Abstract
The effect of preheating temperature (X1), preheating time (X2) and the nature of the extracting solvents (X3) on the antioxidant activity of ultrasonic extracts of hemp cake was evaluated using a factorial design with a general linear multiple regression method using the three variables (X1, X2, and X3) and three levels including low (-1), intermediate (0) and high (+ 1). The results indicated that the extracting solvent and the preheating temperature levels were the principal effects influencing the total phenolic content (TPC), total flavonoid content (TFC), and antioxidant activity (DPPH and FRAP). The highest level of preheating temperature (+ 1 = 180 °C) and extracting solvent (+ 1 = Ac80) were the optimal conditions for enhancing the extraction of the total phenolics and providing the highest antioxidant activity in hemp cake extracts. The interaction between temperature (X1), and the type of solvent (X3) significantly (p < 0.05) affected all the dependent variables examined.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hemp (Cannabis sativa L.) is a high-yielded multi-purpose crop used as a source of fibre, oil and protein. The seeds from hemp contain 26–38% (w/w) oil with demonstrated interesting nutritive, cosmetic and medicinal properties (Liang et al. 2018). Its medicinal properties include lowering blood cholesterol levels, regulating hypertension (Oomah et al. 2002), and mediating atopic dermatitis in humans (Callaway et al. 2005). The oil is separated by cold pressing which involves mechanical pressing of the seeds using temperatures below 50 °C. Since 2009, hemp seeded acres in Canada have been increasing. In 2015, over 84,000 acres were licensed for hemp cultivation and is expected to be over 100,000 acres by 2021 (Canadian Hemp Trade Alliance 2021). The yield of oil varies from 0.2 to 1.0 tons per acre, depending on the seed variety, climatic conditions, and other environmental factors.
Over the past few years by-product utilization of hemp has gained attention including hemp cake, meal, straw, and hulls. Of the different by-products, hempseed cake demonstrated its importance due to being rich in phenolics and other minor components (Pojić et al. 2014). Phenolic compounds have been widely studied and shown to exhibit diverse bioactivities that could be beneficial to human health. In addition to reducing the risk of cancer (Dai and Mumper 2010), heart diseases, and diabetes (Oak et al. 2005), phenolics also exert antibacterial (Suriyarak et al. 2014), anti-inflammatory and anti-allergenic (Nagarkatti et al. 2009) activities.
The main phenolic compounds in oleaginous plants are derivatives of hydroxybenzoic and hydroxycinnamic acids, as well as coumarins and flavonoids (Liang et al. 2018). Cannabisin-B, a bioactive compound unique to hemp, was first isolated and identified as lignanamide by Sakakibara et al. (1992) and later detected as a phenolic compound (Pojić et al. 2014). Recently, cannabisin-B and its derivative N-trans-caffeoyltyramine, isolated from hemp, were identified as potent antioxidants with major free radical scavenging activity and anticancer properties (Chen et al. 2013). The rich phenolic content in hempseed cake enhances its economic value, as well as creates new opportunities for its utilization.
Previous studies claimed that the application of thermal pre-treatments of plant materials (seeds, cake, meal) improved antioxidant activity and the recovery of phenolic compounds (Khattab et al. 2014; Zago et al. 2015). Heat processes can contribute to the hydrolysis of the plant lignin which promotes the release of phenolic compounds with lower molecular weight which may present higher bioactivity (Azadfar et al. 2015). Some studies demonstrated that thermal treatments of plant matrices can modify the plant cell wall by increasing its porosity and facilitating the extraction of phenolic compounds (Azadmard-Damirchi et al. 2010). The ability to increase the recovery of phenolic compounds could be explained by the thermal release of bonded molecules (Li and Guo 2016; Siger and Józefiak 2016). Thermal transformations such as decarboxylation and Maillard reactions can also contribute to the formation of compounds with greater antioxidant activity than the original compounds (Kraljić et al. 2019; Nandasiri et al. 2019, 2020). Prolonged exposure to higher temperatures, however, may deteriorate the more thermally sensitive phenolic compounds. Therefore, it is important to understand the effect of different temperature levels on the antioxidant activity and phenolic contents of the extracts. Extraction efficacy is closely associated with the affinity of the solvent for the phenolics extracted. Thus, the type of solvent appears to be an important criterion in the extraction of phenols. Generally, liquid/solid extractions from the plant materials (seeds, cake, meal) are conducted using polar solvents such as methanol, ethanol, acetone and ethyl acetate (Teh et al. 2014). The selection of the correct solvent would help to minimise the use of large amounts of solvents during the extraction process. Furthermore, our previous study concluded that extractability of individual phenolic compounds was affected by different pre-treatment time, temperature, and solvent conditions (Liang et al. 2018). Hence, a more comprehensive analysis on the behavior of the phenolic compounds on the basis of antioxidant activity is required. The current study was therefore focused on examining the influence of preheating temperature, preheating time and the nature of the extracting solvent on the antioxidant activity of the polar ultrasonic extracts of hempseed cake and their relationship with the major phenolic compounds extracted. Determination of an effective process for the production and recovery of bioactive extracts from hempseed cake could add value to this by-product. Likewise, the use of these antioxidant extracts in food, cosmetic and/or pharmaceutical formulation could help to minimize the environmental impact by the implementation of sustainable processes for obtaining alternatives to synthetic antioxidants no loner permitted for use in industry such as BHT, BHA etc.
Material and methods
Chemicals
Phenolic standards namely gallic acid (> 97%), catechin (≥ 98%), ( ±)-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox, 97%) and Folin-Ciocalteu phenol’s reagent, 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ, ≥ 98%), 1,1-diphenyl-2-picrylhydrazyl (DPPH˙, 97%) sodium acetate (99%), sodium carbonate anhydrous (> 99.5%), Iron (II) chloride hexahydrate (98%) were purchased from Sigma-Aldrich (Ottawa, ON, Canada). Glacial acetic acid (99.8%) and hydrochloric acid (> 36%) Diphenylborinic acid 2-aminoethyl ester (98%) and quercetin hydrate (95%) were from Fisher Scientific (Ottawa, ON, Canada). All extraction solvents were HPLC grade (> 99.9%) purchased from Fisher Scientific (Ottawa, ON, Canada).
Raw materials
Cold-pressed hemp cake was obtained from Hemp Oil Canada (Ste. Agathe, Manitoba, Canada). The pellets were ground into homogeneous powder by a blade grinder (Model PC2770) and stored at -20 °C until analysis. The moisture content of the ground material was determined as 7.49 ± 0.08% (w/w) using an Infrared Moisture Analyzer (Model IR-35, Denver Instrument, USA).
Heat treatments of hemp cakes
The ground hemp cake samples (10 g each) were placed in metal dishes, and loaded into a preheated oven (Thelco, Thermo Scientific, USA) and heated at 140, 160 and 180 °C. The heat treatment was carried out at three different time intervals (5, 15 and 30 min) for each level of temperature. Afterwards, all preheat treated samples were kept in a desiccator to attain ambient temperature. The pre-treated samples were kept at 4 °C until further analysis. The unheated hemp cake was used as a control. All experiments were carried out in triplicate with each replicate analyzed twice for their total phenolic content (TPC), total flavonoid content (TF), antioxidant activity using ferric reducing antioxidant power (FRAP) and DPPH radical activity.
Ultrasound-assisted extraction (UAE) of phenolic compounds
The phenolic compounds were extracted by UAE, according to the method described by Liang et al. (2018) with a few modifications. In brief, each hemp cake sample (1.0 g) was extracted three times with 9.0 mL of methanol (70%, v/v) using a SONOPLUS ultrasonic homogenizer HD 2200 system (BANDELIN electronic GmbH & Co. KG, Heinrichstraße, Berlin, Germany). The ultrasound extraction was carried out at the power of 40% with a frequency of 20 kHz ± 500 Hz for 1 min at room temperature (25 °C). After ultrasonic extraction, extracts were centrifuged at 5000 g for 15 min at refrigeration condition (4 °C) (Sorvall Biofuge Primo R Centrifuge; Thermo Scientific, Asheville, NC, USA). This process was repeated three times and the extracts from the three extractions combined and made up to a total volume of 30.0 mL. The solvents used for extraction were aqueous methanol (70%, v/v) (Me70), aqueous acetone (80%, v/v) (Ac80) and a mixture of the solvents: aqueous methanol (70%, v/v) and aqueous acetone (80%, v/v) in a ratio of 1:1 (v/v) (Me + Ac). The final extracts were stored at -20 °C until further analysis.
Characterization of hemp phenolic extracts
Determination of total phenolic content (TPC)
The total extractable phenolic compounds were determined by the Folin-Ciocalteu procedure as adapted by Thiyam et al. (2006) with a few modifications. The extracts (0.5 mL each) were diluted to 5.0 mL with distilled water, followed by the addition of 0.5 mL Folin-Ciocalteu phenol reagent. The solution was mixed thoroughly and left to stand for 3 min. Then, 1.0 mL of sodium carbonate solution 19% (m/v) was added to the solvent mixture. Total volume was adjusted to 10.0 mL using distilled water. Samples were kept in dark for 60 min at room temperature. After 60 min, absorbance was measured at 750 nm using DU 800 UV/Vis spectrophotometer (Beckman Coulter Inc., Mississauga, ON, Canada). For the blank, a solution was prepared as described with the phenolic extract replaced by the respective solvent (Me70, Me + Ac or Ac80). Gallic acid was used as the standard compound, and the results were expressed as milligrams of gallic acid equivalents per gram of dry matter (mg GAE/gDM). Standard curves were obtained for each extracting solvent at concentrations from 0.08 to 0.20 mg/mL (n = 9 points). Aqueous methanol (70%, v/v): R2 = 0.9947; Aqueous acetone 80% (v/v): R2 = 0.9986; Solvent-mixture (Me + Ac): R2 = 0.9952.
Determination of total flavonoids (TFC)
Flavonoids are another important group of phenolics that play a key role as antioxidants. The quantification of total flavonoids (TFC) in hemp extracts was carried out based on the method described by Oomah et al. (1995) with a few modifications. The extracts (1.0 mL) were diluted in 3.0 mL of distilled water and 100 μL of diphenylboric acid 2-aminoethyl ester solution (1%, v/v) was added into the solvent mixture. The absorbance of the colored complex was measured at 404 nm as described in above. A blank solution was prepared as described for TPC with quercetin as the standard. Standard curves were obtained for each extracting solvent at concentrations from 0.01 to 0.30 mmol/L (n = 7 points). Aqueous methanol (70%, v/v): R2 = 0.9995; Aqueous acetone 80% (v/v): R2 = 0.9993; Solvent-mixture (Me + Ac): R2 = 0.9992. The total flavonoid contents were expressed as millimoles of quercetin equivalents per gram of dried matter (mmol QE/g DM).
Ferric reducing antioxidant power assay (FRAP)
The Ferric Reducing Antioxidant Power assay (FRAP) was used to determine the antioxidant activity of the phenolic extracts as described by Benzie and Strain (1996) with slight modifications. FRAP reagent was prepared freshly by mixing 200 mL of acetate buffer (300 mM, pH 3.6), 20 mL of 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ) solution (10 mM), 20 mL of ferric chloride solution (20 mM) and 24 mL of distilled water and warmed to 37 °C. Briefly, 100 μL hemp cake extracts was mixed with 900 μL of distilled water. To the sample mixture 2.0 mL of freshly prepared FRAP reagent was added vortexed. Sample mixture was incubated in dark at 37 °C for 30 min, and the absorbance of the colored complex was measured at 593 nm and, Trolox was used as standard. The calibration curves were prepared using standard solutions ranging from 0.01 to 1.00 mmol/L (n = 7 points). Aqueous methanol (70%, v/v): R2 = 0.9821; Aqueous acetone 80% (v/v): R2 = 0.9966; Solvent-mixture (Me + Ac): R2 = 0.9932. The results were expressed as millimoles of Trolox equivalents per gram of dry matter (mmol TE/g DM).
DPPH free radical scavenging assay
The DPPH free radical-scavenging effect was determined based on the method described by Thiyam et al. (2006) with a few modifications. Briefly, 50 μL of the phenolic extracts were diluted in 2.95 mL of 0.10 mM DPPH solution in methanol. The samples were stored in the dark for 10 min and the absorbance was measured at 516 nm. The scavenging effect of the extract was calculated by the following Eq. 1:
where Ac, absorbance of DPPH solution control; As, absorbance of the sample.
Experimental design and statistical analysis
The experimental design consisted of three independent variables with each variable examined at three increasing levels. The independent variables and their levels were: X1 [Temperature (140, 160 and 180 °C)], X2 [Time of exposure (5, 15 and 30 min) and X3 [Type of the extracting solvent (Me70, Me + Ac and Ac80)]) (Table 1). Each experiment was carried out in triplicates and the results presented as the mean ± standard deviation of the triplicate analysis. The assumptions of normal distribution and constant variance were verified by examining the residuals (Pallant, 2011). The dependent variables were total phenolic content (TPC), total flavonoid content (TFC), radical scavenging properties (% DPPH), ferric reducing antioxidant power (FRAP) of the polar extracts of preheated hemp cakes. The statistical analysis used a general linear multiple regression model using the statistical software IBM SPSS Statistics 22.0, and the post hoc analysis was conducted using the Tukey’s multiple mean comparison method at 5% significance (p < 0.05).
Results and discussion
Model fit statistics
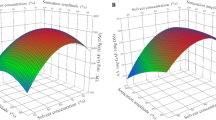
A general linear multiple regression analysis was performed to assess the effect of three different parameters (X1, X2, and X3) at three levels (-1, 0 and + 1) on the ultrasonic extraction of polar phenolic compounds from hemp cake (Table 1). Normality, linearity, univariate outliers, and homogeneity of all dependent variables were evaluated prior to statistical analysis. The results of different combinations of preheating treatments and extracting solvents on TPC, TFC, DPPH, FRAP of the hemp extracts are presented in Table 2. According to the mean sum of squares (Table S1) obtained by the model, the choice of the solvent used in the ultrasonic extraction was the most important factor affecting all parameters (TPC, TFC, DPPH and FRAP). Indeed, the type of solvent and the pre-treatment extraction temperature significantly affected the DPPH radical activity (p < 0.05). The other dependent variables including TPC, TFC and FRAP were significantly (p < 0.05) affected by all X1, X2, and X3 levels.
The predicted and residuals values obtained from the model developed for each parameter were used to conduct the linear regression to understand the relationship between the independent variables (X1, X2, and X3) and the dependent variables. The plot of the predictors versus the residuals is shown in Fig. S1. The upward trend of the data points shown in Fig. S1A and Fig. S1C indicates a positive relationship between the independent variables X1, X2, and X3 which means that positive levels (+ 1) of all independent variables advantageously increased the TPC and the FRAP. The plot of the residuals for TPC and FRAP presented a Gaussian profile and the outliers represent part of the heterogeneity of the samples. For DPPH (Fig. S1B) and total flavonoids (Fig. S1D), the data is distributed vertically and homogenously around zero demonstrating good prediction of the values with no outliers. The total flavonoids residual plot also showed high normality and a homogenous distribution. In these cases, the independent variables X1, X2, and X3 had less influence on the DPPH and total flavonoids than the correlation between these variables.
The total phenolic content (TPC) and total flavonoid content (TFC)
There are few studies on hemp demonstrating its nutraceutical importance and even less on the study of the effect of thermal treatments on the bioactivity of hemp phenolic extracts. Most of the studies were focused on hemp oil as a value addition step. Siger et al. (2008) examined the total phenolic contents (TPC) in methanolic extracts of cold-pressed oil of nine different oleaginous plants including hempseed and found the hemp oil had the second highest TPC content (0.13 ± 0.003 µmol CAE/g). This was later confirmed by Vonapartis et al. (2015) who analyzed ten industrial Canadian hemp cultivars which showed an average phenolic content of 130.8 µmol GAE/g. Concerning hemp seeds with no pre-treatment, Frassinetti et al. (2018) reported 12.99 ± 0.47 µmol GAE/g of TPC in ethanol: water (80:20, v/v) extracts of a French hemp monoecious variety. More recently, Babiker et al. (2021) studied the effect of roasting time in the antioxidant activity and phenolic composition of hemp seeds ethanol: water (80:20, v/v) extracts. In this work, it was demonstrated that thermal treatments of the seeds increased the TPC in 40% when heating the seeds at 160 °C for 21 min in a classic oven (from 0.98 for the non-treated to 1.62 µmol GAE/g for the roasted ones).
The current study analysed the interaction between three independent variables and demonstrated that changes in the temperature of the preheating treatments did not significantly affect TPC regardless of the preheating time (X1*X2: f = 0.790, p = 0.537) and the extracting solvent used (X1*X3: f = 1.669, p = 0.171) (Table 3). Also, despite the main influence of the solvent used for the ultrasonic extraction, the preheating time had no statistical influence on the TPC (X2*X3: f = 2.291, p = 0.072). The interaction of the three independent variables showed no significant correlation for the TPC (X1*X2*X3: f = 1.659, p = 0.131). A Tukey post-hoc analysis was performed for the multiple comparisons of the mean effects. For the TPC, changes in the levels of the independent variables were statistically significant. A significant positive effect was observed for X1 (preheating temperature) and X2 (preheating time) when their levels were increased from -1 (140 °C; 5 min) to + 1 (180 °C; 30 min) and from 0 (160 °C; 15 min) to + 1 (180 °C; 30 min) (Fig. 1A). The higher TPC found in the present study was 30.58 ± 0.89 µmol GAE/g DM for the extract obtained using acetone:water (80:20, v/v) and from the hemp cake preheated at 180 °C for 30 min. The effect of the extracting solvent was significant for all the three levels for the TPC (Fig. 1A) (Mean Square Error = 1.034). All three extracting solvents influenced the TPC statistically (p = 0.00), but aqueous acetone (80%, v/v) was able to extract the greatest amounts of total phenolic compounds (Fig. 1A). Other works led by Do et al. (2014) and Rezaie et al. (2015) showed that a polar-protic solvent (ethanol) followed by a polar-aprotic solvent (acetone) was less shown to be the most effective solvents for the extraction of antioxidant phenolic compounds. Furthermore, the increase of preheating time to 30 min had a positive effect on the TPC, for all solvents. These results as those observed by Babiker et al. (2021) can be explained by the thermal degradation of the chemical bonds between the bioactive phenolic compounds and the plant matrix. Increase in heating time promoted the release of the more strongly linked compounds and even at the highest levels, no deleterious effects were observed.
A similar trend was observed for TFC, where a significant positive effect was observed for X1 (preheating temperature) and X2 (preheating time) when their levels were increased from 0 (160 °C; 15 min) to + 1 (180 °C; 30 min) (Fig. 1D). All three extracting solvents influenced the TFC statistically significantly (p < 0.05), but aqueous acetone (80%, v/v) was able to extract the largest amount of total phenolic compounds (Fig. 1D) compared to other two solvents. Moreover, the total flavonoid content was significantly different regardless of the level of X1 and X3. The preheating time was the only parameter affecting the flavonoid content which increased from level 0 (15 min) to + 1 (30 min) (Mean Square Error = 0.000). Thus, preheating time affected the hemp extracts differently for the flavonoids values as the longer preheat treatment (30 min) had a negative effect for both Me + Ac and Me70 solvents. The amounts of flavonoids, however, were relatively lower compared to other oil crops ranging from 0.17 to 0.39 µmol QE/100 g DM (Nandasiri et al. 2019).
The antioxidant activity
Siger et al. (2008), verified the antioxidant capacity of nine plant oil extracts using the DPPH assay. The highest antioxidant activity was displayed by the extract obtained from hemp and pumpkin oils which was around 70% DPPH• radical scavenging activity. Rapeseed oil also had a radical scavenging activity of over 50%. In a different study Teh et al. (2014), reported that hemp cake extracts obtained by a mixture of methanol:acetone:water (7:7:6, v/v/v) exhibited a relatively higher radical scavenging activity (16.79% ± 0.09) than aqueous acetone (80%, v/v) (12.48% ± 0.46) and aqueous methanol (80%, v/v) (11.01% ± 0.23). The results of the radical scavenging activity of the current study ranged from 9.92% (Me70) to 24.89% (Ac80). In addition, hemp cake extracts exhibited relatively lower reducing power compared to rapeseed and other oilseed crops (Nandasiri et al. 2019). The reducing power of the extracts ranged from 0.27 to 0.92 mmol TE/g DM.
The preheating temperature had a significant influence (p > 0.05) on the antioxidant activity of the ultrasonic phenolic extracts alone (DPPH: X1: f = 3.365, p = 0.042) and when combined with the effect of the extracting solvent (DPPH: X1*X3: f = 14.618, p = 0.000; FRAP: X1*X3: f = 12.433, p = 0.000). The DPPH and FRAP were significantly affected by the interaction between preheating temperature and extracting solvent despite the preheating time (X1*X2*X3: f = 3.097, p = 0.006 and f = 2.989, p = 0.008, respectively) (Fig. 1B, D). However, the radical scavenging activity was affected by both the pre-treatment temperature and extracting solvent. As shown in Table 2 and Fig. 1B, aqueous acetone provided extracts with the highest free radical scavenging activity. Increases in preheating temperature and time levels to + 1 (180 °C; 30 min), however, had a negative impact on the radical scavenging activity of the acetone extracts (Mean Square Error = 1.809). Hence, other levels at 140 and 160 have a positive impact towards the radical scavenging activity. Changes in all levels of X3 significantly affected the reducing power of the extracts. However, only the highest level (+ 1) of X1 (180 °C) and the lowest level of X2 (5 min) significantly affected the FRAP for the three different solvents (Mean Square Error = 0.001). The increase of preheating time to 30 min had a positive effect on the DPPH for only Me + Ac and Ac80 (p = 0.000), while methanol had a negative effect on the radical scavenging activity on prolonged exposure (Fig. 1B). It was observed that the temperature increase is more advantageous for the Me70 solvent compared to the other two solvents (Me + Ac and Ac80) at the point of view of radical scavenging activity (Fig. 1B). For FRAP, increasing the preheating temperature was positive for all the three solvents (Fig. 1C). Preheating time affected the hemp extracts differently for reducing power of the extracts, as the longer preheat treatment (30 min) had a significant effect (p > 0.05) for the Me + Ac solvent at both 140 and 160 °C whereas at 180 °C pre-treatment temperature there was no significant difference. Babiker et al. (2021) observed increases in the TPC of ethanolic extracts when heating hemp seeds for 21 min at 160 °C. Consequently, the antioxidant activity also increased from 18.37 ± 0.01 to 33.08 ± 0.01% of DPPH inhibition for the non-treated and the roasted hemp seeds, respectively.
Moreover, the results obtained for TPC, DPPH, FRAP and TFC of the preheated hemp cake extracts were implicated by high (> 95%) coefficients of variance: R2 = 0.979, 0.946, 0.975 and 0.992, respectively.
Conclusion
These results found that the highest amounts of TPC, TFC, and antioxidant activities were determined by the extracting solvents and the preheating temperatures. TPC, TFC and FRAP were significantly (p < 0.05) affected by the temperature (X1), and the extracting solvent (X3). The highest levels of TPC were obtained at 180 °C and Ac80. For DPPH, both the extracting solvent and pre-treatment temperature had a significant (p < 0.05) effect. Of the three solvents examined, 80% (v/v) aqueous acetone demonstrated the optimum radical scavenging activity as well as the highest level of TPC. Higher temperatures during the preheating treatments of the hemp cakes had a positive impact by increasing the amounts of extractable phenolic compounds. Optimization of the ultrasonic extraction of hemp phenolic compounds was determined by the post-hoc analysis and the calculation of the mean highest values obtained for TPC, DPPH, FRAP and TFC. The optimum conditions established in the present study produced extracts rich in phenolic compounds that exhibited the highest antioxidant activity based on reducing power was observed when preheated at a temperature of 180 °C for 15 min and the highest radical activity was observed at 140 °C for 30 min using aqueous acetone (80%, v/v) as the extracting solvent.
References
Azadfar M, Gao A, Bule M, Chen S (2015) Structural characterization of lignin: a potential source of antioxidants guaiacol and 4-vinylguaiacol. Int J Biol Macromol 75:58–66
Azadmard-Damirchi S, Habibi-Nodeh F, Hesari J, Nemati M, Achachlouei BF (2010) Effect of pretreatment with microwaves on oxidative stability and nutraceuticals content of oil from rapeseed. Food Chem 121:1211–1215
Babiker EE, Uslu N, Juhaimi FA, Mohamed Ahmed IA, Ghafoor K, Özcan MM, Almusallam IA (2021) Effect of roasting on antioxidative properties, polyphenol profile and fatty acids composition of hemp (Cannabis sativa L.) seeds. LWT 139:110537
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76
Callaway J, Schwab U, Harvima I, Halonen P, Mykkänen O, Hyvönen P, Järvinen T (2005) Efficacy of dietary hempseed oil in patients with atopic dermatitis. J Dermatol Treat 16:87–94
Canada Hemp Business, Legalization and Opportunities, New Regulations [Internet document] (2021). https://cannabusinessplans.ca/canada-hemp-business-legalization-opportunities/. Accessed 01 April 2021
Canadian Hemp Trade Alliance (2021) https://www.hemptrade.ca/. Accessed 01 Apr 2021
Chen T, Hao J, He J, Zhang J, Li Y, Liu R, Li L (2013) Cannabisin B induces autophagic cell death by inhibiting the AKT/mTOR pathway and S phase cell cycle arrest in HepG2 cells. Food Chem 138:1034–1041
Dai J, Mumper RJ (2010) Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 15:7313–7352
Do QD, Angkawijaya AE, Tran-Nguyen PL, Huynh LH, Soetaredjo FE, Ismadji S, Ju YH (2014) Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J Food Drug Anal 22:296–302
Frassinetti S, Moccia E, Caltavuturo L, Gabriele M, Longo V, Bellani L, Giorgi G, Giorgetti L (2018) Nutraceutical potential of hemp (Cannabis sativa L.) seeds and sprouts. Food Chem 262:56–66
Khattab RY, Eskin MNA, Thiyam-Hollander U (2014) Production of canolol from canola meal phenolics via hydrolysis and microwave-induced decarboxylation. J Am Oil Chem Soc 91:89–97
Kraljić K, Brkan V, Škevin D, Srček VG, Radošević K (2019) Canolol dimer, a biologically active phenolic compound of edible rapeseed oil. Lipids 54:189–200
Li J, Guo Z (2016) Concurrent extraction and transformation of bioactive phenolic compounds from rapeseed meal using pressurized solvent extraction system. Ind Crops Prod 94:152–159
Liang J, Zago E, Nandasiri R, Khattab R, Eskin NAM, Eck P, Thiyam-Holländer U (2018) Effect of solvent, preheating temperature, and time on the ultrasonic extraction of phenolic compounds from cold-pressed hempseed cake. JAOCS J Am Oil Chem Soc 95:1319–1327
Nagarkatti P, Pandey R, Rieder SA, Hegde VL, Nagarkatti M (2009) Cannabinoids as novel anti-inflammatory drugs. Future Med Chem 1:1333–1349
Nandasiri R, Eskin NAM, Thiyam-Höllander U (2019) Antioxidative polyphenols of canola meal extracted by high pressure: impact of temperature and solvents. J Food Sci 84:3117–3128
Nandasiri R, Eskin NAM, Komatsu E, Perreault H, Thiyam-Holländer U (2020) Valorization of canola by-products: concomitance of flavor-active bitter phenolics using pressurized heat treatments. LWT 138:110397
Oak MH, Bedoui JE, Schini-Kerth VB (2005) Antiangiogenic properties of natural polyphenols from red wine and green tea. J Nutr Biochem 16:1–8
Oomah BD, Kenaschuk EO, Mazza G (1995) Phenolic acids in flaxseed. J Agric Food Chem 43:2016–2019
Oomah BD, Busson M, Godfrey DV, Drover JC (2002) Characteristics of hemp (Cannabis sativa L.) seed oil. Food Chem 76:33–43
Pallant J (2011) SPSS survival manual: a step by step guide to data analysis using SPSS version 18, 4th edn. Open University Press, McGraw-Hill Education, Maidenhead
Pojić M, Mišan A, Sakač M, Hadnađev D, Tamara Šarić B, Milovanović I, Hadnađev M (2014) Characterization of byproducts originating from hemp oil processing. J Agric Food Chem 62:12436–12442
Rezaie M, Farhoosh R, Iranshahi M, Sharif A, Golmohamadzadeh S (2015) Ultrasonic-assisted extraction of antioxidative compounds from Bene (Pistacia atlantica subsp. mutica) hull using various solvents of different physicochemical properties. Food Chem 173:577–583
Sakakibara I, Ikeya Y, Hayashi K, Mitsuhashi H (1992) Three phenyldihydronaphthalene lignanamides from fruits of Cannabis sativa. Phytochemistry 31:3219–3223. https://doi.org/10.1016/0031-9422(92)83479-I
Siger A, Józefiak M (2016) The effects of roasting and seed moisture on the phenolic compound levels in cold-pressed and hot-pressed rapeseed oil. Eur J Lipid Sci Technol 118:1952–1958
Siger A, Nogala-Kalucka M, Lampart-Szczapa E (2008) Compounds in cold-pressed plant oils. J Food Lipids 15:137–149
Suriyarak S, Gibis M, Schmidt H, Villeneuve P, Weiss J (2014) Antimicrobial mechanism and activity of dodecyl rosmarinate against Staphylococcus carnosus LTH1502 as influenced by addition of salt and change in pH. J Food Prot 77:444–452
Teh S-S, Bekhit AE-D, Birch J (2014) Antioxidative polyphenols from defatted oilseed cakes: effect of solvents. Antioxidants (basel, Switzerland) 3:67–80
Thiyam U, Stöckmann H, Felde TZ, Schwarz K (2006) Antioxidative effect of the main sinapic acid derivatives from rapeseed and mustard oil by-products. Eur J Lipid Sci Technol 108:239–248
Vonapartis E, Aubin MP, Seguin P, Mustafa AF, Charron JB (2015) Seed composition of ten industrial hemp cultivars approved for production in Canada. J Food Compos Anal 39:8–12
Zago E, Lecomte J, Barouh N, Aouf C, Carré P, Fine F, Villeneuve P (2015) Influence of rapeseed meal treatments on its total phenolic content and composition in sinapine, sinapic acid and canolol. Ind Crops Prod 76:1061–1070
Acknowledgements
University of Manitoba’s GETS scholarship for Ruchira Nandasiri and University of Manitoba’s postdoctoral fellowship for Dr. Erika Zago is kindly acknowledged. In addition, we would like to acknowledge Dr. Rasheda Rabbani, Faculty Biostatistician, Department of George & Fay Yee Centre for Healthcare Innovation (CHI), Community Health Sciences (CHS), University of Manitoba is kindly acknowledged for her statistical advice.
Funding
This project was funded by NSERC Discovery Grant RGPIN‐2015‐03809. The grant holder, Dr. Usha Thiyam‐Holländer, sadly passed away on December 24th, 2020 and this publication is one several manuscripts submitted after her death. Dr. Michael Eskin was a close collaborator of Dr. Thiyam-Holländer.
Author information
Authors and Affiliations
Contributions
Dr. EZ designed the study, conducted the statistical analysis and drafted the manuscript. Both Dr. EZ and Dr. RN performed experiments, and interpreted and reviewed the results. Dr. ME, Dr. EZ and Dr. RN reviewed and approved the final version of the manuscript. Dr. UT-H conceived the research idea and funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Zago, E., Nandasiri, R., Thiyam-Holländer, U. et al. Influence of thermal treatments on the antioxidant activity of hemp cake polar extracts. J Food Sci Technol 59, 3256–3265 (2022). https://doi.org/10.1007/s13197-021-05325-9
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-021-05325-9