Abstract
In order to understand the reason why glycerin pre-treatment can accelerate the deformation fixation of compressed wood, the interaction between glycerin and wood at various temperatures was investigated in this study from stress relaxation approach. The compression stress relaxation curves of poplar (Populus cathayana Rehd.) samples impregnated with glycerin were measured at temperatures ranging from 25 to 180°C, together with the curves of oven-dry wood at temperatures between 100 and 180°C for comparison. The activation energy was calculated according to the Eyring’s absolute rate reaction theory. The results showed that temperature had very obvious effect on stress relaxation for both glycerin-treated wood (GTW) and oven-dry wood. The stress released very fast at higher temperatures. Glycerin showed an accelerating effect on stress relaxation. At temperatures exceeding 120°C, a complete relaxation of the stress could be expected. While for untreated wood, it cannot be reached until 160°C. By calculating the apparent activation energy (ΔE) of GTW at different temperatures, it is clear that two mechanisms are responsible for different temperature ranges. From 40 to 100°C, ΔE is only 8.24 kJ/mol, which corresponds to the hydrogen bonds formed between wood and glycerin molecules; from 120 to 180°C, ΔE reached 81.38 kJ/mol, which corresponds to the degradation of hemicelluloses or lignin, and during this process, new cross-linking would happen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wood is a porous material. Its density is tightly related to the porosity since all woods have a similar specific gravity around 1.5 for dry solid cell wall substance. In order to improve its mechanical properties, many researchers have proposed to use compression to increase the density of wood, especially fast-growing woods with wide-grown rings. Under hygro-thermal conditions, wood can easily be compressed along transverse direction, but the problem is the recovery of the compression deformation when the external force is removed. In previous studies, many methods including heating (Stamm 1964; Higashihara et al. 2001), steaming (Ito et al. 1998; Fukuta et al. 2007; Inoue et al. 2008), resin impregnation (Inoue et al. 1991a, b) and cross-linking (Inoue et al. 1994, 2000; Higashihara et al. 2001) have been applied to fix the deformation of compressed wood. Organic liquids such as glycerin and ethylene glycol were used as swelling agents in cross-linking method to accelerate the fixation of compression deformation. High dimensional stability was obtained when wood was pretreated with aqueous glycerin solution of 40% or higher concentrations prior to compression (Inoue et al. 2000).
The effect of different methods on deformation fixation can be studied by stress relaxation approach. Wood is also a viscoelastic material. It exhibits time-dependent mechanical behavior such as stress relaxation during compression process. Stress relaxation refers to the fact that at a fixed strain, the stress resistance to the deformation decreases with increasing time. If all the stress release at a certain condition, it means that the deformation is completely fixed. Iida et al. (1984) and Iida and Norimoto (1987) studied the mechanisms of the formation and recovery of drying set and proposed that the recovery of compression set was mainly due to the elastic recovery stress. In order to clarify the mechanisms for good fixation of compressive deformation of wood at high temperatures, Dwianto et al. (1998) measured the stress relaxation of oven-dried Albizia (Paraserienthes falcata Becker) wood specimens at temperatures ranging from 20 to 200°C for 24 h and a strain level of 50% in the radial direction. They also measured the stress relaxation and stress–strain relationships in the radial compression of Sugi (Cryptomeria japonica D. Don) wood under steaming at temperatures up to 200°C and proposed that the permanent fixation of deformation resulted from the release of stresses stored in the cell wall polymers by their degradation and the increase in regularity of the crystalline lattice space of microfibrils or the formation of cross-linkings between the cell wall polymers (Dwianto et al. 1999). Higashihara et al. (2001) measured the stress relaxation in radial compression of Sugi wood saturated with glycerin and compared the residual stress and deformation recovery of glycerin-treated wood and steaming post-treated wood in boiling water and then suggested that glycerin treatment at high temperatures might cause structural changes in wood cell walls similar to those by steaming. But the role of glycerin in deformation fixation process and the interaction between glycerin and wood during compressive process were not mentioned. Therefore, in this study, the stress relaxation approach was used to understand the mechanism of compressed deformation fixation of GTW. The kinetics of stress relaxation was also used to study the interaction between glycerin and wood.
The objective of this study is to analyze the effect of glycerin treatment on the compression stress relaxation behavior of wood at different temperatures, and the stress relaxation of oven-dry wood was also tested for comparison. The result of this study can provide a better understanding of the reaction or interaction between glycerin and wood during hot pressing or heat treatment.
Materials and methods
Materials
The samples used in this study were made from poplar (Populus cathayana Rehd.) from Kashi, Xinjiang Province of China. The average growth ring width was 7.18 mm. Samples were 10 mm cubes cut from the air-dried sapwood. All the samples were free of knots, cracks, decay and other visible defects. They were dried at 60°C for further testing. The glycerin used in this study had a concentration of 99.0% A.P.
The samples were impregnated with glycerin by 30 min vacuum at −0.09 Mpa, followed by an immersion at room temperature for another 48 h.
Testing apparatus of stress relaxation
Testing apparatus for stress relaxation experiments were self-assembled in the wood science lab of Beijing Forestry University, China. The schematic diagram of this apparatus is shown in Fig. 1. It essentially consists of electric system (A), mechanical system (B, E, G), heating system (I), cooling system (C, D) and control system (B, J). A set of softwares was designed to control the mechanical system and to record the stress relaxation curves. The sample was compressed by two compression plates (E, G). The temperature of the sample capsule (H) was controlled by the heating system (I), and the range of temperature was 25–180°C. When the sample was compressed to a small fixed deformation, the force could be measured every 0.1 min by the sensor (B) and transmitted to the computer (J).
Determination of stress relaxation
The GTW was compressed at nine temperatures (25, 40, 60, 80, 100, 120, 140, 160 and 180°C) for 1 mm in radial direction, 10% of its original thickness. For oven-dry wood, five temperatures (100, 120, 140, 160 and 180°C) were used. Prior to the determination, the temperature of sample capsule was elevated to testing temperature. Then, the sample was fixed between upper and down compression plates. The temperature in the sample capsule would fall when the door of capsule opens. So determination began while the temperature reached the testing temperature again and finished when the stress relaxation curve became calm. Three replicates were used for each condition.
Results and discussion
Effect of temperature on stress relaxation of oven-dry wood
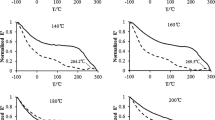
For all conditions, the stress relaxation curves of three replicates are very close suggesting good reproducibility. The intermediate curve of the three was taken as the final result. The stress relaxation curves of oven-dry wood at different temperatures are shown in Fig. 2. It is clear from the figure that temperature plays a significant role in the stress relaxation of oven-dry wood. With the increase of temperature, the residual stress and relaxation time decreased. The compressive stresses were released completely at 1300 and 90 min at 160 and 180°C, respectively. The stress relaxation curves of oven-dry wood showed two relaxation stages at 100–160°C. The stresses reduced sharply in a short period around 260 min of the early stage, and then the stress relaxation curves became almost straight lines declining slowly. According to Kuriyama (1967), hemicelluloses, cellulose and lignin in wood decompose dramatically at 180–300°C, 40–400°C and 280–550°C, respectively. So the main chemical components of oven-dry wood could not decompose at 120–160°C. At the first relaxation stage, the stress relaxation was mainly due to the slide between wood molecules and the break-down of the intermolecular hydrogen bonds. At the second relaxation stage, it was mainly due to the forming of new hydrogen bonds between wood molecules. When the temperature reached 180°C, the stress in wood released rapidly and disappeared in about 90 min. It can be assumed that the molecular chains of main wood components especially hemicelluloses were cut off and then new chemical bonds were formed between wood molecules. In this way, cross-linking formed between wood molecules, which is considered to be the main reason for the rapid stress relaxation at 180°C.
Effect of temperature on stress relaxation of GTW
The stress relaxation curves of GTW at various temperatures are given in Fig. 3a. Similar to oven-dry wood, temperature also played a significant role in the stress relaxation of GTW. The residual stress decreased with increased temperature. A complete stress relaxation was observed when the temperature reached 120°C. The residual stress disappeared even in 20 min at 180°C. Therefore, it is obvious that the stress relaxation of wood was accelerated by glycerin treatment.
In order to show more clearly the relaxation rates of GTW at different temperatures, Fig. 3a was transformed to Fig. 3b by using double logarithmic plots of f (t) versus time. The slope of the double logarithmic plots represents the relaxation rate of stress. The curves at 25–100°C can be regarded as linear suggesting that the relaxation rates are steady within this temperature range. For temperatures within the range of 120–180°C, the double logarithmic plots are almost linear at the beginning, and then fall dramatically with time until they disappeared. Therefore, the temperature of 120°C seems the critical temperature for stress relaxation of GTW, which must correspond to a change of mechanism.
Kinetics of stress relaxation of GTW
Wood is a polymer that has various relaxation processes. The relationship of relaxation time and temperature followed the Eyring’s absolute rate reaction theory (Glasstone et al. 1941) as expressed in Eq. 1.
where t0 is average relaxation time (s), A is a constant, R is gas constant, T is the absolute temperature (K), and ΔE is apparent activation energy (KJ/mol). Eq. 1 can be transformed to Eq. 2.
where ln A is a constant, so dln A equals zero. Then, Eq. 2 can be written as
The average relaxation time was defined as the point on the stress relaxation curve that starts contact with the tangent parallel to the abscissa axis in this study. At each temperature, there is a corresponding value of ln t0. When ln t0 was plotted against 1/T, the apparent activation energy ΔE can be calculated from the slope of the curves according to Eq. 3.
The plots of relaxation time to the reciprocal of absolute temperature for GTW are given in Fig. 4. Clearly, the data from 25 to 180°C conformed to two linear relationships with correlation coefficient R 2 of 0.9773 and 0.9878, respectively, one is within the temperature range of 25–100°C (Phase I) and the other one within 120–180°C (Phase II). According to Eq. 3, the apparent activation energy calculated for Phase I (ΔE1) and Phase II (ΔE2) is 8.18 and 81.38 kJ/mol, respectively. ΔE1 is low, which supports the explanation given above that within a low temperature range below 100°C, the stress relaxation of GTW is mainly caused by the sliding of wood molecules, the break-down of intermolecular hydrogen bonds and the forming of new hydrogen bonds between wood molecules or between wood and glycerin molecules. The value of ΔE2 for higher temperature range is much higher compared to ΔE1. Toshimi (1995) proposed that the apparent activation energy for the thermal decomposition of hemicelluloses and/or lignin is generally 80–125 kJ/mol. The value of ΔE2 is right within this range. Therefore, it can be assumed that some molecular chains of hemicelluloses and/or lignin were cut off above 120°C in GTW and formed new cross-linking. It will be further studied in FT-IR analysis to understand whether the chemical reaction occurred between glycerin and the products formed after the molecular chains of hemicelluloses and/or lignin broken.
Conclusion
Temperature played a significant role in the stress relaxation of wood. The stress relaxation was increased and the residual stress was reduced with rising temperature. The stress relaxation was aggravated by glycerin treatment. The compressive stress of oven-dry wood released completely at 180°C and that of GTW even released completely at 120°C. The molecular chains of hemicelluloses and lignin in GTW began to break and formed new cross-linking because of the strong hydrogen bonds between wood and glycerin at above 120°C. That is the main reason for stress relaxation of GTW. But the mechanism of the intermolecular hydrogen bonding between wood and glycerin also needs further study.
References
Dwianto W, Morooka T, Norimoto M (1998) The compressive stress relaxation of Albizia (Paraserienthes falcata Becker) wood during heat treatment. Mokuzai Gakkaishi 44(6):403–409
Dwianto W, Morooka T, Norimoto M, Kitajima T (1999) Stress relaxation of Sugi (Cryptomeria japonica D.Don) wood in radial compression under high temperature steam. Holzforschung 53(5):541–546
Fukuta S, Takasu Y, Sasaki Y, Hirashima Y (2007) Compressive deformation process of Japanese cedar (Cryptomeria japonica). Wood Fiber Sci 39(4):548–555
Glasstone S, Laidler KJ, Eyring H (1941) The theory of rate processes. McGraw Hill, New York, pp 544–551
Higashihara T, Morooka T, Norimoto M (2001) Permanent fixation of transversely compressed wood by heating and its mechanism. Mokuzai Gakkaishi 47(3):205–211
Iida I, Norimoto M (1987) Recovery of compression set. Mokuzai Gakkaishi 33(12):929–933
Iida I, Norimoto M, Imamura Y (1984) Hygrothermal recovery of compression set. Mokuzai Gakkaishi 30(5):354–358
Inoue M, Norimoto M, Otsuka Y, Yamada T (1991a) Surface compression of coniferous wood lumber II: Permanent set of compression wood by low molecular weight phenolic resin and some physical properties of the products. Mokuzai Gakkaishi 37(3):223–227
Inoue M, Norimoto M, Otsuka Y, Yamada T (1991b) Surface compression of coniferous wood lumber III: Permanent set of the surface compressed layer by a water solution of low molecular weight phenolic resin. Mokuzai Gakkaishi 37(3):234–240
Inoue M, Minato K, Norimoto M (1994) Permanent fixation of compressive deformation of wood by crosslinking. Mokuzai Gakkaishi 40(9):931–936
Inoue M, Hamaguchi T, Morooka T, Higashihara T (2000) Fixation of compressive deformation of wood by wet heating under atmospheric pressure. Mokuzai Gakkaishi 46(4):298–304
Inoue M, Sekino N, Morooka T, Rowell RM, Norimoto M (2008) Fixation of compressive deformation in wood by pre-steaming. J Trop For Sci 20(4):273–281
Ito Y, Tanahashi M, Shigematsu M, Shinoda Y (1998) Compressive-molding of wood by high-pressure steam-treatment: Part 2. Mechanism of permanent fixation. Holzforschung 52(2):217–221
Kuriyama A (1967) On the changes in the chemical composition of wood within the temperature range up to 200°C. Materials 16(169):772–776
Stamm AJ (1964) Wood and cellulose science. Ronald Press, New York
Toshimi H (1995) Kinetics of pyrolysis of wood and cellulose. Mokuzai Gakkaishi 41(10):879–886
Acknowledgments
This study was supported by the National Natural Science Foundation of China (NSFC) (No. 30500386).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yan, L., Cao, J., Gao, W. et al. Interaction between glycerin and wood at various temperatures from stress relaxation approach. Wood Sci Technol 45, 215–222 (2011). https://doi.org/10.1007/s00226-010-0322-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-010-0322-x