Abstract
As water moves inside the wood, the gluelines might act as barriers that are potentially leading to local moistening. Even low amounts of water may influence the mechanical performance of glued wood products. Dynamic vapor sorption analysis was performed to assess the sorption processes of six commercial wood adhesives. Vapor sorption isotherms were compared with vapor uptake dynamics. Phenol–resorcinol–formaldehyde showed high moisture uptake of 18 %, while vapor diffusion speed was low. Fish glue showed a water uptake of 45 % at otherwise moderate vapor uptake speed. Melamine–formaldehyde resin gained 22 % water, and polyvinyl acetate absorbed 10 %. The latter was also the fastest vapor absorbing adhesive. Polyurethane only absorbed 3.5 % of moisture at medium uptake speed. Mechanisms of water diffusion seem to be driven by (1) the available free volume in the polymer and (2) the interacting ionic groups of the polymer chain. While the free volume could be linked to the accumulated moisture, the ionic group interaction might determine the measured vapor diffusion dynamics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Absorption of water in adhesives has the potential of causing undesirable effects. Even a small amount of water uptake in adhesives may influence the mechanical performance of gluelines, with the risk of jeopardizing the safety of glued wood products or wood-based structural elements (Hakala et al. 2001). It was found that under water-immersed condition, the elastic modulus and hardness of various wood adhesives were reduced by up to half of the dry-state values (Konnerth et al. 2010). As water flows or diffuses within wood, the present glue layers may act as barriers, potentially leading to local moistening or swelling of the used adhesive. In addition to a loss of mechanical performance, a possible glueline swelling may cause additional internal stresses, or fungal attack may occur in case polymer moisture is reaching higher levels.
For hardened phenol–formaldehyde resins, Pizzi (2003) reported that the mechanical properties are affected by the concurrent moisture content. This applies even more when additives, fillers, and other ingredients are present. In thermosetting resins, various additives are common, that is, strengthener, extenders, stabilizer to improve storage-life properties, or functional fillers such as wood flour. Such additives or fillers may amount to up to 50 % of the total polymer formulation. They strongly influence the polymer properties, including strength, water uptake as well as swelling and shrinking (Kaiser 2007). Ito et al. (2005) found that different dental resins stored in water experienced a decrease in the elastic modulus, which was proportional to the degree of water sorption.
In studying wood adhesives, the vapor sorption phenomenon may not only cause mass gain. Vapor sorption also creates structural changes, such as relaxational effects of polymer side chains, free volume variations, glass transition temperature lowering, and also changes in the viscoelastic behavior (Smith et al. 2004). The glass transition temperature lowering might result in a decrease in thermal stability as well as in a polymer plasticization. Further, degradation of the matrix/fiber or matrix/filler interface may evolve with polymer chain scission through hydrolytic cleavage (Marom 1985).
Polymer films absorb and desorb water at different vapor pressures in ambient air. Here, sorption isotherms are useful tools as they describe the equilibrium of sorption of a material at constant temperature, representing a measure for the amount of vapor being absorbed or desorbed. Water vapor might penetrate into micro- to nanometer-sized free volume spaces between polymer chains (Soles et al. 1998; Soles and Yee 2000), or clustering around functional groups of the polymer, the latter being capable of hydrogen bonding (Takizawa 1967; Herrera-Gómez et al. 2001).
The adhesive type and its affinity to water are both indicated in the shape of sorption isotherms. Following the theory by Brunauer et al. (1938), a sorption isotherm divides into three zones: a first zone corresponding to bound water monolayer, a second one linked to weaker and capillary adsorbed water, and a third zone representing mainly free water. Although the Brunauer et al. (1938) theory is criticized as being oversimplified, the model is still used in the discussion of sorption phenomena (Sing 1998, 2004; van der Wel and Adan 1999). While vapor sorption mechanisms have been extensively investigated for many polymers, dentine adhesives or epoxy-based composites, there is little knowledge on how wood adhesives interact with water vapor. When water gets in contact with gluelines of wooden pieces, absorption and diffusion processes through the adhesive layers may occur (Bowditch 1996). In addition to sorption isotherms that describe the equilibrium of the sorption of a material, the moisture diffusion dynamics through the glueline adhesive becomes a subject of interest. Here, the kinetics of occurring mass exchanges could become a key property for the bonding performance, especially when adhesive layers experience temporary wetting. Mannes et al. (2012) have shown that one-component moisture-curing polyurethanes (1C PURs) adhesives as well as emulsion–polymer–isocyanate (EPI) adhesives may act like barriers for water and water vapor in wooden glued joints.
Because wood adhesives are used with many different applications, a wide variety of adhesive types are commercially available (Vick 1999; Zeppenfeld and Grunwald 2005). A common group of wood adhesives is based on reactions of formaldehyde with phenol, resorcinol, urea, melamine, or mixtures thereof (Frihart 2005). Formaldehyde-reacting adhesives produce rigid polymers having high glass transition temperatures. Phenol–resorcinol–formaldehyde (PRF) and melamine–urea–formaldehyde (MUF) as thermosetting resins are known for their high polymer strength providing excellent stability (Dunky and Niemz 2002; Brunner et al. 2010). The cold setting PRF adhesives are commonly produced in a two-step procedure. In a first step, phenol reacts with an excess of formaldehyde under alkaline conditions resulting in a linearly condensated phenol–formaldehyde (PF) resin polymer that contains reactive methylol groups. In a second step, a surplus of highly reactive resorcinol is added. The result is a thermosetting PRF adhesive of characteristically dark-brownish color with resorcinol “grafted” onto the PF polymers. Finally, the curing of PRF is driven by polycondensation between the resorcinol surplus and the finally added formaldehyde hardener (Pizzi 2003).
The aminoplastic MUF resin requires a suitable acid hardener, which is delivered through direct additions of acids (e.g., formic acid) in aqueous solutions. Formaldehyde reacts with urea or melamine monomers leading to quasi-linear polymer chains. These chains are going to be crosslinked by reactions with the formaldehyde surplus forming the finally cured polymer. MUF-bonded joints are considered suitable for structures when weathering and water exposure is limited, as MUF is subjected to partial hydrolysis. On the contrary, cold setting PRFs are less prone to hydrolyzation, which makes this adhesive the preferred one in load-bearing timber constructions across North America (Dunky and Niemz 2002; Pizzi 2003). Present hygroscopicity of these adhesives is expected to be linked to the number of the prevalent hydroxyl groups (Miyazaki and Nakano 2005). Another common wood adhesive is polyvinyl acetate (PVAc), a waterborne vinyl polymer. It belongs to the group of thermoplastic polymers, and it is able to deform reversibly within a specified temperature range. PVAc is known as white glue or carpenter’s glue and is widely used in the processing of solid wood boards, veneers, furniture manufacturing, or old furniture restoration (Frihart and Hunt 2010). In rooms with warm and humid climates, furniture glued with PVAc may face delamination.
Recently, polyurethanes have increasingly gained acceptance in wood bonding. Polyurethanes are either one- (1C PUR) or two-component systems, and for good wetting, the components need to be of low molecular weight or with a solvent added (Frihart 2005). By using polyurethane as a one-component adhesive, isocyanate-terminated polyurethane–prepolymers are reacting with the adherent water. They are produced in a two-step-process starting with a polyaddition reaction of partly branched polyols with diisocyanate isomer excess, leading to oligomeric isocyanate-terminated urethane prepolymers (Clauss 2011; Kaiser 2007). The second step comprises the final tailoring of the prepolymer, with additives such as catalysts, defoamers, or fillers.
Protein-based glues have been known for centuries, and their wide variety of functional groups makes them well suited for making wood adhesives (Frihart and Hunt 2010). These adhesives are often derived from by-products of other industries, for example, blood and fish skins. Due to the organic nature of the adhesives used for wood gluing, water molecules potentially penetrate the adhesive films and will cause a net-water uptake as vapor pressures rise.
In this paper, data from two sorption-relevant processes are presented: (1) Determination of vapor sorption isotherms of different wood adhesives, including hysteresis analysis. Such sorption isotherms reflect the equilibrium amount of vapor sorbed as a function of relative vapor pressure at constant temperature. (2) Determination of the water vapor uptake dynamics through assessing diffusion coefficients. It is hypothesized that conclusions derived from static water vapor sorption analysis of wood adhesives differ axiomatically from those obtained through related dynamic vapor processes. Comparative results on static sorption isotherms and dynamic vapor sorption processes of wood adhesives have not been communicated so far. The present work is therefore scientifically seen inductive and should encourage continuative studies.
Materials and methods
Adhesive film preparation
In this research, different adhesive films derived from commercially available wood adhesives were tested. Films were made through fill-spreading the liquid adhesive onto a table and smoothing the surface with a film applicator frame. After this casting process, the frame holding the film was carefully moved onto a PVC foil. The ambient climate was individually adjusted to meet the reactivity and the curing characteristics of each adhesive type. After curing, the achieved film thickness was about 0.15 mm for all types. As for the tested film sizes, it was attempted to cut same sizes across all adhesive types. Strips were about 20 mm long and 6 mm wide throughout, equaling in a surface area of 240 mm2 for both film sides. The masses of the adhesive films varied between 13 and 23 mg. Pretesting revealed that the varying masses at constant film sizes had negligible effects on the obtained sorption data.
As commercially available adhesives, a melamine–urea–formaldehyde resin (MUF) with resin:hardener ratio of 100:60 (Kauramin® 683, with Kauramin® hardener 688, BASF) and a phenol–resorcinol–formaldehyde (PRF) resin with a resin:hardener ratio of 100:20 (Aerodux® 185 with hardener HRP 155, Dynea) were used. As a commercial PVAc adhesive, Miracol® 630 (Geistlich Ligamenta AG) was used, following EN 204-D standard. Further, two one-component polyurethanes were tested, that is, HB S 309 and HB S 709, both commercialized by Purbond®. These two PU adhesives differ in curing speed and open assembly time. Open assembly times for HB S309 and HB S709 were 30 and 70 min, respectively. To calculate the curing time of the respective product, the open assembly time is multiplied by the factor 2.5 (e.g., 10-min open assembly time = 25 min cure time; www.purbond.com). Finally, the bio-based fish gelatin adhesive, or fish glue, was used (Product 63550 by Kremer Pigmente GmbH & Co. KG,). This adhesive is a proteinaceous material extracted from the skins of codfish. The provided glue was also casted into a chamber knife, which was then carefully drawn over a PVC foil.
Measurement of water sorption
Sorption isotherms of the cured adhesive films were determined using a DVS Advantage apparatus [DVS, Surface Measurement Systems Ltd (SMS), London, UK]. With this equipment, it was possible to accurately determine the sorption isotherms at constant temperature using a range of preset relative humidity values. The apparatus holds two measurement pans, a sample and reference pan, suspended by arms of a SMS UltraBalance™ microbalance at a sensitivity of 0.1 μg. Sample and reference holders were connected to the microbalance by hanging wires on two arms in a chamber, both being thermostatically controlled. A constant flow of nitrogen gas was mixed into a preset stream of nitrogen containing water vapor at saturation, and this stream passed through the chamber to maintain a set of relative humidity levels. Runs started at zero percent relative humidity and increased in 5 and 10 % steps, respectively, up to a maximum of 98 %, before descending to zero relative humidity. Before moving to a next humidity level, the instrument maintained the adhesive film at constant humidity until the weight change per minute (dm/dt) has achieved 0.0008 % (Fig. 1). Humidity and temperature probes were located in close proximity to the sample and reference holders providing accurate data. The adhesive films were predried in an oven at 40 °C before placing them onto the clean sample pan, which was then put on the hang down wire connected to the microbalance. The sample chamber was then closed and clamped. Predrying of the films was continued at 0 % in the chamber, and as soon as the stability was reached, the actual measurement started. All water sorption experiments were done at a temperature of 25 °C in the chamber.
Through preruns of different adhesive film types, the data reproducibility was assured. Isotherms were calculated based on sample’s equilibrium uptakes at each relative humidity step at constant temperature. From the sorption and desorption data, hysteresis curves were also produced. Hysteresis describes the difference in the amount adsorbed between the sorption and desorption phases of the isotherm. With a built-in video camera, images were taken at different sorption stages to assess the dimensional changes of the tested films.
Calculation of diffusion coefficients
The rate of adsorption and desorption for each adhesive film was obtained following Fick’s law of diffusion (van der Wel and Adan 1999; Dhanpal et al. 2009). Adhesive films had the thickness d (150 μm; Fig. 2) and were exposed on both sides to the same environment (length ~20 mm, width ~6 mm).
At early stages (M t /M ∞ < 0.4), the diffusion process can be approximated with the diffusion equation by Crank and Park (1968). The initial kinetics into the bulk was therefore calculated according to (1):
where M t is the absorbed amount of water vapor at time t, M ∞ is the amount of adsorbed vapor at the thermodynamic equilibrium, and D is the diffusion constant. The diffusion constant D was calculated from the slope of this line.
The data were plotted as M t /M ∞ against t 1/2, and the diffusion coefficients were obtained from the slopes of the initially linear parts of the plots using least-square fitting. Sorption kinetic data were taken from the initial phases when at weight constancy, the humidity was changed to the next level. Sorption kinetics of several humidity steps were analyzed, and since coefficients were more or less consistent, the 20–30 % of steps was plotted.
Results and discussion
Sorption isotherms and hysteresis analysis
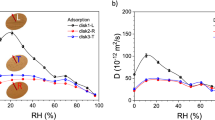
Major differences in equilibrium sorption behavior were seen among the tested wood adhesives (Fig. 3). The two thermosetting resins achieved high weight gains, with PRF accumulated 18 % water, while MUF absorbed 22 %. PVAc also incorporated 10 % water in the film. In contrast, moisture uptake for both one-component polyurethane adhesives remained rather low at 3.5 %. At a relative humidity of maximally 70 %, the vapor sorption of fish glue was similar to PRF. At 70 % r.h. and higher strong water vapor, absorption took place. In total, fish glue gained 45 % in weight at full vapor saturation. At higher humidity levels, the fish glue film lost its inherent rigidity by showing strong warping. Since desorption took place with a heavily warped film, no hysteresis and desorption diffusion coefficients were derived for fish glue.
While adsorption and desorption of PRF and PUR demonstrated linearity, fish glue, MUF, and PVAc had sigmoid shapes with expressed upswings between 70 % and full vapor saturation. Between 80 and 98 % r.h., water uptake doubled for MUF and even tripled for fish glue (Fig. 3). Dimensional changes of film sizes during sorption cycles were determined. While the film width of fish glue increased by 5 %, the other adhesive films remained under 1.5 % of dimensional swelling.
Hysteresis curves representing the differences between absorption and desorption branches are drawn in Fig. 4. Hysteresis was observed for all adhesives and shapes are close to hysteresis loop type H3, according to IUPAC classification (Sangwichien et al. 2002; Dolmaire et al. 2003; Di Vona et al. 2008). Type H3 isotherms should generally indicate weaker adsorbent–adsorbate interactions with reduced uptake at low concentrations and an increase in sorption at higher vapor concentrations (Sing 1985; Rouquerol et al. 1999).
Hysteresis for the PRF adhesive reached a maximum value of 2.6 % at 70 % relative humidity. MUF hysteresis also peaked at 70 % r.h. but was only half of PRF. Polyurethane hysteresis remained under 0.5 %; with the faster curing PUR being moderately higher. Slightly negative hysteresis shift was observed for PVAc. Among the most prominent explanations for the hysteresis phenomenon, the polarity of sites for the water bonding and the capillary condensation are discussed. During desorption, the polymer shrinks and molecules and their water-holding sites are drawn closer together, which reduces the water-holding capacity of the material (Sing 1985). Another discussed theory is capillary condensation. This theory deals with the non-uniformity of pore geometries, which leads to differences in adsorption and desorption. The suggestion is that hysteresis takes place due to a delay in forming a meniscus in the capillaries of the sorbent. When desorption takes place, the water remains filled in larger pores until an equilibrium vapor pressure is reached, which then satisfies a filling of the smaller pore radii (e.g., Bell and Labuza 2000; Thommes et al. 2002).
As for PRF, the condensation of formaldehyde, resorcinol, and phenol takes place in the presence of a basic catalyst that produces a methylol phenol derivative. The involved OH-groups are capable of holding two water molecules through hydrogen bonding. In addition, the OH-groups can also hold an indefinite number of water molecules by electromagnetic attractions between the free electrons of the hydroxyl-oxygen atom and the hydrogen of the highly polarized water molecule (Benz and Neville 1949). The tested formaldehyde-reacting adhesives PRF and MUF both accumulated considerably more moisture in their polymer network than, for example, PU. Especially MUF is known as a “high-energy material,” which is characterized by a strong attraction to water due to hydrophilicity, forming water contact angles <30° on flat surfaces (Wang et al. 2009). In the monolayer region, the MUF adhesive shows a reduced vapor uptake, while uptake rates were higher in the free-water region. The same was observed for fish glue and for PVAc.
The investigated 1C PURs originated from the reaction of a polyether–polyol with a stoichiometric excess of diisocyanate under hot conditions, which has led to a prepolymer. Subsequently, the prepolymer was custom-tailored with specific additives, that is, fillers, catalysts, or defoamers, forming a 1C PUR adhesive. The curing process is driven by the adherent moisture or by moisture coming from the ambient atmosphere. Hydrophilic tertiary amines are utilized as catalysts. The technical properties of 1C PURs heavily depend on the precursors and the process parameters during polymerization. As there is a large variety of precursors, the properties of the customized 1C PUR polymers are highly versatile. Therefore, 1C PURs may behave like duromers, elastomers, or even like thermoplastic polymers (Habenicht 2012). Dolmaire et al. (2003) reported that in polyurethanes, the two linear segmented polyurethanes, based on poly(oxyethylene), have played a major role in the water sorption mechanisms. Thermoplastic polyurethane with longer poly(oxyethylene) segments displayed also higher water diffusion rates.
Finally, gelatin in fish glue is chemically a long-chain protein molecule containing 20 different amino acids. It is of amphoteric nature and can react as a base or an acid. End groups include OH−, carboxy, and amino groups. Reactivity of each group will depend on the pH of the gelatin solution. At higher relative humidity levels, the molecular structure of this adhesive obviously opened up and vapor was able to move (Norland 2006).
Water uptake rates and diffusion coefficients
Water uptake mass ratio M t /M ∞ versus t 1/2 plots of the tested adhesive films are shown in Fig. 5. Calculated absorption and desorption coefficients are listed in Table 1. PRF, fish glue, and MUF were categorized as slow absorbing adhesives with diffusion coefficients <1 × 10−8 cm2 s−1. The two PU adhesives formed the medium speed group, while PVAc was clearly the fast absorbing adhesive type. Desorption coefficients were generally higher than the absorption diffusion coefficients. The M t /M ∞ versus t 1/2 plots was linear in the initial stages of absorption (M t /M ∞ < 0.6) for all tested adhesive types (Fig. 5). This confirms that the dynamic of water uptake is Fickian diffusion (case I) controlled, which occurs when the speed of water diffusion is much slower than the speed of polymer segmental relaxation (Dhanpal et al. 2009). Discrimination between Fickian and non-Fickian diffusion is more relevant at higher relative humidity as kinetic curves are likely to show S shapes with visible inflection points. Non-Fickian kinetics is also present when the kinetic curves are linear but sorption is accompanied by considerable amount of swelling. As dimensional swelling rates were found low, it is assumed that deviations from Fickian law are limited (van der Wel and Adan 1999). Fish glue was the only adhesive showing higher dimensional swelling (~5 %). It can be assumed that water vapor diffusion gets higher as the film swells in dimension (van der Wel and Adan 1999). When water molecules enter the film, they might “clear the way” for successive molecules and this continues until the diffusion fronts have met in the center of the film. To understand sorption dynamics of wood adhesives, a discussion about mechanisms of water vapor entering the polymer network is required. Three mechanisms are of relevance: (1) diffusion processes, (2) capillarity, and (3) transport via microcracks. Of these, three mechanisms diffusion is generally considered to be the most critical one (Marom 1985). In addition, the following molecular and microstructural adhesive facts can be seen as important in relation to water uptake: (1) polarity of the molecular structure, with the presence of chemical groups capable of forming hydrogen bonds with water; (2) degree of molecular crosslinking in the film; (3) presence of residual monomers and/or other water-attracting components; and (4) crystallinity of the polymer with crystallites being inaccessible to water.
Rate of water uptake as a function of square root of time for the tested six commercial wood adhesives fish glue, melamine–urea–formaldehyde (Kauramin, MUF), phenol–resorcinol–formaldehyde (Aerodux, PRF), polyvinyl acetate (Miracol, PVAc), and one-component polyurethane (HB S 307 and HB S 307, PUR), analyzed from the 20–30 % adsorption step
PVAc as an amorphous thermoplast has a fully accessible molecular network, which should have allowed rapid access of vapor, which was according to the authors` data 30 times higher than in PRF. Both tested duroplasts (PRF, MUF) have accessible inner spaces. Fish glue as a slowly absorbing adhesive was highest in weight gain at full vapor saturation. The fish glue film was most obvious in losing its internal mechanical integrity at constant high humidity levels. The strength of the protein-colloid fish glue depends on the interaction of apolar and polar groups between the protein and the wooden material. Fish glue is widely used in art conservation and restoration, and exposure of glued artefacts facing constant high humidity levels is not recommended, as significant reductions in glue strength (shear test) of fish-glued materials have been shown (Buck 1990). As a slowly responding adhesive, fish glue is less prone to possible damage during temporary moistening. Sonderegger et al. (2010) have determined diffusion properties through gluelines for different adhesive types. It was found that one-component PU adhesives are more diffusion resistant, while water-swelling and diffusion of PVAc was much greater due to the high pore ratio. These findings are in accordance with the data determined in this research. Since Sonderegger et al. (2010) measured the adhesives in situ in glued wooden pieces, the overall moisture content was of influence as well.
Conclusion
The effect of water on the adhesive itself is of great importance when considering the durability of adhesive joints. By using DVS analysis, the significance of sorption isotherms versus the dynamic of water uptake using Fick’s law of diffusion was shown. Adhesives types with strong water uptake at higher humidity levels under equilibrium sorption conditions were not identical with those showing most rapid water uptake. Water uptake processes in wood adhesives need to be seen as a function of time. The best examples of the measured adhesives were PRF and fish glue. PRF showed the lowest diffusion coefficients, and at the same time, a total weight gain of 20 % was reached. Also, slow absorbing fish glue showed a water uptake of even 50 %. According to the results found, mechanisms of water diffusion can be hypothesized by two theories: (1) free volume theory, where water diffuses through voids within the polymer, and (2) interaction theory, where water binds to specific ionic groups of the polymer chain (Mortier et al. 2004; Fabre et al. 2007). On the one hand, the free volume theory is seen as being linked to the maximum moisture sorption capacity, while on the other hand, the interaction theory should be more related to the absorption and diffusion dynamics, respectively. Further research is required here to better understand the varying water vapor sorptivity mechanisms in wood adhesives.
References
Bell LN, Labuza TP (2000) Moisture sorption: practical aspects of isotherms measurements and use. AACC International, St. Paul
Benz RW, Neville H (1949) Water content of hydrophilic phenol–formaldehyde resins: vapor pressure–temperature relationships. J Polym Sci 4:673–688
Bowditch MR (1996) The durability of adhesive joints in the presence of water. Int J Adhes Adhes 16:73–79
Brunauer S, Emmet PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319
Brunner M, Lehmann M, Kraft S, Fankhauser U, Richter K, Conzett J (2010) A flexible adhesive layer to strengthen glulam beams. J Adhes Sci Technol 24(8–10):1665–1701
Buck SL (1990) A study of the properties of commercial liquid hide glue and traditional hot hide glue in response to changes in relative humidity and temperature. In: Wooden artifacts group. Specialty sessions, June 2 and 3, 1990. AIC Annual meeting, Richmond, VA
Clauss S (2011) Structure–property relationships of one-component moisture-curing polyurethane adhesives under thermal load. Dissertation at ETH Zürich, No. 20060
Crank J, Park GS (1968) Diffusion in polymers. Academic Press, London
Dhanpal P, Yiu CKY, King NM, Tay FR, Hiraishi N (2009) Effect of temperature on water sorption and solubility of dental adhesive resins. J Dent 37:122–132
Di Vona ML, Sgreccia E, Licoccia S, Khadhraoui M, Denoyel R, Knauth P (2008) Composite proton-conducting hybrid polymers: water sorption isotherms and mechanical properties of blends of sulfonated PEEK and substituted PPSU. Chem Mater 20:4327–4334
Dolmaire N, Espuche E, Mechin F, Pascault JP (2003) Water transport properties of thermoplastic polyurethane films. J Polym Sci B 42:473–492
Dunky M, Niemz P (2002) Holzwerkstoffe und Leime: Technologie und Einflussfaktoren. Springer, Heidelberg
Fabre HS, Fabre S, Cefaly DF, de Oliveira Carrilho MR, Garcia FC, Wang L (2007) Water sorption and solubility of dentin bonding agents light-cured with different light sources. J Dent 35:253–258
Frihart CR (2005) Wood adhesion and adhesives. In: Rowell RM (ed) Handbook of wood chemistry and wood composites. CRC Press, Boca Raton, pp 215–287
Frihart CR, Hunt CG (2010) Adhesives with wood materials: bond formation and performance. Wood handbook: wood as an engineering material, chap 10. Centennial ed. General technical report FPL; GTR-190. US Department of Agriculture, Forest Service, Forest Products Laboratory, Madison, pp 10.1–10.24
Habenicht G (2012) Kleben- erfolgreich und fehlerfrei, 6th edn. Springer Fachmedien, Wiesbaden
Hakala H, Vatanparast R, Vuorimaa E, Lemmetyinen H (2001) Monitoring water uptake of polyurethanes by in situ fluorescence technique. J Appl Polym Sci 82:1593–1599
Herrera-Gómez A, Velázquez-Cruz G, Martín-Polo MO (2001) Analysis of the water bound to a polymer matrix by infrared spectroscopy. J Appl Phys 89:5431–5437
Ito S, Hashimoto M, Wadgaonkar B et al (2005) Effects of resin hydrophilicity on water sorption and changes in modulus of elasticity. Biomaterials 26:6449–6459
Kaiser W (2007) Kunststoffchemie für Ingenieure. Von der Synthese bis zur Anwendung. Carl Hanser, München
Konnerth J, Stockel F, Muller U, Gindl W (2010) Elastic properties of adhesive polymers. III. Adhesive polymer films under dry and wet conditions characterized by means of nanoindentation. J Appl Polym Sci 118:1331–1334
Mannes D, Schmidt J-A, Volkmer T, Niemz P (2012) Untersuchungen zum Einfluss der Klebstoffart auf den kapillaren Wassertransport in Holz parallel zur Faserrichtung. Bauphysik 34:61–65
Marom G (1985) The role of water transport in composite materials, chap 9. In: Comyn J (ed) Polymer permeability. Elsevier Applied Science, Great Britain, pp 341–374
Miyazaki J, Nakano T (2005) Hygroscopicity of resorcinol–formaldehyde resin and aqueous vinyl polymer-isocyanate resin in high humidity. Holzforschung 59:342–346
Mortier E, Gerdolle DA, Jacquot B, Panighi MM (2004) Importance of water sorption and solubility studies for couple bonding agent—resin-based filling materials. Oper Dent 29:669–676
Norland RE (2006) Fish gelatin and fish flue. In: Tracton AA (ed) Coatings technology handbook, 3rd edn. CRC Press, Boca Raton, pp 65-1–65-4
Pizzi A (2003) Phenolic resin adhesives. In: Pizzi A, Mittal KL (eds) Handbook of adhesive technology, chap 29, 2nd edn. Marcel Decker, New York
Rouquerol F, Rouquerol J, Sing K (1999) Adsorption by powders and porous solids: principles, methodology and applications. Academic Press, London
Sangwichien C, Aranovich GL, Donohue MD (2002) Density functional theory predictions of adsorption isotherms with hysteresis loops. Colloids Surf A 206:313–320
Sing KSW (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl Chem 54:2201–2218
Sing KSW (1998) Adsorption methods for the characterization of porous materials. Adv Colloid Interface Sci 76–77:3–11
Sing KSW (2004) Characterization of porous materials: past, present and future. Colloid Surf A 241:3–7
Smith AL, Mulligan RB Sr, Shirazi HM (2004) Determining the effects of vapor sorption in polymers with the quartz crystal microbalance/heat conduction calorimeter. J Polym Sci Part B 42:3893–3906
Soles CL, Yee AF (2000) A discussion of the molecular mechanisms of moisture transport in epoxy resins. J Polym Sci B 38:792–802
Soles CL, Chang FT, Bolan BA, Hristov HA, Gidley DW, Yee AF (1998) Contribution of the nanovoid structure to the moisture absorption properties of epoxy resins. J Polym Sci B 36:3035–3048
Sonderegger W, Hering S, Mannes D et al (2010) Quantitative determination of bound water diffusion in multilayer boards by means of neutron imaging. Eur J Wood Prod 68:341–350
Takizawa A (1967) Analysis of the high-pressure region of certain sorption isotherms. J Phys Chem 71:1611–1616
Thommes M, Köhn R, Fröba M (2002) Sorption and pore condensation behavior of pure fluids in mesoporous MCM-48 silica, MCM-41 silica, SBA-15 silica and controlled-pore glass at temperatures above and below the bulk triple point. Appl Surf Sci 196:239–249
van der Wel GK, Adan OCG (1999) Moisture in organic coatings—a review. Prog Org Coat 37:1–14
Vick CB (1999) Adhesive bonding of wood materials. In: Wood handbook: wood as an engineering material. General technical report FPL; GTR-113. USDA Forest Service, Forest Products Laboratory, Madison, pp 9.1–9.24
Wang H, Yuan Y, Rong M, Zhang M (2009) Microencapsulation of styrene with melamine–formaldehyde resin. Colloid Polym Sci 287:1089–1097
Zeppenfeld G, Grunwald D (2005) Klebstoffe in der Holz- und Möbelindustrie, 2nd edn. DRW-Verlag Weinbrenner GmbH & Co. KG, Leinfelden-Echterdingen
Acknowledgments
The analytical assistance by Monika Funk from the University Göttingen is greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wimmer, R., Kläusler, O. & Niemz, P. Water sorption mechanisms of commercial wood adhesive films. Wood Sci Technol 47, 763–775 (2013). https://doi.org/10.1007/s00226-013-0538-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-013-0538-7