Abstract
Dissolving pulps are the raw materials for the production of many different end-products. Jute is a very good source of cellulose. In this investigation, jute fiber was subjected to pulping in soda process in order to produce dissolving pulp under different prehydrolysis conditions and compared with prehydrolysed kraft pulp from jute. An increase of the prehydrolysis temperature or H2SO4 in prehydrolysis liquor increased the α-cellulose content and decreased the viscosity of pulp. The effect of ethylenediamine in soda liquor was also investigated when producing dissolving pulp. Jute fiber produced pulp having 90–97% α-cellulose. Ethylenediamine in soda liquor produced pulp of higher yield, viscosity and higher α-cellulose content than that of prehydrolysis soda or kraft pulp. The α-cellulose content and viscosity were increased with the increase of amine in soda liquor. The kappa number of dissolving pulp from jute was very low (9–5), which indicated that less bleaching chemicals are required for bleaching. The bleachability of soda-ethylenediamine pulp was lower than prehydrolysed soda and kraft pulp in ECF bleaching sequences. The bleachability of soda-ethylenediamine pulp was improved at the sacrifice of pulp yield when prehydrolysis was done prior to pulping. The alkali solubility S 10 and S 18 were 4–9 and 2–4%, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Jute used to play an important role in the socio-economic development of Bangladesh. A significant portion of the total export earnings was dependent on jute and related products in those days (Jahan et al. 2007). The chemical and morphological characteristics of jute favor it as pulping raw material (Nahar 1987). Therefore, many studies have been done on paper grade pulp from jute at home and abroad (Akhtaruzzamen and Shafi 1995; Jahan 2001; Roy et al. 1998). Retted jute fiber contains a very high α-cellulose and low hemicelluloses as compared to wood or other nonwood (Nahar 1987). So, it may be used in producing dissolving pulp.
Most chemical cellulose or dissolving pulp comes from wood using the prehydrolysis kraft or acid sulfite processes (Biermann 1993; Hinck et al. 1985). Dissolving wood pulp is a chemically refined bleached pulp composed of more than 90% pure cellulose. The end uses of dissolving pulp include cellophane and rayon, cellulose esters (acetates, nitrates, etc.), cellulose ethers (carboxymethyl cellulose, etc.), graft and cross-linked cellulose derivatives (Sjöström 1981). When producing dissolving pulp for making products such as carboxymethyl cellulose, viscose, cellulose film and sausage skin, determining the pulp quality is essential. The dissolving pulp quality depends on both properties of the raw wood material and the pulp processing. The reactivity of cellulose pulp can refer to its capacity to participate in diverse chemical reactions. The two secondary hydroxyl groups on carbons two and three are more reactive than the primary hydroxyl group on carbon six (Krässig 1993). For derivatization reactions, it is important to note that reactions with the hydroxyl groups on carbons two and three are kinetically favorable, while substitution on carbon six is thermodynamically more stable (Schlotter 1988; Krässig 1993). Both celluloses I and II have been found in pulp. Cellulose II is more thermodynamically stable than cellulose I. This may make the dissolving pulps with large proportions of cellulose II more resistant to heating than pulps with large proportions of cellulose I (Lennholm and Iversen 1995a, b). Dissolving pulp should have special properties, such as a high level of purity, uniform molecular-weight distribution and the reactivity and accessibility of the cellulose to chemicals (Krässig 1993). The structure and morphology of the fibers determine these properties. To achieve maximum reactivity of pulp, acid hydrolysis, mechanical and swelling treatments, enzyme treatment, etc. is done (Engström et al. 2006; Tang et al. 2002). For example, endoglucanase preferably degrades amorphous rather than crystalline cellulose and cleaves the cellulose randomly within the chain (Rabinovich et al. 2002; Henriksson et al. 1999). Since less ordered or amorphous regions occur on the surface and between the microfibrils (Wickholm 2001; Vietor et al. 2002) endoglucanse treatment leads to a swelling of the cell wall and thus an increase in accessibility to solvents and reagents. Even though dissolving pulp is a highly purified pulp, it possesses some disadvantages, for example, a broad molecular weight distribution and a low viscosity at a given purity level (Sixta et al. 2004). Many attempts have been made to find a correlation between the hydroxyl group reactivity and the microstructure of the cellulose. Both X-ray diffraction and iodine sorption measurements are commonly used to determine the crystallinity and accessibility, respectively. The iodine sorption method measures the amorphous part or the accessible hydroxyl groups, whereas X-ray diffraction measures the crystalline fraction (Hessler and Power 1954; Racz et al 1996). It is not clear, how well these methods correlate with the reactivity in industrial production.
In recent years, various innovative pulping methods have been developed primarily in response to environmental considerations (Sixta et al. 2004; Vila et al. 2004; Kirci and Akgul 2002). New acidic pulping processes, such as acetosolv, formacell, milox, promise to have superior potential regarding purification selectivity, e.g., expressed by the viscosity–pentosan relationship and specific investment costs (Pulps et al. 1999; Sixta et al. 2004). It has been reported in our earlier studies (Jahan and Farouqui 2000, 2001) that ethylenediamine (EDA) in soda liquor increased delignification and hemicelluloses dissolution of jute. MacLeod et al. (1984) also observed that the soda-EDA process dissolved the greatest amount of xylan from spruce. Therefore, EDA in soda liquor can produce high α-cellulose containing dissolving pulp from jute.
In this article, an effort was exerted to produce dissolving pulp from jute by prehydrolysed soda and kraft processes. The effect of ethylenediamine (EDA) in soda liquor on producing dissolving pulp was also assessed. The effect of acid and temperature on the prehydrolysis soda pulp was studied.
Experimental
Raw materials
Retted jute fiber was collected from the BJRI, Dhaka. It was sun-dried and cut to 2–3 cm in length. The moisture content of the raw materials was determined according to TAPPI Standard Methods (1953) (T 18 m-53). After determination of the moisture content of air-dried raw materials equivalent to 250 gm oven-dried (o.d.) was weighed separately in a polyethylene bag for subsequent cooking experiments.
Prehydrolysis
The prehydrolysis was carried out in an electrically heated stainless steel digester of 5 l capacity, rotating at 1 rpm. Water prehydrolysis was carried out either with or without the addition of sulfuric acid at 150 and 170°C. The jute to liquor ratio was 1:5. The time required to raise maximum temperature was 60 min. The liquor was drained after prehydrolysis. The prehydrolysate contained high amounts of sugars. It may be a valuable source of ethanol or other valuable chemicals (Rath et al. 2005). This will be studied in our next projects.
Cooking
All pulping experiments were performed in the same digester. The following parameters were kept constant in the soda process:
-
active alkali charge was 18% on o.d jute
-
liquor to fiber ratio was 5:1
-
temperature was 170°C
-
cooking time was 60 min
Two sets of experiments were carried out in soda-EDA process, one with prehydrolysis at 150°C and 0.25% H2SO4 and the other one without prehydrolysis. The prehydrolysed jute was subjected to the soda-EDA pulping. The proportions of EDA in soda liquor were 10, 20, 30 and 40% (v/w), respectively. All other parameters were kept constant.
During prehydrolysis kraft cooking, the active alkali charge was adjusted to 18% Na2O on oven dry wood and 25% sulfidity at 170°C for 60 min. Material to liquor ratio was 1:5.
Bleaching
Pulps were bleached in DoED1 bleaching sequences. The bleaching was done in polyethylene bags. The kappa factor 0.22 was used in D0 stage. The pH of bleach liquor was 2–2.5 in D0. The bleaching continued for 60 min at 70°C. Alkaline extraction was carried out with 2% NaOH at 70°C for 60 min. The consistency was 5 and 10 in chlorine dioxide and extraction stage, respectively. In the final stage (D1), half of the ClO2 applied in the D0 stage was used. The temperature was 70°C for 120 min.
Evaluation of pulps
Pulp tests were performed according to the Standard Methods of the Technical Association of the Pulp and Paper Industry (TAPPI, Atlanta, GA): kappa number (T 236 cm-85); brightness (T 452 om-92); viscosity (T 230 om-89); carbohydrate (T249 cm00); α-cellulose (T 203 om-88); and alkali solubility S 10 and S 18 (T 235 cm-85). Alpha-cellulose is the pulp fraction resistant to a treatment in an aqueous solution containing 17.5% sodium hydroxide and indicates undegraded, high molecular weight cellulose content in pulp. Alkali solubilities S 10 and S 18 provide information on the low molecular weight carbohydrates (degraded cellulose and hemicellulose) in pulp. A 10% sodium hydroxide solution dissolves both degraded cellulose and hemicelluloses (S 10) whereas hemicelluloses are soluble in an 18% sodium hydroxide solution (S 18). All pulp properties were analyzed in duplicate.
Results and discussion
Pulping
Soda process
To determine the optimum prehydrolysis temperature and H2SO4 concentration for getting high purity dissolving pulp, various experiments were carried out as shown in Table 1. A simple prehydrolysis (without H2SO4 and 60 min at 150°C) with water is insufficient for the thorough removal of hemicelluloses from jute in a simple soda process. Under identical cooking conditions, the cellulose content was raised when more severe prehydrolysis was applied (Table 1). Addition of H2SO4 in prehydrolysis regardless of temperature increased the α-cellulose content in pulp. At a given temperature (150 or 170°C), the addition of 1% sulphuric acid increased the alpha-cellulose content by 4.1 and 2.3% (from 91.3 to 95.4 and from 93.9 to 96.2) at the expense of a severe pulp yield drop.
However, the jute was satisfactorily delignified under any prehydrolysis conditions. Pulp obtained after prehydrolysis at 150°C and without H2SO4 followed by pulping achieved a kappa number of 9.0. The kappa number was reduced to 6.1 when 0.25% H2SO4 was added in prehydrolysis under similar conditions. The delignification degree of pulping was increased with increasing H2SO4 concentration in prehydrolysis (Table 1).
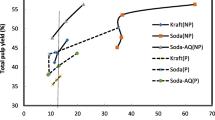
The pulp yield dropped sharply from 52.9 to 45.3% when H2SO4 concentration was increased from 0 to 1.0% at 150°C during prehydrolysis. The pulp yield severely dropped (31.7–37.8%) when the prehydrolysis temperature was 170°C. The delignification selectivity was lower at 170°C (Fig. 1). The pulp yield was 11% lower at 170°C than at 150°C at kappa number 5. The dissolving pulp yield from jute by acidic prehydrolysis soda process was better than the corresponding pulp yield from bamboo or jute stem at the same level of purity and viscosity (Bhowmic 1999). The main target parameter in dissolving pulp production besides purity is viscosity as a measure of the degree of polymerization (D.P.) of the cellulose. In dissolving pulp production, the viscosity must always be considered in relation to purity, i.e. cellulose content of the pulp. In this case, a cellulose content of 91.3% was obtained under mild prehydrolysis conditions (without H2SO4 and 60 min at 150°C), which is the lower limit of the satisfactory range. Figure 2 shows the viscosity versus the cellulose content. In each case, the prehydrolysis conditions were variable and pulping was carried out under identical conditions (Alkali 18% as NaOH, cooking temperature 170°C, cooking time 60 min and liquor ratio 1:5). High cellulose content can be achieved at the expense of a severe degradation of molecular weight at a higher temperature of prehydrolysis. The effect of prehydrolysis on the purification efficiency or on the removal efficiency of short-chain carbohydrates, etc. is determined by the intensity of prehydrolysis, which may be calculated by using the P-factor concept, which is expressed by the prehydrolysis time and temperature as a single variable and is defined as
where k rel is the relative rate of acid-catalyzed hydrolysis of glycosidic bonds. The activation energy was increased when the prehydrolysis temperature increased. The α-cellulose content was increased with the increase of H2SO4 concentration in prehydrolysis (Fig. 2). The addition of strong mineral acid contributes to the prehydrolysis intensity, meaning that at a given P-factor, the addition of e.g., H2SO4 enhances the prehydrolysis efficiency (Masura 1987). Consequently, to obtain a high degree of polymerization (D.P.) of cellulose a lower degree of purity of cellulose has to be accepted. Pulp of this kind is only suitable for dissolving grade pulp, where the viscosity requirements are in the low to medium range (e.g., for rayon stable, rayon filament, CMC technical use, cellophane, etc.) (Sixta et al. 1994). Compared to a reference pulp produced by prehdrolysis kraft process, prehydrolysis soda pulp showed poorer properties (Fig. 2). The pulp yield and kappa number were also better (data not shown).
Soda-EDA process
The jute was prehydrolysed with 0.25% H2SO4 at 150°C for 60 min and cooked with varied proportion of ethylenediamine (EDA) in 18% soda liquor. The pulp yield and kappa number were decreased with increasing EDA in soda liquor (Table 2). Julien and Sun (1979) also observed better delignification when EDA was added in soda liquor. The presence of amine in soda liquor altered the molecular weights of the dissolved lignin fraction and delignification is associated with negative reduction potential of pulping additive (Kubes and Bolker 1978). The viscosity of pulp was improved when EDA was used. The α-cellulose content was also increased with the addition of EDA. At 40%, EDA in soda liquor produced pulp of 95.3% α-cellulose content with viscosity of 11.7 mPs.s. Helmy and State (1991) observed increasing pulp yield, α-cellulose content and the D.P. and decreasing pentosan of bagasse pulp with the increase of EDA in soda liquor. Slight increase in viscosity and the substantial increase in alpha-cellulose could also be attributed to the selective removal of hemicelluloses, e.g., xylan that is rather more likely than a “stabilization” of the alpha-cellulose as indicated by Kubes and Bolker (1978) and Kubes et al. (1978). The slightly decreasing trend in pulp yield with increasing EDA addition may be explained by the dissolution of hemicelluloses (Jahan and Farouqui 2000). Table 2 also shows the effect of EDA in soda liquor on jute pulping without prehydrolysis. The pulp yield was 63–65% that was about 15% higher than prehydrolysed soda-EDA process. The high yield combined with high alpha-cellulose content has to be attributed to high cellulose yield, high viscosity and possibly the preservation of a high amount of high-molecular weight xylan. The α-cellulose content in pulp was increased with increasing EDA in soda liquor. Pulp yield was decreased slightly with increasing EDA. This may be attributed to the dissolution of hemicelluloses. The viscosity of soda-EDA pulp without prehydrolysis was 15–16 mPa s, which was 3–6 mPa s higher than prehydrolysed soda-EDA pulp.
Bleaching
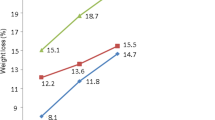
Pulps obtained under different conditions in soda and soda-EDA processes were subjected to DED bleaching. All pulps were bleached under identical conditions. Increasing H2SO4 concentration in prehydrolysis liquor or increasing prehydrolysis temperature improved pulp bleachability (Table 3). Pulp obtained under simple water prehydrolysis (without H2SO4, 150°C) followed by pulping had a brightness of 78.3%, but increased to 82.2% when 0.25% H2SO4 was added during prehydrolysis. The solubility in 10% alkali (S 10) was increased with increasing H2SO4 in prehydrolysis liquor or prehydrolysis temperature. The higher S 10 value indicated that the H2SO4 attacked both cellulose and hemicelluloses of jute. Therefore, the hemicelluloses content in pulp was decreased (S 18) with increasing H2SO4. The viscosity of bleached pulp was decreased from 7.1 to 3.8 mPa s at 150°C and 6.1 to 3.4 mPa s at 170°C with increasing H2SO4 from 0 to 1.0% in prehydrolysis liquor.
Table 4 shows the bleachability and dissolving pulp properties of soda-EDA pulp. α-cellulose and viscosity were increased and alkali solubility (S 10 and S 18) decreased with increasing EDA in soda liquor. Soda-EDA pulp showed poor bleachability (Table 4). There was no change of brightness within the applied EDA in soda liquor. Prehydrolysed soda-EDA pulp showed a slightly better brightness than the one without prehydrolysed soda-EDA pulp. Lower bleachability of soda-EDA pulp is under investigation in our laboratory.
Figure 3 shows the comparison of soda (obtained from prehydrolysed at 150°C and 0.25% H2SO4 concentration) and soda-EDA (obtained at 20% EDA with and without prehydrolysis) process for producing dissolving pulp from jute. Soda-EDA pulp without prehydrolysis showed the highest and soda the lowest viscosity (14.5 and 5.3 mPa.s) among these three pulps. Prehydrolysed soda-EDA pulp had the highest and soda the lowest α-cellulose, which indicates the better solubility of hemicelluloses and stabilization of α-cellulose in soda-EDA process. S 10–S 18 values indicate the content of degraded cellulose with a degree of polymerization between approximately 50 and 150 (Hinck et al. 1985). Soda-EDA pulp showed better S 10–S 18 values. These results are consistent with the data of α-cellulose and viscosity. The main problem of soda-EDA process was bleachability. Unbleached brightness of soda-EDA pulp was lower, which indicates its lower bleachability. Using the same kappa factor, soda pulp gave 82.2% brightness that was 8.8% unit higher than soda-EDA pulp (with an EDA concentration of 20%).
The amount of residual hemicelluloses (Table 5) reflects the degree of purity, which is crucial for the production of rayon fibers and products made from cellulose acetate. In the case of hardwood, the major hemicelluloses component is 4-O-methyl glucuronoxylan, which can be easily identified by xylose. Prehydrolysed-soda-EDA pulp showed the highest purity, when the residual xylose content was 3.0% (Table 5). The xylose content in jute was lower as compared to hardwood as shown in Table 5. However, the produced dissolving pulp from jute is not as pure as wood. This is maybe due to some part of hemicelluloses being entrapped within the cellulose matrix and some part was associated with the lignin–carbohydrate complex (Gübitz et al. 1998).
Conclusion
The aim of the present study was to investigate the ability of extracting hemicelluloses of ethylenediamine (EDA) in soda liquor and the effect of prehydrolysis on soda-EDA pulp. Prehydrolysis temperature and H2SO4 had great influences on the properties of dissolving pulp. An increase of the prehydrolysis temperature or H2SO4 in prehydrolysis liquor increased the α-cellulose content and decreased the pulp yield and kappa number and viscosity of pulp. Jute fiber prehydrolysis at 170°C had a pulp yield of 37.8% only. The pulp yield was further decreased to 31.7% when 1.0% H2SO4 was used in prehydrolysis. EDA in soda liquor had also a good effect on dissolving hemicelluloses. The α-cellulose content and viscosity were increased with the increase of amine in soda liquor. EDA in soda liquor produced pulp of higher yields (63–65%), viscosity (14–16 mPa s) and α-cellulose (91–93%) content when prehydrolysis was not used. However, the bleachability of soda-EDA pulp was very poor. Soda-EDA pulp without prehydrolysis showed a brightness of about 72% only. The bleachability of soda-EDA pulp was improved with prehydrolysis. The α-cellulose content in prehydrolysed soda-EDA (40%) pulp was 96.2%, where the pulp brightness was 80%. EDA is a highly protective agent for α-cellulose and extracting agent for hemicelluloses, thus better viscosity was obtained. Degraded cellulose (S 10) in pulp was lower in soda-EDA pulp than that of soda pulp.
References
Akhtaruzzamen AFM, Shafi M (1995) Pulping of jute. Tappi 78(2):106
Bhowmic K (1999) Dissolving pulp from jute stem by water prehydrolysis kraft process. IPPTA J 11(1):37–42
Biermann CJ (1993) Essentials of Pulping and Papermaking. Academic Press, New York, pp 72–100
Engström AC, Monica E, Henriksson G (2006) Improved accessibility and reactivity of dissolving pulp for the viscose process: pretreatment with monocomponent endoglucanase 7:2027–2031
Gübitz GM, Stebbing DW, Johansson CJ, Saddler JN (1998) Lignin-hemicellulose complexes restrict enzymatic solubilization of mannan and xylan from dissolving pulp. Appl Microbiol Biotechnol 50:390–395
Helmy SA, State MA (1991) Viscose pulps from Egyptian bagasse with high chemical reactivity. Holzforschung 45:433–436
Henriksson G, Nutt A, Henriksson H, Pettersson B, Stahlberg J, Johansson G, Pettersson G (1999) Endoglucanase 28 (Cel12A)—a new Phanerochaete chrysosporium cellulase. Eur J Biochem 259(1/2):88–95
Hessler LE, Power RE (1954) The use of iodine adsorption as a measure of cellulose fiber crystallinity. Textile Res J 24:822–827
Hinck JF, Casebier RL, Hamilton JK (1985) Pulp and paper manufacture. In: Ingruder OV, Kocurek JJ, Wong W (eds) vol. 4 Tappi Press, Atlanta, pp 213–243
Jahan MS (2001) Evaluation of additives in soda pulping of jute. Tappi J 84(8):1–11
Jahan MS, Farouqui FI (2000) Pulping of whole jute plant (C. Capsularis) by soda-amine process. Holzforschung 54(6):625–630
Jahan MS, Farouqui FI (2001) Pulping of jute with amine. Cellulol Chem Technol 35(1–2):177–187
Jahan MS, Al-Maruf A, Quaiyyum MA (2007) Comparative studies of pulping of jute fiber, jute cutting and jute caddis. Bangladesh J Sci Ind Res 42(4):425–434
Jahan MS, Kanna GH, Mun SP, Nasima Chowdhury DA (2008) Variations in chemical characteristics and pulpability within jute plant (Chorcorus capsularis). Ind Crops Prod 28:199–205
Julien LM, Sun BCH (1979) Pulping with amine and soda-amine systems. Tappi J 62(8):63–65
Krässig HA (1993) Cellulose—structure, accessibility and reactivity. In: Huglin MB (ed) Polymer monographs, vol 11. Gordon and Breach science Publishers, Amsterdam
Kirci H, Akgul M (2002) Production of dissolving pulp from poplar wood by ethanol-water process. Turk J Agric For 26:239–245
Kubes GJ, Bolker HI (1978) Sulphur free delignification I. Alkaline pulping with monoethanolamine and ethylene diamine. Cellulose Chem Technol 12(5):621–645
Kubes GJ, Fleming BI, MacLeod JM, Bolker HI (1978) Sulphur free delignification. Tappi 61(8):46–50
Lennholm H, Iversen T (1995a) Classification of pulp fibers from different wood species by multivariate data-analysis of C-13-CP/MAS-NMR-spectra. Holzforschung 49(5):462–464
Lennholm H, Iversen T (1995b) The effect of laboratory beating on cellulose structure. Nordic Pulp Paper Res J 10(2):104–109
MacLeod JM, Iwase H, Bolker HI (1984) The carbohydrate composition of soda-additive pulps. Tappi J 67(5):123–124
Masura M (1987) Prehydrolysis of beechwood. Wood Sci Technol 21(1):89–100
Nahar N (1987) Studies on carbohydrates in jute and pigeon pea. Swedish University of Agriculture Sciences, Uppsala, p 42
Pulps J, Saake B, Parajo JC, Abad S,Sixta H, Harms H, Fink HP Weigel P, Al Ghatta H, Glionna A (1999) Comparative production of dissolving pulps by acetosol, formacell and milox pulping. Proceedings of ISWPC, June 7–10
Rath RL, Bhattacharjee C, Jain S, Bhattacharya PK (2005) Treatment of prehydrolysis liquor from pulp mill using a biological route followed by reverse osmosis. Chem Eng Technol 28(10):1201–1211
Racz I, Borsa J, Bodor B (1996) Crystallinity and accessibility of fibrous carboxymethylcellulose by pad-roll technology. J Appl Polym Sci 62(12):2015–2024
Rabinovich ML, Melnik MS, Bolobova AV (2002) Microbial cellulases (review). Appl Biochem Microbiol 38(4):305–321
Roy TK, Mohindru VK, Behera NC, Kulkarni AG, Prasad A (1998) Jute for speciality pulp. Ippta J 10(3):81–86
Schlotter NE (1988) Rayon. In: Mark HF (ed) Encyclopedia of polymer science and engineering, vol 14 (CD-ROM), Paulo Alto, Dialog information service, pp 45–69
Sixta H, Schuster J, Krotscheck AW, Ruckl W (1994) Towards effluent-free TCF-bleaching of eucalyptus prehydrolysis kraft pulp. Proc non-chlorine bleaching conf, Amelia Island, USA
Sixta H, Harms H, Dapia S, Parajo JC, Pulps J, Saake B, Fink HP, Roeder T (2004) Evaluation of new organosolv dissolving pulps, Part 1: preparation, analytical characterization and viscose processability. Cellulose 11:73–83
Sjöström E (1981) Wood chemistry: fundamentals and applications. Academic Press, New York, pp 169–189
Tang A, Zhang H, Chen G, Wu S, Xie G, Liang W (2002) Emerging technologies of pulping and papermaking. Proceedings of the international symposium on emerging technologies of pulping and papermaking, 2nd, Guangzhou, China, October 9–11, 152–158
TAPPI Standard Methods (1953) Specific gravity (density) and moisture content of pulpwood (T 18 m-53). Tappi Press, GA
Vila C, Santos V, Parajo C (2004) Dissolving pulp from TCF bleached acetosolv beech pulp. J. Chem. Technol Biotechnol. 79:1098–1104
Vietor RJ, Newman RH, Ha M, Apperley DC, Jarvis MC (2002) Conformational features of crystal-surface cellulose from higher plants. Plant J. 30(6):721–731
Wickholm K (2001) Structural elements in native celluloses. PhD thesis. Royal Institute of Technology, Stockholm, Sweden
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jahan, M.S. Studies on the effect of prehydrolysis and amine in cooking liquor on producing dissolving pulp from jute (Corchorus capsularis). Wood Sci Technol 43, 213–224 (2009). https://doi.org/10.1007/s00226-008-0213-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-008-0213-6