Abstract
The liquors from steam explosion of Olea europea wood carried out at temperatures in the range of 190–240°C were analysed for phenolic content, radical scavenging capacity and reducing power. Increased ethyl acetate solubles (EAS) and phenolics [as Gallic Acid Equivalents, (GAE)] extraction yield with increasing temperature were observed. At the higher temperature tested, up to 2.3 g EAS/100 g dry wood were obtained with 0.7 g GAE/100 g dry wood. The purity of the phenolic extracts was independent of the temperature (30 g GAE/100 g EAS). The lignin derived compounds increased steadily with temperature in the studied range, whereas the sugar decomposition products showed a maximum at 230°C. The radical scavenging capacity was slightly higher for the samples produced at the lowest temperature, and comparable to those of synthetic antioxidants. At 0.5 g/L the FRAP (Ferric Reducing Antioxidant Power) and Trolox Equivalent Antioxidant Activity (TEAC) values were equivalent to 1 and 2–3 mM ascorbic acid, respectively. EA extracts were less effective than Butylated hydroxyanisole (BHA) for protecting the oxidation in emulsions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydrothermolysis of lignocellulosic materials is an environmentally benign process for biomass fractionation (Garrote et al. 1999). These treatments can be a preliminary step for the utilization of polysaccharides, since the production of organic acids from hemicelluloses during autohydrolysis leads to acidolysis of cell wall components. Among hydrothermal methods, steam explosion has been widely applied to lignocellulose residues, including olive tree wood (Cara et al. 2006), a renewable, largely available, low cost residue obtained from pruning. In steam explosion, biomass is exposed to pressurized steam followed by rapid reduction in pressure. Typical effects of this treatment are substantial breakdown of the lignocellulosic structure, hydrolysis of the hemicellulosic fraction, depolymerization of the lignin components and defibration. As a consequence, the accessibility of the cellulose components to degradation by enzymes is increased (Moniruzzaman 1996). Compared with other pre-treatments, the advantages of hydrothermal technology include a significantly lower environmental impact, lower capital investment and less hazardous process chemicals. These treatments lead to solutions containing xylooligosaccharides, monomeric sugars, acetic acid, furfural and compounds derived from the acid soluble lignin and extractives (fatty and resin acids, waxes and sterols).
The non-saccharide compounds present in hydrolyzates inhibit microbial metabolism if the sugar solutions are destined to bioconversion, and lower the purity of xylooligosaccharides. Extraction with ethyl acetate in order to improve fermentability of sugar solutions has been proposed (Frazer and McCaskey 1989; Wilson et al. 1989; Parajó et al. 1997). The byproduct from ethyl acetate extraction is a phenolic-rich extract, containing a variety of potentially valuable compounds with antioxidant, antimicrobial and biological activities. Their utilization would favour the economical feasibility of the fractionation process. The effect of the process severity on the yield and activity of the solubilized fraction was observed during autohydrolysis (Garrote et al. 2003, 2004; Cruz et al. 2004; Moure et al. 2005) and during steam explosion (Fernández-Bolaños et al. 1998, 2002). This approach has been addressed by isothermal autohydrolysis of grape pomace (Cruz et al. 2004), by non-isothermal autohydrolysis Eucalyptus globulus wood, corncobs (Garrote et al. 2003) and barley husks (Garrote et al. 2008).
The ethyl acetate fraction obtained from the solubilized liquors after steam explosion of olive cake contained low molecular weight phenols (Felizón et al. 2000). Fernández-Bolaños et al. (2002) proposed the hydrothermal treatment of the solid waste from two-phase olive oil extraction to fractionate and recover hydroxytyrosol and the saccharide fraction. Olive wood extracts in conventional solvents showed radical scavenging activity (Altarejos et al. 2005), the most active compounds detected were hydroxytyrosol, cycloolivil and oleuropein (Pérez-Bonilla et al. 2006), but scarce literature on the activity of fractions from biomass pre-treatment is available.
The aim of the present work is to evaluate the phenolic content and antioxidant activity of the ethyl acetate solubles (EAS) of Olea europea wood present in the liquors from steam explosion under experimental conditions leading to a cellulose residue suitable for ethanol production (Ruiz et al. 2006).
Materials and methods
Materials
Raw material. Wood of olive tree branches (more than 5 cm diameter) was locally collected after fruit-harvesting, air-dried at room temperature to equilibrium moisture content of about 10%, milled using a laboratory hammer mill (Retsch) to a particle size smaller than 10 mm, homogenised in a single lot and stored until used.
The standard gallic acid, Trolox, butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT) were purchased by Sigma (St. Louis, MO, USA). A standard of hydroxytyrosol was obtained from oleuropein (Extrasyntheses, Genay, France) after acid hydrolysis with 3 N HCl at 100°C (Fernández-Bolaños et al. 2002). Tyrosol was also purchased from Extrasyntheses.
Steam explosion
The raw material was submitted to steam explosion in a custom-built batch pilot unit based on Masonite technology and equipped with a 2 L reaction vessel designed to reach a maximum operating pressure of 4.12 MPa. The steam explosion device is described in detail in Ballesteros et al. (2004). The reactor was charged with 200 g (dry matter) of feedstock per batch and heated to the desired temperature (190, 210, 230 or 240°C) with saturated steam for 5 min. The exploded material was recovered in a cyclone and after cooling to about 40°C filtered for liquid and solid recovery. The liquid phase was extracted with ethyl acetate under previously reported conditions (Cruz et al. 2004). The organic phase was vacuum evaporated to remove and recover the solvent and the extract was freeze-dried, and analysed for total phenolics and antioxidant activity.
Conditioning of samples for GC-MS. About 1 mL of a 10 mg/mL ethanolic solution of the extract was diluted with distilled water up to 25 mL. About 0.25 mL of 3-octanol (10 ppm) and 3,4-dimethylphenol (100 ppm) were added to this solution as internal pattern. This mixture was extracted with DCM under the next conditions, a first extraction with 2.5 mL of DCM for 5 min at 600 rpm, and subsequently 5 min to pour off. The aqueous phase was again extracted with 1.25 mL of DCM two times with same conditions. The total organic phase was transferred to a graduate glass tube and concentrated with nitrogen stream up to a final volume of 0.5 mL.
Analytical methods
The total phenolics content was determined by Folin Ciocalteu method (Singleton and Rossi 1965) using gallic acid as standard.
Determination of the antioxidant activity
α,α-Diphenyl-β-picrylhydrazyl (DPPH) radical scavenging
About 2 mL of a 6 10−5 M methanolic solution of DPPH were added to 50 μL of a methanolic solution of the antioxidant, and the decrease in absorbance at 515 nm after 16 min was recorded in an Agilent 8453E UV-Visible spectrophotometer. The inhibition percentage (IP) of the DPPH radical was calculated as the percent reduction of absorbance at 16 min with respect to the initial value. EC50 was calculated as the concentration of phenolic compounds required to quench 50% of the initial DPPH radical.
TEAC (trolox equivalent antioxidant capacity)
This assay proposed by Re et al. (1999) is based on the scavenging of ABTS radical [2,2′-azinobis (3-ethyl-benzothiazoline-6-sulfonate)]. ABTS radical cation (ABTS+) was produced by reacting 7 mM ABTS stock solution with 2.45 mM potassium persulfate (final concentration) and allowing the mixture to stand in the dark at room temperature for 12–16 h before being used. Next ABTS+ solution was diluted with PBS (Phosphate Buffer Saline) (pH 7.4) to an absorbance of 0.70 at 734 nm and equilibrated at 30°C. After addition of 1.0 mL of diluted ABTS+ solution to 10 μL of antioxidant compounds or Trolox standards in ethanol or PBS, the absorbance reading was taken at 30°C exactly 1 min after initial mixing and for up to 6 min. Appropriate solvent blanks were run in each assay. The percentage inhibition of absorbance at 734 nm was calculated as a function of the concentration of extracts and Trolox.
β-Carotene bleaching method
The oxidative bleaching of β-carotene in a β-carotene/linoleic acid emulsion was measured using the following procedure: 1 mL of a solution prepared with 2.0 mg sample of crystalline β-carotene in 10 mL of chloroform was pipetted into a flask containing 20 mg of purified linoleic acid and 200 mg of the non-ionic detergent Tween 40. After removing chloroform by evaporation, 50 mL of oxygenated, distilled water (prepared using an Ultraturrax T-50 homogenizer, 5 min, 10,000 rpm) were added to the flask under vigorous stirring, and 5 mL aliquots of the emulsion formed were pipetted into test tubes containing 0.2 mL of ethanolic antioxidant solution. The test and control (ethanol) tubes were stoppered and placed in a water bath at 50°C. Absorbance readings at 470 nm were taken at regular intervals until the carotene was decolorized. The antioxidant activity was measured by the Antioxidant Activity Coefficient (AAC), calculated as:
Ferric reducing antioxidant power (FRAP)
The reactive solution was prepared with 25 mL of 300 mmol/L acetate buffer (pH 3.6), 2.5 mL of a 10 mmol/L 2,4,6-tripyridyl-s-triazine (TPTZ) solution in 40 mmol/L HCl and 20 mmol/L FeCl3·6 H2O in distilled water. The reactive solution was always freshly prepared and was used as a blank. An aqueous solution of known Fe (II) concentration was used for calibration (in a range of 100–1,000 μmol/L). Samples (100 μL) were mixed with 3 mL of the FRAP reagent and the absorbance was monitored at 593 nm.
Reducing power
About 1 mL of extract dissolved in methanol with concentration in the range of 50–250 ppm was mixed with 2.5 mL of 0.2 M phosphate buffer (pH 6.6) and 2.5 mL of 1.0% potassium ferricyanide, and the mixture was incubated at 50°C for 30 min. After the addition of 2.5 mL of 10% trichloroacetic acid the mixture was centrifuged and the supernatant (2.5 mL) was mixed with water (2.5 mL) and 0.5 mL of 0.1% ferric chloride before reading the absorbance at 700 nm. The reducing power was expressed as absorbance of the reaction mixture.
All tests and analyses were run in triplicate, and the average values are presented.
HPLC
Identification was carried out in an Agilent HPLC 1100 instrument equipped with a DAD detector and a Supelcosil LC18 column. The injection volume was 20 μL and a flow rate of 1 mL/min was used. A non-linear gradient of the solvent mixture MeOH : H2O : CH3COOH (10:89:1, v:v:v) (solvent A) and MeOH : H2O : CH3COOH (90:9:1, v:v:v) (solvent B) was used. Elution gradients were used as follows: 0 min, 100% A, 0% B; 30 min, 60% A, 40% B; 40 min, 100% A, 0% B.
GC–MS
Analysis was carried out in splitless mode in a Hewlett-Packard 5890-II gas chromatograph coupled to a mass spectrometer HP-5970 using He as carrier gas. Separation was performed using a 60 m × 0.25 mm × 0.25 μm film thickness HP-Innowax capillary column. Temperature was maintained at 45°C for 1 min, increased to 230°C at 3°C/min, and then held for 30 min. Mass spectrometer was in EI mode (electron energy 70 eV; source temperature 250°C), and data acquisition was made in scanning mode from 30 to 300 amu/s and 1.9 spectra/s. Compounds were identified by comparison of the retention time and mass spectra with library data of mass spectra and authentic compounds. Quantization was performed by the internal standard method (using 3-octanol and 3,4-dimethylphenol as standards) using model compounds for calibration.
Results and discussion
Ethyl acetate solubles and Total Phenolic yield
The total phenolics (estimated by the Folin-Ciocalteu method) and expressed as grams of gallic acid equivalents (GAE) in the hydrolyzate produced by steam explosion of olive wood, the EAS extraction yield from this hydrolyzate and the phenolics in the ethyl acetate extracts as a function of temperature are summarized in Table 1. Increasing phenolic content with temperature was observed both in the hydrolyzates and in the ethyl acetate phase after solvent extraction. The EAS of non-phenolic nature also increased with temperature since the phenolics purity in the extracts did not differ significantly among the tested treatment conditions (0.3 g of gallic acid/g ethyl acetate extract). Other compounds (condensation and degradation products derived from hemicelluloses, oligomeric substances from lignin) are also soluble in the ethyl acetate extracts. The ethyl acetate recovers only half of the phenolic compounds present in the hydrolyzates (42–57%). The olive antioxidants are amphiphilic in nature and are more soluble in the water than in the oil phase, particularly 3,4-dihydroxyphenylethanol, tyrosol, caffeic and protocatechuic acid (Rodis et al. 2002).
The extraction yield 0.43–0.70 g GAE/100 g dry wood was comparable to that reported for solid olive residues (Mulinacci et al. 2005), or for olive mill waste (Obied Jr et al. 2007) extracted with conventional solvents, and two to five times lower than extracts obtained from other olive residues treated with steam explosion (Fernández-Bolaños et al. 1998, 2002).
In this temperature range, a continuous increase with the severity on the extraction of hydroxytyrosol from olive waste was reported (Fernández-Bolaños et al. 2002). Depolymerisation of lignin during steam explosion increases its extractability by various solvents and, when followed by a solubilization post-treatment, it can be proposed to isolate cellulose and hemicelluloses mono- and oligosaccharides (Sun et al. 2004). In this case of wheat straw, an increase in steam pre-treatment time or in temperature from 200 to 220°C did not significantly increase the total lignin solubilized, the yield ranging between 1.9 and 2.1% of the raw matter, a degradation of 11.2–12.4% of the original lignin (Sun et al. 2004). Ethyl acetate extraction has also been addressed by isothermal autohydrolysis of grape pomace (Cruz et al. 2004), by non-isothermal autohydrolysis of E. globulus wood, corncobs (Garrote et al. 2003) and barley husks (Garrote et al. 2008). The yield of EAS substances ranged from 2.06 to 8.72 g solubles/100 g dry raw material, the total phenolics content accounting for up to 1 g GAE/100 g dry material. Stable values of the total phenolics yield from barley husks during non-isothermal autohydrolysis have been observed at temperatures higher than 225°C (Garrote et al. 2008). Above a certain value, a slight decrease was observed from whole stones (Fernández-Bolaños et al. 1998, 2000), probably due to condensation reactions leading to repolymerisation of the lignin fragments reducing its solubility (Sun et al. 2004). Fernández-Bolaños et al. (2002) reported that the optimal conditions for the maximum recovery of hydroxytyrosol are not coincident with the maximum recovery of other constituent fractions, such as hemicelluloses, cellulose or oil. Similarly, the maximum values for phenolics solubilization occurred at higher severity than the solubilization of monomeric sugars from barley husks (Garrote et al. 2008).
Antioxidant activity
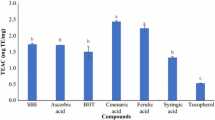
The DPPH test is simple and rapid, the radical is stable and commercially available, but it has no similarity to the radicals involved in lipid peroxidation. However, the DPPH scavenging reaction has been proposed as a measure of rapid reactions of radicals, and thus it could be relevant for predicting the lipid oxidation, since an effective antioxidant must interrupt the propagation step of lipid oxidation by reacting much faster with lipid radicals than linoleic acid does (Gordon et al. 2001). TEAC has been proposed as one of the methods considered for standardization for the antioxidant activity in food and nutraceuticals (Prior et al. 2005).
Hydroxytyrosol was a more active DPPH scavenger than BHT, Vit E and Vit C (Visioli et al. 1998), BHA and BHT (Fki et al. 2005), Trolox and α-tocopherol (Gordon et al. 2001), oleuropein and tyrosol (Carrasco-Pancorbo et al. 2005; Pérez-Bonilla et al. 2006). Hydroxytyrosol is one of the most active antioxidants as radical scavenger (Gordon et al. 2001; Pellegrini et al. 2001; Carrasco-Pancorbo et al. 2005), but also as protector from the oxidation of β-carotene-linoleic acid (Fki et al. 2005), and refined oil (Gordon et al. 2001; Fki et al. 2005). Hydroxytyrosol was the most abundant phenolic in some olive residues such as olive oil mill wastewaters (Visioli et al. 1998; Fki et al. 2005), and in the ethyl acetate extracts from hydrothermal treatments of olive oil processing wastes, exhibiting high thermal stability during processing (Fernández-Bolaños et al. 2002). However, in studies with oil fractions, the antiradical capacity was affected by high temperature, and protocatechuic acid was more active than hydroxytyrosol, tyrosol and syringic and caffeic acids (Espin et al. 2000). In the solvent extracts from olive wood the most abundant compound was oleuropein (Pérez-Bonilla et al. 2006), but the mild acid hydrolysis (pH samples in the range of 3.26–3.75) during steam explosion can cause the decomposition into hydroxytyrosol, as observed from the increase in the corresponding peak area (Fig. 1); this increase being closely related to the temperature during steam explosion. Hydroxytyrosol is part of other molecules (oleuropein, dimethyloleuropein, verbascoside and hydroxytyrosol glucosides), and the cell wall disruption caused by the treatment led to the production of free hydroxytyrosol.
In mixtures of compounds present in olive products it may be difficult to discern which compound contributes more significantly to antioxidant activity. The biological activities of the major phenolics in olive oil wastewaters have been revised by Obied Jr et al. (2005). It is generally agreed that hydroxytyrosol shows a high antioxidant activity, although a positive correlation cannot be found always (Del Carlo et al. 2004).
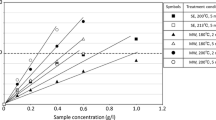
The most active radical scavenger, ethyl acetate (EA) extract from the liquors of steam exploded olive tree wood, was obtained at 210°C, both against ABTS and DPPH radicals (Fig. 2 and Table 1, respectively). The EA extracts showed potency comparable to that of synthetic antioxidants. Increased TEAC-values with extract concentration were observed (Fig. 2). The extracts produced during steam explosion at 210°C were slightly but significantly more active than those produced at other temperatures. The values are comparable to those already reported for tyrosol, hydroxytyrosol, and oleuropein (Pellegrini et al. 2001).
Since the antioxidant potency of an extract is related to its reducing ability, the reducing power is often evaluated. The FRAP values of the EA extracts from hydrolyzates produced at different temperatures tested are shown in Fig. 3. A linear behaviour of the reducing power up to 250 ppm and of FRAP up to 1,000 ppm was noticed. A linear relationship was also reported for olive o-diphenols and their FRAP-values in the range of 0–200 mg/kg (Manna et al. 2002). No significant differences among the extracts obtained at the different temperatures of steam explosion were observed. The FRAP of EA extracts from the liquors of steam exploded olive tree wood was lower than the value observed for extracts from autohydrolysis liquors of pine wood (Moure et al. 2005).
The effect of extract concentration on the antioxidant activity in emulsion is shown in Fig. 4 in relation to the activity of BHA. BHA and BHT provided higher protection against oxidation of β-carotene in this assay than the ethyl acetate extracts from olive mill wastewaters (Fki et al. 2005), although pure hydroxytyrosol was as active as BHT. The lower activity of the extracts in emulsion, compared to control antioxidants, is not surprising since some phenolic and cinnamic acids, which are active DPPH scavengers, are less potent than BHA or BHT in the β-carotene bleaching test (von Gadow et al. 1997). Although the ethyl acetate selectively extracts the more lipophilic fraction, which according to the Polar Paradox would be active in emulsion, other features influence this activity (the partitioning of components between phases, the interactions at the oil-water interfaces, the radical-scavenging potency, and the stability of the antioxidants).
Composition
The liquid separated in the cyclone after steam explosion was extracted with dichloromethane (DCM) to yield the soluble compound, which can be classified into three categories (sugar-derived compounds, lignin-derived compounds and nitrogen-containing compounds). The temperature during steam explosion of olive tree wood strongly influenced the composition of the DCM soluble fraction of the hydrolyzates (Table 2).
An increase in the peak area of identified compounds with steam explosion temperature was also observed. The total area of compounds identified at 240°C was 50% higher than at 210°C. A corresponding increase in both the total phenolics present in the aqueous phase and in the ethyl acetate with the total compounds identified in GC-MS was observed. Whereas the maximum value of the peak areas of the identified sugar derived compounds and nitrogen derived compounds was found at 230°C, a continuous increase in the range of temperatures studied for lignin derived compounds was noticed. With increasing temperature in the studied range, sugar derived compounds accounted for 33.9–28.7% of total peak area of identified compound, whereas the fraction of the total peak area corresponding to nitrogen-containing compounds was reduced from 22.5 to 7.2%, and that of lignin derived compounds was increased from 43.5 to 64% when the treatment temperature increased from 190 to 240°C.
The major compounds found in the lignin derived compounds fractions were syringaldehyde, vanillin, 4-formyl benzoic acid methyl ester and desaspidinol. Syringol, guaiacol, homosyringic acid, methoxyeugenol were also identified. Even when hydroxytyrosol was expected, either the GC conditions or the method were not suited to identify it, since in an experiment with a synthetic solution of this compound it could not be detected even at prolonged analysis times. Vanillin, syringaldehyde, guaiacol were among the major components in other liquors alkaline wet oxidation of wheat straw (Klinke et al. 2002), steam explosion liquors from aspen (De Bari et al. 2002), autohydrolysis of wheat straw (Sun et al. 2004), autohydrolysis from corn cobs and rice (Garrote et al. 2007). Cleavage of the ester bonds of the lignin fraction and release of phenolic acids (coumaric, ferulic, syringic and vanillic) was reported during wheat straw fractionation by steam explosion coupled with ethanol extraction (Hongzhang and Liying 2007).
Conclusions
In this work it was observed that the liquid phase generated during steam explosion of olive tree can be extracted with ethyl acetate to obtain an extract showing antioxidant activity comparable to that of synthetic antioxidants. The utilization of these compounds could benefit the economy of the whole fractionation process by facilitating the utilization of the hemicellulosic sugars in the liquid phase and by adding commercial value to a residual fraction.
References
Altarejos J, Salido S, Pérez-Bonilla M, Linares-Palomino PJ, van Beek TA, Nogueras M, Sánchez A (2005) Preliminary assay on the radical scavenging activity of olive wood extracts. Fitoterapia 76:348–351
Ballesteros M, Oliva JM, Negro MJ, Manzanares P, Ballesteros I (2004) Ethanol from lignocellulosic materials by a simultaneous saccharification and fermentation process (SFS) with Kluyveromyces marxianus CECT 10875. Process Biochem 39:1843–1848
Cara C, Ruiz E, Ballesteros I, Negro MJ, Castro E (2006) Enhanced enzymatic hydrolysis of olive tree wood by steam explosion and alkaline-peroxide delignification. Process Biochem 41:423–429
Carrasco-Pancorbo A, Cerretani L, Bendini A, Segura-Carretero A, Del Carlo M, Gallina-Toschi T, Lercker G, Compagnone D, Fernández-Gutiérrez A (2005) Evaluation of the antioxidant capacity of individual phenolic compounds in virgin olive oil. J Agric Food Chem 53:8918–8925
Cruz JM, Domínguez H, Parajó JC (2004) Assessment of the production of antioxidants from winemaking waste solids. J Agric Food Chem 52:5612–5620
De Bari I, Viola E, Barisano D, Cardinale M, Nanna F, Zimbardi F, Cardinale G, Braccio G (2002) Ethanol production at flask and pilot scale from concentrated slurries of steam-exploded aspen. Ind Eng Chem Res 41:1745–1753
Del Carlo M, Sacchetti G, Di Mattia C, Compagnone D, Mastrocola D, Liberatore L, Cichelli A (2004) Contribution of the phenolic fraction to the antioxidant activity and oxidative stability of olive oil. J Agric Food Chem 52:4072–4079
Espin JC, Soler-Rivas C, Wichers HJ (2000) Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphenyl-1-picrylhydrazyl radical. J Agric Food Chem 48:648–656
Felizón B, Fernández-Bolaños J, Heredia A, Guillén R (2000) Steam-explosion pre-treatment of olive cake. J Am Oil Chem Soc 77:15–22
Fernández-Bolaños J, Felizón B, Brenes M, Guillén R, Heredia A (1998) Hydroxytyrosol and tyrosol as the main compounds in the phenolic fraction of steam-exploded olive stones. J Am Oil Chem Soc 75:1643–1649
Fernández-Bolaños J, Heredia A, Felizón B, Brenes M, Guillén R, Rodríguez R (2000) Procedimiento de obtención de hidroxitirosol a partir de hueso de aceituna. Span Pat ES 2,145.701
Fernández-Bolaños J, Rodríguez G, Rodríguez R, Heredia A, Guillén R, Jiménez A (2002) Production in large quantities of highly purified hydroxytyrosol from liquid-solid waste of two-phase olive oil processing or “alperujo”. J Agric Food Chem 50:6804–6811
Fki I, Allouche N, Sayadi S (2005) The use of polyphenolic extract, purified hydroxytyrosol and 3,4-dihydroxyphenyl acetic acid from olive mill wastewater for the stabilization of refined oils: a potential alternative to synthetic antioxidants. Food Chem 93:197–204
Frazer FR, McCaskey TA (1989) Wood hydrolyzate treatments for improved fermentation of wood sugars to 2,3-butanediol. Biomass 18:31–42
Garrote G, Cruz JM, Domínguez H, Parajó JC (2008) Non-isothermal autohydrolysis of barley husks: product distribution and antioxidant activity of ethyl acetate soluble fractions. J Food Eng 84:544–552
Garrote G, Cruz JM, Domínguez H, Parajó JC (2003) Valorisation of waste fractions from autohydrolysis of selected lignocellulosic materials. J Chem Technol Biotech 78:392–398
Garrote G, Cruz JM, Moure A, Domínguez H, Parajó JC (2004) Antioxidant activity of byproducts from the hydrolytic processing of selected lignocellulosic materials. Trends Food Sci Technol 15:191–200
Garrote G, Domínguez H, Parajó JC (1999) Hydrothermal processing of lignocellulosic materials. Holz Roh- Werkst 57:191–202
Garrote G, Falqué E, Domínguez H, Parajó JC (2007) Autohydrolysis of agricultural residues: study of reaction byproducts. Biores Technol 98:1951–1957
Gordon MH, Paiva-Martins F, Almeida M (2001) Antioxidant activity of hydroxytyrosol acetate compared with that of other olive oil polyphenols. J Agric Food Chem 49:2480–2485
Hongzhang C, Liying L (2007) Unpolluted fractionation of wheat straw by steam explosion and ethanol extraction. Biores Technol 98:666–676
Klinke HB, Ahring BK, Schmidt AS, Thomsen AB (2002) Characterization of degradation products from alkaline wet oxidation of wheat straw. Biores Technol 82:15–26
Manna C, D’Angelo S, Migliardi V, Loffredi E, Mazzoni O, Morrica P, Galletti P, Zappia V (2002) Protective effect of the phenolic fraction from virgin olive oils against oxidative stress in human cells. J Agric Food Chem 50:6521–6526
Moniruzzaman M (1996) Effect of steam explosion on the physicochemical properties and enzymatic saccharification of rice straw. Appl Biochem Biotechnol 59:283–297
Moure A, Domínguez H, Parajó JC (2005) Antioxidant activity of liquors from aqueous treatments of Pinus radiata wood. Wood Sci Technol 39:129–139
Mulinacci N, Innocenti M, La Marca G, Mercalli E, Giaccherini C, Romani A, Erica S, Vincieri FF (2005) Solid olive residues: insight into their phenolic composition. J Agric Food Chem 53:8963–8969
Obied HK Jr, Bedgood DR, Prenzler PD, Robards K (2007) Bioscreening of Australian olive mill waste extracts: biophenol content, antioxidant, antimicrobial and molluscicidal activities. Food Chem Toxicol 45:1238–1248
Obied HK, Allen MS, Bedgood DR, Prenzler PD, Robards K, Stockmann R (2005) Bioactivity and analysis of biophenols recovered from olive mill waste. J Agric Food Chem 53:823–837
Parajó JC, Domínguez H, Domínguez JM (1997) Xylitol production from Eucalyptus wood hydrolysates extracted with organic solvents. Process Biochem 32:599–604
Pellegrini N, Visioli F, Buratti S, Brighenti F (2001) Direct analysis of total antioxidant activity of olive oil and studies on the influence of heating. J Agric Food Chem 49:2532–2538
Pérez-Bonilla M, Salido S, van Beek TA, Linares-Palomino PJ, Altarejos J, Nogueras M, Sánchez A (2006) Isolation and identification of radical scavengers in olive tree (Olea europaea) wood. J Chromatogr A 1112:311–318
Prior RL, Wu X, Schaich K (2005) Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem 53:4290–4302
Rodis PS, Karathanos VT, Mantzavinou A (2002) Partitioning of olive oil antioxidants between oil and water phases. J Agric Food Chem 50:596–601
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad Bio Med 26:1231–1237
Ruiz E, Cara C, Ballesteros M, Manzanares P, Ballesteros I, Castro E (2006) Ethanol production from pretreated olive tree wood and sunflower stalks by an SSF process. Appl Biochem Biotechnol 129–132:631–643
Singleton VL, Rossi SA (1965) Colorimetric of total phenolics with phosphomolibic-phosphotungstic acid reagents. J Enol Vitic 16:144–158
Sun XF, Xu F, Sun RC, Wang YX, Fowler P, Baird MS (2004) Characteristics of degraded lignins obtained from steam exploded wheat straw. Polym Degrad Stabil 86:245–256
Visioli F, Bellomo G, Galli C (1998) Free radical-scavenging of olive oil phenols. Biochem Biophys Res Commun 247:60–64
von Gadow A, Joubert E, Hansmann CF (1997) Comparison of the antioxidant activity of aspalathin with that of other plant phenols of rooibos tea (Aspalathus linearis), α-tocopherol, BHT and BHA. J Agric Food Chem 45:632–638
Wilson JJ, Deschatelets L, Nishikawa N (1989) Comparative fermentablility of enzymatic and acid hydrolysates of steam-pretreated aspenwood hemicellulose by Pichia stipitis CBS 5776. Appl Microbiol Biotechnol 31:592–596
Acknowledgements
This work was partially financed by Ministerio de Educación y Ciencia (Projects ENE2005-08822, and AGL2003-03596), by Xunta Galicia (Research Project PGIDT, 04PXI38301PN) and FEDER funds. Financial support from Azucareras Reunidas de Jaén, S.A. is also gratefully acknowledged. Dr. Andrés Moure thanks Xunta de Galicia for his “Isidro Parga Pondal” contract.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Castro, E., Conde, E., Moure, A. et al. Antioxidant activity of liquors from steam explosion of Olea europea wood. Wood Sci Technol 42, 579–592 (2008). https://doi.org/10.1007/s00226-007-0169-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-007-0169-y