte L (2018) Polyurethanes from Kraft Lignin without Using Isocyanates. J Renew Mater 6(4):413–425
Serier A, Pascault JP, My LT (1991) Reactions in aminosilane–epoxy prepolymer systems I. Kinetics of epoxy–amine reactions. J Polym Sci Part A Polym Chem 29(2):209–218
Stoeckel F, Konnerth J, Gindl-Altmutter W (2013) Mechanical properties of adhesives for bonding wood—a review. Int J Adhes Adhes 45:32–41
Thébault M, Pizzi A, Dumarçay S, Gerardin P, Fredon E, Delmotte L (2014) Polyurethanes from hydrolysable tannins obtained without using isocyanates. Ind Crops Prod 59:329–336
Thébault M, Pizzi A, Essawy H, Baroum A, Van Asche G (2015) Isocyanate free condensed tannin-based polyurethanes. Eur Polym J 67:513–523
Tomita H, Sanda F, Endo T (2001a) Polyaddition behavior of bis(five- and six-membered cyclic carbonate)s with diamine. J Polym Sci Part A Polym Chem 39:860–867
Tomita H, Sanda F, Endo T (2001b) Model reaction for the synthesis of polyhydroxyurethanes from cyclic carbonates with amines: substituent effect on the reactivity and selectivity of ring-opening direction in the reaction of five-membered cyclic carbonates with amine. J Polym Sci Part A Polym Chem 39:3678–3685
Xi X, Pizzi A, Delmotte L (2018a) Isocyanate-Free Polyurethane Coatings and Adhesives from Mono-and Di-Saccharides. Polymers 10(4):402
Xi X, Pizzi A, Gerardin C, Du G (2018b) Glucose-biobased Non-Isocyanate Polyurethane Rigid Foams. J Renew Mater 1–11
Zhang L, Hu Y (2014) Novel lignocellulosic hybrid particleboard composites made from rice straws and coir fibers. Mater Des 55:19–26
Acknowledgements
This work was supported by National Natural Science Foundation of China (31660176, 31800481 and 31870546). The first author thanks the China Scholarship Council for the study bursary granted to him. The LERMAB of the University of Lorraine is supported by a grant overseen by the French National Research Agency (ANR) as part of the Laboratory of Excellence (Labex) ARBRE.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xi, X., Wu, Z., Pizzi, A. et al. Non-isocyanate polyurethane adhesive from sucrose used for particleboard. Wood Sci Technol 53, 393–405 (2019). https://doi.org/10.1007/s00226-019-01083-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-019-01083-2