Abstract
The aim of this research was to characterise the bio-oil produced by pyrolysis of Acacia mangium wood through gas chromatography–mass spectrometry (GC–MS). Experimental study was employed using two experiment models: two-level factorial design (TLFD) and response surface methodology–Box–Behnken (RSM–BB). TLFD was used to analyse the final temperature, heating rate and particle size effect on the bio-oil yield, while RSM–BB was conducted to determine the optimum conditions for bio-oil production. The statistical analysis showed that the factors of pyrolysis temperature and particle size had the greater effect, while the heating rate was significant, but had a lesser effect. By utilising RSM, these factors presented the optimal conditions obtained at pyrolysis temperature of 499.57 °C, heating rate of 12 °C min−1 and particle size of 0.46 mm. With the GC–MS result, it was observed that the percentage of phenol and derivatives was much higher than the rest of the components.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
At present, one recognises the problem of the limited quantity of fossil fuel resources and their environmental impacts, such as the carbon dioxide emissions released through the burning of fossil fuels. The need for reducing carbon dioxide emissions from energy consumption has stimulated the development of sustainable technologies based on renewable energy sources (Mohan et al. 2006).
In contrast to fossil fuels, the use of biomass for energy provides significant environmental advantages. Plant growth needed to generate biomass feedstocks removes atmospheric carbon dioxide, i.e. utilising forest material which will not create a carbon imbalance over the life cycle of the forest, which can have a duration of 5–80 years for managed forests (Amidon and Liu 2009). In a neutral balanced operation, carbon dioxide is simply recycled during the life span of plant growth and bio-products (Liu et al. 2006). Biomass is known as the third largest primary energy resource in the world. Woody biomass represents the most abundant and available biomass source (Vamvuka et al. 2003).
The lignocellulosic biomass represents a sustainable choice for the production of fuels and raw materials for the chemical processing industry (Czernik and Bridgwater 2004; Liu 2010). The conversion of biomass to chemicals and energy is imperative to sustaining modern ways of life. Various possible uses of bio-oil have been reported in the literature in recent years. For example, in the energy sector, bio-oils can be used as fuels for diesel engines, gas turbines, boilers or raw materials to obtain hydrocarbons by catalytic transformation (Czernik and Bridgwater 2004; Gayubo et al. 2010). Bio-oils could also be used as raw materials for the production of resins and pharmaceutical products (Czernik and Bridgwater 2004; Effendi et al. 2008). The production of some high demand chemicals, such as methanol, acetic acid, acetone and particularly phenols, can also be obtained from bio-oils (Amen-Chen et al. 1997; Choi et al. 2015). Pyrolysis liquids can also be useful for soil improvement, vermin extermination and deodorant effects. However, it would be helpful to clarify their characteristics to establish new uses such as the development of new biocides effective against wood biodegradation (Donald 2004). However, these uses could be hindered by certain properties of the bio-oils, such as high acidity, high water content or storage instability, but thermal, physical or chemical upgrading could improve them (Bertero et al. 2012; Valle et al. 2007; Zhang et al. 2007).
Most processes that convert biomass to liquid fuels begin with pyrolysis, followed by the catalytic upgrading of the resulting bio-crude liquids. The research and technology concerning bio-oil have advanced significantly. More than two hundred publications have been reviewed, discussed and summarised by Jahirul et al. (2012). Other studies have recently been published, among them: a review on the parameters that affect biomass pyrolysis and a summary of the state of industrial pyrolysis technologies by Kan et al. (2016) and the importance of catalytic effects on pyrolysis (Sharma et al. 2015).
Nevertheless, the chemical composition of biomass is very different from that of coal oil and oil shales, etc. The major constituents consist of cellulose (a polymer glucosan), hemicelluloses (which are also called polyose), lignin, organic extractives and inorganic minerals. The weight per cent of cellulose, hemicellulose and lignin varies in different biomass species of wood (McKendry 2002).
The presence of large amounts of oxygen in plant carbohydrate polymers means the pyrolytic chemistry differs sharply from these other fossil feeds. The liquid fraction is a complex mixture of water and organic chemicals. For highly cellulosic biomass feedstocks, the liquid fraction usually contains complex mixtures of oxygenated aliphatic and aromatic compounds, resins, intermediate carbohydrates, phenols, aromatics, aldehydes, their condensation products and other derivatives, which can be produced by the pyrolysis of the biomass (Güllü and Demirbas 2001; Meier and Faix 1999). Liquid yield and liquid physicochemical properties, such as water content, viscosity or composition, depend on the feedstock, the operational conditions, the liquid recovery system and type of reactor. However, each reactor type has specific characteristics, bio-oil yielding capacity, advantages and limitations (Jahirul et al. 2012).
Various studies have been published about the different types of biomass for the production of pyrolysis liquids. For example, in countries like Finland or Canada, wood pyrolysis and forestry residue pyrolysis have been investigated for several years (Ba et al. 2004). Other suitable types of feedstock for the pyrolysis process are all kinds of waste, such as agricultural waste (Bertero et al. 2014), urban waste (Islam and Beg 2004), meat and bone meal (Ayllón et al. 2006) and sewage sludge (Fonts et al. 2009).
The results, which were strongly dependent on the sources and the technological operations of the process, showed that the liquid yields range from 30 to 50 wt%, oxygen between 25 and 40 wt% and water from 20 to 50 wt% contents (Bertero et al. 2014).
Among the fast-growing species is Acacia mangium, which may be a promising species for energy production. This species has been planted extensively in the humid tropics of Southeast Asia because of its fast growth in a wide range of places, including degraded land, and because it yields an acceptable quality of wood for pulp and paper (Krisnawati et al. 2011; Turnbull et al. 1988). This plant could be a possible alternative to wood for short-rotation forestry due to its fast growth, the fact that it does not require annual reseeding or agricultural inputs and that it can grow in poor soils with low fertility.
Recent studies published show an increasing interest in the use of biomass from A. mangium such as renewable energy source. Arteaga Crespo et al. (2015) analysed the thermal and kinetic behaviour of the A. mangium and compared it with other species that have been proposed as raw materials with great potential to be used as raw material in thermochemical processes. The proposed model predicts the curves of biomass pyrolysis of A. mangium with a deviation of less than 3% between calculated and experimental curves at the heating rates studied. On the other hand, the potential bioenergy of A. mangium wood as a heating fuel resource was examined using the basic properties (anatomical, physical and chemical related) by Marsoem and Irawati (2016). Additionally, the thermal evolution profile of the pyrolysis process of A. mangium wood to be used as a mixture with coal was investigated. This study showed that the reactivity of the fuel increased with the increasing proportion of this biomass in the fuel. A high content of volatile matter found in the blends compared to coal may also improve the amount of the fuel (Barlin et al. 2016).
However, these studies did not deal with the liquids and their characteristics, not even in the revised version. A study of that type is necessary to increase knowledge of A. mangium which would allow the development of technical and economically feasible systems of conversion of this raw material.
In this study, the chemical components of pyrolysis liquids obtained from Acacia mangium Willd wood were analysed by gas chromatography–mass spectroscopy (GC–MS).
Materials and methods
Samples
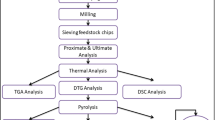
Woody biomass of A. mangium samples were obtained from an energy plantation. A selection of young trees up to 8 years old was performed by Empresa Forestal Pinar del Río, Cuba. They were reduced to small particles of 2.36–0.40 mm in accordance with ASTM-E1757-01 (2007) using a set of Tyler mesh sieves. The proximate and ultimate analysis of A. mangium has previously been determined and published by Arteaga Crespo et al. (2015). The main information concerning the composition is shown in Table 1.
Pyrolysis experimental procedure
Experimental study was conducted in two stages:
The objective of the first stage was to determine the main characteristics of degradation of A. mangium wood pyrolysis through thermogravimetric analysis (TGA). This TGA was carried out with a thermobalance (TGA-TA Instruments 2050, New Castle, DE). The sample weight loss and the rate of weight loss were recorded continuously under dynamic conditions, as functions of time or temperature and at a heating rate of 15 °C min−1 under a nitrogen flow of 100 mL min−1 from room temperature to about 700 °C. The mass of the initial samples was approximately 8 mg. For the determination of the main degradation characteristics, the procedure suggested by Grønli et al. (2002) was used taking into account:
-
1.
T onset(hc) temperature calculated from the extrapolation of the partial peak of the decomposition of the hemicellulose, which marks the beginning of the active zone, and the decomposition of hemicellulose.
-
2.
(−dX/dt)sh and T sh are the maximum overall decomposition rates of the hemicellulose and the corresponding temperature, respectively.
-
3.
(−dX/dt)peak and T peak are the maximum overall decomposition rates of the cellulose and the corresponding temperature, respectively.
-
4.
T offset is the extrapolated temperature of the (−dX/dt) curve. This value marks the end of the cellulose decomposition.
In this study, the decomposition zones of hemicellulose, cellulose and lignin (Jeguirim and Trouvé 2009) were observed. Based on results of the TGA, the temperature ranges were selected for the laboratory-scale experiments.
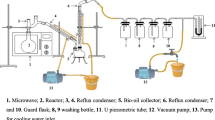
The second stage was carried out in a laboratory-grade furnace with temperatures at a heating rate of 15 °C min−1 being controlled with thermocouples inside the furnace. A constant rate of nitrogen gas flow at 100 mL min−1 was maintained in the furnace throughout the pyrolysis reaction. The mass of the initial samples was approximately 10 g. The system consisted of a trap bottle to collect the liquid fraction of a cleaning system consisting of electrostatic precipitator capacitor and filters, leads and a vacuum system (Fig. 1). The liquid fraction retained in the condenser and the electrostatic precipitator system was separated in bio-oil and water (see Fig. 1). A qualitative analysis was then performed for bio-oil by means of GC–MS gas chromatography.
Experimental design
It is possible to investigate a number of variables and their effects using a factorial or screening design. In this experiment, the effect of temperature, heating rate and particle size was used and the bio-oil yield was measured as a response variable. The three factors and the design point for 23 (low, medium, high) in coded and uncoded independent variables are listed in Table 2. The significant factors which affected the response were identified by means of a two-level factorial design (TLFD) and investigated in the RSM experiment. The significant variables were analysed by employing a Box–Behnken design (BBD). BBD can observe the interaction effect of the independent variables on the response (Pappa et al. 2000). TLFD and RSM were carried out through Design Expert version 10.0.2 (Stat Ease, USA). RSM was applied to identify the optimum levels of three variables—pyrolysis temperature, heating rate and particle size—in regard to one response (liquid production). The level of the three factors and the design point for RSM (low, medium, high) in coded and uncoded independent variables is shown in Table 2. BBD is an experimental design, useful for response surface methodology, for building a second-order (quadratic) model for the response variable and usually works well for the process optimisation. That is why it was used for the experimental design (Aslan and Cebeci 2007; Oramahi and Diba 2013).
Experiments were carried out according to the design point with independent variables including pyrolysis temperatures at 270, 385 and 500 °C, heating rate of 5, 10 and 15 °C min−1 and particle size of 0.4, 1.38 and 2.36 mm with total of 17 experimental runs (5 centre points and 12 non-centre points). The numbers of experiments \(\left( N \right)\) were calculated as follows:
where \(k\) is the factor number and \({\text{cp}}\) is the replicate number of the central point. RSM was applied to analyse the effect of independent variables on response parameter (production of liquid, %) by matching the responses studied (Y) using the second-order polynomial equation:
where \(\beta_{0}\), \(\beta_{i}\), \(\beta_{ii}\) and \(\beta_{ij}\) are the regression coefficients for intercept, linear, quadratic and interaction terms, respectively, and \(x_{i}\), \(x_{j}\) are the independent variables (Kiran et al. 2016).
Characterisation of pyrolysis liquids
Gas chromatography–mass spectroscopy (GC–MS) (Shimadzu QP5000, Japan) was employed for the chemical analysis of the liquid components. A column (DB1701) (J&W Scientific) with a diameter of 0.25 mm and length of 50 m was maintained at 45 °C for 4 min; then, the temperature was raised to 270 °C at a heating rate of 3 °C min−1 and held at 280 °C min−1. The Wiley275 Spectra library was employed.
Results and discussion
Pyrolysis is one of the thermochemical technologies for converting biomass into energy and chemical products consisting of liquid bio-oil, solid biochar and pyrolytic gas. It is derived from the complex conversion network of the three main components in biomass: cellulose, hemicellulose and lignin (Kan et al. 2016).
Degradation characteristics of A. mangium wood
The purpose of this research is to determine the main points of A. mangium thermal degradation, based on DTG data. The data of the thermal stability of biomass were analysed. The time derivative of the mass fraction and derivative second of the mass fraction are reported as functions of temperature in Fig. 2, since the A. mangium shows the typical thermal decomposition for lignocellulosic materials.
This diagram was used to determine the main temperatures of the A. mangium wood decomposition (T onset(hc), T shoulder, T peak and T offset(c)), and it established the temperature range of work for the experimental design. The minimum level of temperature was selected as a value higher than T onset(hc) and lower than T shoulder, associated with the beginning and maximum hemicellulose decomposition, respectively. The devolatilisation of A. mangium occurs at temperatures similar to the ones obtained for the same kind of wood—hardwood at 237 °C (Grønli et al. 2002), Arundo donax which occurs at 200 °C (Jeguirim and Trouvé 2009) and Dichrostachys cinerea at 222 °C (Abreu et al. 2010).
Moreover, for the maximum temperature level, a value higher than T offset was taken into account, known as the end of the active decomposition zone, a factor affecting bio-oil yield.
Factors affecting bio-oil yield
It can be observed that factors A and C produced the highest effect. The significant effects fall to the right on this graph, with the biggest effects starting on the right. Factors B and AC also were significant, but had a lesser effect. However, the interactions of AB, BC and ABC are insignificant with p value > 0.05, as shown in Fig. 3a. Analysis of variance (ANOVA) was performed, and the “prob > F” less than 0.05 indicates factors of heating rate, pyrolysis temperature and particle size are statistically significant. Pyrolysis temperature shows strong influences on producing bio-oil and is presented by F value of 99.42. The F values for particle size and heating rate were 37.06 and 5.29, respectively. The equation in terms of coded factors can be used to make predictions about the response for levels of each factor. The coded equation is useful for identifying the relative impact of the significant factors by comparing the factor coefficients (Eq. 3):
ANOVA test was conducted and shows that A, B and C are significant with a p value of <0.05. Figure 3b shows that the data are normally distributed and stable. The curvature test is significant and shows that optimisation can be investigated.
Optimisation of bio-oil yield from A. mangium
The main objective of the optimisation study was to find the finest combination values of all parameters (pyrolysis temperature, heating rate and particle size) within their particular range in order to maximise the production of bio-oil yield percentage from the studied biomass. The quadratic model was suggested with R2 value of 0.962 and indicated that 91.54% of the total variation in the bio-oil yield was attributed to the experimental variables studied. The “Pre R-Squared” of 0.9146 is in reasonable agreement with the “Adj R-Squared” of 0.7512 with a smaller difference of 0.2 as suggested by Anderson and Whitcomb (2015). According to ANOVA results, for lack of fit using F value of 0.68 is not significant while its p value is 0.4158. The p value for lack of fit indicates that there is only 41.58% chance that a “Lack of fit F value” this large could occur due to noise. Furthermore, non-significant “Lack of fit” is good for experimental data to fit in predicted model. The most important portion of the data analysis is to verify model adequacy. Since this model yielded a result of 15.065, which is higher than 4, the model is adequate for representing the experimental data.
The predicted response values of the quadratic model and actual response values obtained from the experimental results were compared and are shown in Fig. 4. The graph distribution confirms the ability of the model to cover the whole range of experiments studied.
The coefficients of the independent variables (in terms of coded values) were determined for the second-order polynomial model. Only the significant terms (p ≤ 0.05) are presented as follows:
The result of regression analysis seems to suggest that bio-oil yield was only affected by the main factors since the interactions of AB, AC and BC are insignificant with p value >0.05.
By using RSM, every factor was analysed and a high bio-oil yield could be obtained at a temperature of nearly 500 °C, a higher heating rate and smaller particle size for studied conditions. An optimisation process was conducted to determine the optimal conditions of the process to obtain a high percentage of bio-oil yield. The results from the software suggested the optimal conditions can be attained at a pyrolysis temperature of 499.57 °C, a heating rate of 12 °C min−1 and a particle size of 0.46 mm. The predicted bio-oil yield was 33.13% at the researched conditions (Fig. 5). Previous studies (Akhtar and Saidina Amin 2012; Aysu and Küçük 2014; Yorgun and Yıldız 2015) suggest that a final pyrolysis temperature close to 500 °C for biomass pyrolysis maximises the liquid product yield. At temperatures higher than 500 °C, pyrolysis process favours gas generation and liquid yield begins to decrease, as reported by Luo et al. (2004). Moreover, Yorgun and Yıldız (2015) discovered that the bio-oil yield for paulownia wood reached a maximum value of 26% at a final temperature of 500 °C, particle size range of 0.425 < Dp < 1.00 mm and a heating rate of 10 °C min−1.
The production of liquid ranged from 24.88 to 32.94% which is displayed in Table 3. Similar results were obtained by Demiral and Şensöz (2006) for Corylus Avellana L. bagasse with values between 24.53 and 33.19%, at a heating rate of 10 °C min−1 and temperature of 500 °C. A similar behaviour with different biomass samples was also obtained in the literature (Zanzi et al. 2002). Nevertheless, another study at a higher heating rate and with similar values of temperature and particle size reported results with a maximum pyrolysis oil yield of 48% (Isa et al. 2011).
The reason for the lower liquid yield at lower temperatures may be due to the temperature rise which was not enough for complete pyrolysis to take place, thus yielding less liquid product. On the other hand, at a higher temperature, the secondary decomposition reaction could possibly take place. The yield and quality of the pyrolysis products mainly depend on operating parameters, type of biomass and type of the pyrolysis process. The operating parameters include the final pyrolysis temperature, heating rate, residence time, sweep gas flow rate, moisture content, mineral matter and catalysts (Akhtar and Saidina Amin 2012; Önal et al. 2011; Xiu and Shahbazi 2012).
Chemical composition of bio-oil from A. mangium
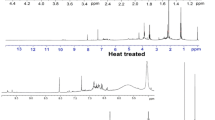
GC–MS has become a quick, convenient and powerful tool for characterising complex and heterogeneous bio-oil samples (Sobeih et al. 2008). GC–MS analysis was carried out in order to determine the component of organic compounds in the bio-oil produced at the nearly optimum pyrolysis conditions (reaction temperature 500 °C, heating rate 10 °C min−1 and particle size of 0.4 mm). The compounds detected (see Fig. 6) have been grouped according to their chemical nature (see Table 4). Around 40 compounds were identified in the liquid fraction, consistent with previously reported studies (Bhattacharjee 2016; Chen et al. 2016; Qu et al. 2011; Shen et al. 2011). The highest peak areas of the chemical compound analysed by GC–MS correspond to cyclopropyl carbinol; 2-hydroxy-3-methyl-2-cyclopenten-1-one; 1,2-benzenediol; phenol, 4-ethyl-2-methoxy; 2-methoxy-4-methylphenol. In general, chemical composition was similar to the poplar wood sawdust reported by Gu et al. (2013).
The percentage area corresponding to phenol and its derivatives was much higher than the rest of the components. The GC–MS data revealed that the samples were rich in phenolic compounds; similar results have been reported in the literature (Qu et al. 2011; Yorgun and Yıldız 2015). This result is mainly attributed to the lignin degradation in phenol and their derivatives compounds (Bridgwater 2003). The presence of acids and aldehydes is attributed to the degradation of phenolic. On the other hand, phenols are one of the degradation products of furans and derivatives. The presence of alcohol and ketones was higher than that reported by Muley et al. (2016) regarding pine sawdust in all ranges of temperatures studied by these authors.
Higher carbon content could be contributed to the bio-oil produced from hemicellulose, as it has previously been reported that bio-oil from hemicellulose has a high content of acids, aromatics, esters and ketones (Qu et al. 2011; Xiu and Shahbazi 2012). The oxygen content of the liquids obtained from the pyrolysis of cellulose was much higher than lignin and sawdust. This can be shown by the higher initial oxygen content of the cellulose based upon its chemical formula (C6H10O5)n as compared to (C9H10O2, C10H12O3, C11H14O4,)n of lignin.
Conclusion
Thermogravimetric analysis shows the main zones of the A. mangium wood thermal degradation. This allowed defining the experimental temperature ranges. The heating rate, pyrolysis temperature and particle size have significant effects on the production of bio-oil. The pyrolysis temperature and particle size had the highest effects, while the heating rate was significant, but had a lesser effect. Optimisation by RSM–BB suggested an optimal bio-oil yield of 33.13%. It was observed through GC–MS that there were 42 types of chemical compounds with an area percentage of phenol and derivatives of around 40%.
References
Abreu NR, Foppa PE, Riva G, Romero RO (2010) Caracterización energética del Marabú (Energetic characterization of Marabu). DYNA Ingeniería e Industria 85(7):581–592 (In Spanish)
Akhtar J, Saidina Amin N (2012) A review on operating parameters for optimum liquid oil yield in biomass pyrolysis. Renew Sustain Energy Rev 16:5101–5109
Amen-Chen C, Pakdel H, Roy C (1997) Separation of phenols from Eucalyptus wood tar. Biomass Bioenerg 13:25–37
Amidon TE, Liu S (2009) Water-based woody biorefinery. Biotechnol Adv 27:542–550
Anderson MJ, Whitcomb PJ (2015) DOE simplified: practical tools for effective experimentation. CRC Press, New York
Arteaga Crespo Y, Abreu Naranjo R, Vargas Burgos JC, Glauco Sanchez C, Sanchez Sanchez EM (2015) Thermogravimetric analysis of thermal and kinetic behavior of acacia mangium wood. Wood Fiber Sci 47:327–335
Aslan N, Cebeci Y (2007) Application of Box-Behnken design and response surface methodology for modeling of some Turkish coals. Fuel 86:90–97
ASTM-E1757-01 (2007) Standard practice for preparation of biomass for compositional analysis, ASTM International, West Conshohocken, PA
Ayllón M, Aznar M, Sánchez JL, Gea G, Arauzo J (2006) Influence of temperature and heating rate on the fixed bed pyrolysis of meat and bone meal Chem Eng J 121:85–96 d a fixed-bed reactor: effects of pyrolysis parameters on product yields and characterization of products. Energy 64:1002–1025
Aysu T, Küçük MM (2014) Biomass pyrolysis in a fixed-bed reactor: Effects of pyrolysis parameters on product yields and characterization of products. Energy 64:1002–1025. doi:10.1016/j.energy.2013.11.053
Ba T, Chaala A, Garcia-Perez M, Rodrigue D, Roy C (2004) Colloidal properties of bio-oils obtained by vacuum pyrolysis of softwood bark. Characterization of water-soluble and water-insoluble fractions. Energy Fuels 18:704–712
Barlin B, Gunawan G, Arifin A, Pratiwi DK (2016) Thermal evolution profile analysis for pyrolysis of coal—Acacia Mangium Wood Blends. IJTech 7(5):881–888
Bertero M, de la Puente G, Sedran U (2012) Fuels from bio-oils: bio-oil production from different residual sources, characterization and thermal conditioning. Fuel 95:263–271
Bertero M, Gorostegui HA, Orrabalis CJ, Guzmán CA, Calandri EL, Sedran U (2014) Characterization of the liquid products in the pyrolysis of residual chañar and palm fruit biomasses. Fuel 116:409–414
Bhattacharjee N (2016) Bio-oil production from fast pyrolysis of aquatic prostate herb (Achyranthes paludosa) Int J Latest Tech Eng Manag Appl Sci vol:9
Bridgwater AV (2003) Renewable fuels and chemicals by thermal processing of biomass. Chem Eng J 91:87–102
Chen T, Zhang J, Wu J (2016) Kinetic and energy production analysis of pyrolysis of lignocellulosic biomass using a three-parallel Gaussian reaction model. Bioresour Technol 211:502–508
Choi G-G, Oh S-J, Lee S-J, Kim J-S (2015) Production of bio-based phenolic resin and activated carbon from bio-oil and biochar derived from fast pyrolysis of palm kernel shells. Bioresour Technol 178:99–107
Czernik S, Bridgwater AV (2004) Overview of applications of biomass fast pyrolysis oil. Energy Fuels 18:590–598
Demiral İ, Şensöz S (2006) Fixed-bed pyrolysis of Hazelnut (Corylus Avellana L.) Bagasse: influence of pyrolysis parameters on product yields. Energy Source Part A Recovery Util Environ Eff 28:1149–1158
Donald LK (2004) Biomass for renewable energy and fuels. In: Cutler JC (ed) Encyclopedia of Energy. Elsevier, New York, pp 193–212
Effendi A, Gerhauser H, Bridgwater AV (2008) Production of renewable phenolic resins by thermochemical conversion of biomass: a review. Renew Sustain Energy Rev 12:2092–2116
Fonts I, Azuara M, Gea G, Murillo MB (2009) Study of the pyrolysis liquids obtained from different sewage sludge. J Anal Appl Pyrolysis 85:184–191
Gayubo AG, Valle B, Aguayo AT, Olazar M, Bilbao J (2010) Olefin production by catalytic transformation of crude bio-oil in a two-step process. Ind Eng Chem Res 49:123–131
Gronli MG, Várhegyi G, Di Blasi C (2002) Thermogravimetric analysis and devolatilization kinetics of wood. Ind Eng Chem Res 41:4201–4208
Gu X, Ma X, Li L, Liu C, Cheng K, Li Z (2013) Pyrolysis of poplar wood sawdust by TG–FTIR and Py–GC/MS. J Anal Appl Pyrolysis 102:16–23
Güllü D, Demirbas A (2001) Biomass to methanol via pyrolysis process. Energy Convers Manag 42:1349–1356
Isa KM, Daud S, Hamidin N, Ismail K, Saad SA, Kasim FH (2011) Thermogravimetric analysis and the optimisation of bio-oil yield from fixed-bed pyrolysis of rice husk using response surface methodology (RSM). Ind Crop Prod 33:481–487
Islam M, Beg M (2004) The fuel properties of pyrolysis liquid derived from urban solid wastes in Bangladesh. Bioresour Technol 92:181–186
Jahirul MI, Rasul MG, Chowdhury AA, Ashwath N (2012) Biofuels production through biomass pyrolysis—a technological review. Energies 5:4952–5001
Jeguirim M, Trouvé G (2009) Pyrolysis characteristics and kinetics of Arundo donax using thermogravimetric analysis. Bioresour Technol 100:4026–4031
Kan T, Strezov V, Evans TJ (2016) Lignocellulosic biomass pyrolysis: a review of product properties and effects of pyrolysis parameters. Renew Sustain Energy Rev 57:1126–1140
Kiran B, Pathak K, Kumar R, Deshmukh D (2016) Statistical optimization using central composite design for biomass and lipid productivity of microalga: a step towards enhanced biodiesel production. Ecol Eng 92:73–81
Krisnawati H, Kallio M, Kanninen M (2011) Acacia mangium Willd.: ecology, silviculture and productivity. CIFOR (C. f. I. F. Research Ed.). Indonesia
Liu S (2010) Woody biomass: niche position as a source of sustainable renewable chemicals and energy and kinetics of hot-water extraction/hydrolysis. Biotechnol Adv 28:563–582
Liu S, Amidon TE, Francis RC, Ramarao BV, Lai Y-Z, Scott GM (2006) From forest biomass to chemicals and energy Biorefinery initiative in New York State. Ind Biotechnol 2:113–120
Luo Z, Wang S, Liao Y, Zhou J, Gu Y, Cen K (2004) Research on biomass fast pyrolysis for liquid fuel. Biomass Bioenergy 26:455–462
Marsoem SN, Irawati D (2016) Basic properties of Acacia mangium and Acacia auriculiformis as a heating fuel. In: AIP Conference Proceedings, 2016. doi:10.1063/1.4958551
McKendry P (2002) Energy production from biomass (part 1): overview of biomass. Bioresour Technol 83:37–46
Meier D, Faix O (1999) State of the art of applied fast pyrolysis of lignocellulosic materials—a review. Bioresour Technol 68:71–77
Mohan D, Pittman CU, Steele PH (2006) Pyrolysis of wood/biomass for bio-oil: a critical review. Energy Fuels 20:848–889
Muley PD, Henkel C, Abdollahi KK, Marculescu C, Boldor D (2016) A critical comparison of pyrolysis of cellulose, lignin, and pine sawdust using an induction heating reactor. Energy Convers Manag 117:273–280
Önal EP, Uzun BB, Pütün AE (2011) Steam pyrolysis of an industrial waste for bio-oil production. Fuel Process Technol 92:879–885
Oramahi HA, Diba F (2013) Maximizing the production of liquid smoke from bark of durio by studying its potential compounds. Procedia Environ Sci 17:60–69
Pappa A, Tzamtzis N, Statheropoulos M, Fasseas C (2000) The pyrolytic behavior of Pinus halepensis needles observed by transmission light microscopy and stereoscopy. J Anal Appl Pyrolysis 55(2):195–202
Qu T, Guo W, Shen L, Xiao J, Zhao K (2011) Experimental study of biomass pyrolysis based on three major components: hemicellulose cellulose, and lignin. Ind Eng Chem Res 50:10424–10433
Sharma A, Pareek V, Zhang D (2015) Biomass pyrolysis—a review of modelling, process parameters and catalytic studies. Renew Sustain Energy Rev 50:1081–1096
Shen DK, Gu S, Jin B, Fang MX (2011) Thermal degradation mechanisms of wood under inert and oxidative environments using DAEM methods. Bioresour Technol 102:2047–2052
Sobeih KL, Baron M, Gonzalez-Rodriguez J (2008) Recent trends and developments in pyrolysis–gas chromatography. J Chromatogr A 1186:51–66
Turnbull JW, Midgley SJ, Cossalter C (1988) Tropical acacias planted in Asia: an overview ACIAR Proc 82
Valle B, Gayubo Ana G, Atutxa A, Alonso A, Bilbao J (2007) Integration of thermal treatment and catalytic transformation for upgrading biomass pyrolysis oil. Int J Chem React Eng. doi:10.2202/1542-6580.1559
Vamvuka D, Kakaras E, Kastanaki E, Grammelis P (2003) Pyrolysis characteristics and kinetics of biomass residuals mixtures with lignite☆. Fuel 82:1949–1960
Xiu S, Shahbazi A (2012) Bio-oil production and upgrading research: a review. Renew Sustain Energy Rev 16:4406–4414
Yorgun S, Yıldız D (2015) Slow pyrolysis of paulownia wood: effects of pyrolysis parameters on product yields and bio-oil characterization. J Anal Appl Pyrolysis 114:68–78
Zanzi R, Sjöström K, Björnbom E (2002) Rapid pyrolysis of agricultural residues at high temperature. Biomass Bioenergy 23:357–366
Zhang Q, Chang J, Wang T, Xu Y (2007) Review of biomass pyrolysis oil properties and upgrading research. Energy Convers Manag 48:87–92
Acknowledgements
The authors are grateful to the Biomass Laboratory of the Department of Thermal and Fluid Engineering, State University of Campinas, Brazil. The authors would also like to thank the anonymous reviewers and editor for their thoughtful comments that contributed to the improvement in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Crespo, Y.A., Naranjo, R.A., Quitana, Y.G. et al. Optimisation and characterisation of bio-oil produced by Acacia mangium Willd wood pyrolysis. Wood Sci Technol 51, 1155–1171 (2017). https://doi.org/10.1007/s00226-017-0913-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-017-0913-x