Abstract
Preclinical studies have shown a potential osteoanabolic effect of metformin but human studies of how metformin affects bone turnover are few. A post hoc sub-study analysis of an 18-month multicenter, placebo-controlled, double-blinded trial in type 2 diabetes mellitus (T2DM), randomizing participants to metformin versus placebo both in combination with different insulin analogue regimens (Metformin + Insulin vs. Placebo + Insulin). Patients were not treatment naive at baseline, 83% had received metformin, 69% had received insulin, 57.5% had received the combination of metformin and insulin before entering the study. Bone formation and resorption were assessed by measuring, N-terminal propeptide of type I procollagen (P1NP) and C-terminal telopeptide of type I collagen (CTX) at baseline and end of study. The influence of gender, age, smoking, body mass index (BMI), T2DM duration, glycosylated hemoglobin A1c (HbA1c), c-reactive protein (CRP) and insulin dosage was also included in the analyses. The levels of bone formation marker P1NP and bone resorption marker CTX increased significantly in both groups during the trial. P1NP increased less in the Metformin + Insulin compared to the placebo + insulin group (p = 0.001) (between group difference change), while the increases in CTX levels (p = 0.11) were not different. CRP was inversely associated (p = 0.012) and insulin dosage (p = 0.011) was positively related with change in P1NP levels. BMI (p = 0.002) and HbA1C (p = 0.037) were inversely associated with change in CTX levels. During 18 months of treatment with metformin or placebo, both in combination with insulin, bone turnover increased in both groups. But the pattern was different as the bone formation marker (P1NP) increased less during Metformin + Insulin treatment, while change in bone resorption (CTX) was not significantly different between the two groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with type 2 diabetes mellitus (T2DM) have an increased risk of fractures [1, 2]. Hyperglycemia, hyperinsulinemia, adipokines, inflammation, mechanical loading, accelerated aging with increased oxidative stress, advanced glycation end products (AGEs) and microvascular disease have all been suggested to play a part in the pathophysiology of diabetic bone disease [3]. In most studies, but not in all, a reduced bone turnover is reported in people with T2DM [4] which might play a role in the observed increased bone fragility. Because of the link between T2DM and increased bone fragility, as well as the damaging effect of hyperglycemia on the skeleton, antihyperglycemic drugs could affect bone both directly and indirectly by improving glycemic control.
The results regarding a direct effect of insulin on bone cells have been contradictory [5, 6]. Animal studies have indicated that insulin stimulates osteoblast proliferation and bone formation, but in vitro studies have also reported that insulin signaling in osteoblasts promotes bone resorption by osteoclasts. A higher fracture risk has been found in patients with T2DM treated with insulin, compared to patients treated with oral antidiabetic agents [5, 6]. It is unclear whether the increased risk of fractures is due to the insulin treatment itself, longer disease duration, higher complication rate, traumatic falls due to hypoglycemic episodes associated with insulin treatment or something else. One large case–control study showed a trend towards a lower fracture risk with insulin use in a combined group of people with type 1 and 2 diabetes [7]. A stabilization in bone mineral density (BMD) with intensive insulin treatment has been reported in type 1 diabetes [8], but in T2DM some studies have also found a decrease in BMD during insulin treatment [9, 10].
In vitro studies suggest that Metformin affects bone metabolism by a promotion of osteoblast differentiation and mineralization and reduction in osteoclast formation [3, 5]. Clinical observational and case–control studies on the effect of metformin on fracture risk reports mixed results, but several studies point to a beneficial effect with reduced risk of fractures [5, 7]. Studies on the effect of metformin on bone turnover are few and point towards a reduction in turnover [5]. Taken together these observations suggest that metformin may be advantageous, but the mechanism is unresolved. Bone turnover markers are fairly easy to obtain from serum or urine and are indicative of bone formation and resorption rates [3].
The purpose of the present study was to examine this by studying markers of bone formation and resorption, the hypothesis being that the difference between those treated with metformin plus insulin and those only treated with insulin, would be an increase in markers of bone formation.
Several common diabetes specific risk factors for fractures have been suggested, especially in regard to the management of bone fragility in patients with diabetes such as diabetes duration, some diabetes medications, level of HbA1c and microvascular complications [11]. Therefore, we also had the opportunity to explore the influence of glycemic control, metformin treatment, insulin treatment, interaction between metformin and insulin, inflammation and several other potential risk factors on changes in the separate markers. We expected a potential bone anabolic effect of insulin and metformin. The study specifically addressed the interaction between the two drugs.
Materials and Methods
This sub-study used data and blood samples from a multicenter, randomized, placebo-controlled, blinded trial (the Copenhagen Insulin and Metformin Therapy (CIMT) Trial). The primary purpose of this trial was to study effects of metformin or placebo in combination with insulin on progression of atherosclerosis as measured by Carotis Intima Media Thickness [12,13,14]. Approval by the Regional Committee on Biomedical Research Ethics (region of Copenhagen journal number H–D-2007–112), and the Danish Medicines Agency (journal number 2612–3648) was obtained, and the trial was reported to the Danish Data Protection Agency (NOH-2015–034) and registered with ClinicalTrials.gov (NCT00657943). All participants provided a written informed consent and the trial was conducted in accordance with the Helsinki Declaration and guidelines of Good Clinical Practice.
Eight Danish hospitals in the Capital Region of Denmark took part in the recruitment of participants. Patients were included if they had T2DM, body mass index (BMI) 25–40 kg/m2, HbA1c ≥ 7.5% (≥ 58 mmol/mol), were treated with oral antihyperglycemic drugs for ≥ 1 year and/or insulin treatment for ≥ 3 months and were older than 30 years of age. Exclusion criteria were recent cardiovascular disease (previous myocardial infarction, stroke, coronary or vascular surgery), cancer, renal or liver disease, and other chronic diseases as determined in the original study protocol [12], as well as pregnant or breastfeeding women and fertile women not using oral contraceptives.
A computer-generated allocation sequence performed the central randomization, stratified by treatment center, age (> 65 years) and prior insulin treatment. The options were 1:1 randomization to 18 months blinded treatment with metformin or placebo, in addition with randomization 1:3 to three different open labeled insulin regimens: basal insulin, biphasic insulin or basal-bolus insulin [14]. Prior antihyperglycemic therapy was discontinued at trial entry. Previously metformin treated participants (81–85%) initiated a dose of metformin or placebo of 1 g twice daily and metformin naïve participants were titrated up weekly from an initial dose of 500 mg once daily to 1 g twice daily. Insulin dose was titrated aiming at an HbA1c ≤ 7% (≤ 53 mmol/mol). A detailed description of the design of the trial and outcomes has previously been published [13].
Fasting morning blood samples were collected at trial entry, before starting treatment with metformin or placebo and one of the three insulin regimens, and again after 18 months and initially analyzed as part of the CIMT trial. Blood was also collected and frozen for later use. Participants that discontinued the trial were invited to take part in collection of blood samples at end of trial. The reasons for drop-outs in the original CIMT trial have previously been described [13]. The primary focus of this sub-study was to measure P1NP and CTX concentrations in the previously collected samples. In addition, C-reactive protein (CRP) was analyzed as part of this sub-study.
The associations between CRP, BMI, duration of T2DM, HbA1c and insulin treatment with P1NP and CTX were explored for the whole study population.
Serum Analyses
Serum P1NP was measured using the IDS-iSYS intact P1NP assay (Immunodiagnostic Systems). Serum CTX was measured using the IDS-iSYS CTX (CrossLaps®) assay (Immunodiagnostic Systems, plc, Tyne and Wear, UK). Serum C-reactive protein (CRP) was measured on the Vitros 5.1 FS instrument (Ortho Clinical Diagnostics, Raritan, NJ, USA) using an immunoturbidimetric method.
All analyses were done with serum as the sample material. For each assay the sample aliquots were kept frozen at − 80 °C until the day of analysis. None of the samples had previously been thawed, and all analyses were performed immediately after thawing the samples. All samples were analyzed using one single batch of each assay. Assay performance was verified using the manufacturers’ control specimens. The intermediary precisions expressed as coefficients of variation for P1NP were 5.4% (18.96 µg/L), 6.5% (48.48 µg/L), and 6.1% (122.10 µg/L) and for CTX they were 5.3% (at CTX concentration 213 ng/L), 3.4% (869 ng/L), and 3.5% (2,113 ng/L). For CRP the intermediary precisions were 10% (at CRP concentration 0.8 mg/L) and 6% (23 mg/L).
Vitamin D was measured as part of the original sample analysis. The sample material was serum. Total 25(OH)D (25-hydroxy vitamin D3 plus 25-hydroxy vitamin D2) was measured with an electro-chemiluminescence immunoassay (ECLIA) competitive assay using the Cobas e411, on Roche equipment based on a one-step sandwich assay. All samples were measured in one batch using identical batch numbers. The coefficients of variation (CV) for 25(OH)D was 6.4% and analytical sensitivity was 10.0 nmol/l.
Statistical Analysis
Participants who discontinued the trial medication but accepted the invitation for the last follow-up with collection of blood samples were analyzed according to the randomized treatment group. The intention-to-treat principle was used in the post hoc statistical analyses of this sub-study.
The unadjusted analysis of changes over time was done using paired samples t-test, while analyses between groups were done with the independent samples t-test/ANOVA analysis of the delta value of the dependent variable without the use of covariates.
Adjustment for relevant covariates; gender, age, vitamin D, smoking, BMI, duration of T2DM, CRP, HbA1c and insulin dose were part of the secondary analyses. The delta value of the dependent variable with an adjustment for baseline levels of named covariates was used in the ANOVA analyses.
Exploratory analysis of the associations between CRP, BMI, duration of T2DM, HbA1c and insulin treatment with P1NP and CTX was done using a mixed effect regression model. This analysis considers the fact that baseline and endpoint values are related and therefore can include both, for the dependent variables and covariates. In addition to the named variables also gender and age were included in this model.
Histograms and Q–Q plots were used to test for normal distribution of data. Both raw data and residuals for the delta values used in unadjusted and adjusted analyses were normally distributed. The residuals in the explorative analysis especially regarding CTX did not have a normal distribution but analysis using log transformed values yielded the same significant p-values. Therefore, we chose to display the associations as changes in ng/l instead of percentage change. To test if the baseline characteristics were comparable student’s t-test and Chi-square test/ Fisher’s exact test (fewer than 5 observations) were used.
In the supplementary material we present sub-analyses on the subgroup of patients that were either metformin naïve (15–19%) or insulin naive (app 35%) at baseline, but not necessarily treatment naive. The metformin naïve group is therefore showing the effect of adding metformin to insulin and the insulin naïve group is showing the effect of adding insulin to metformin. Due to smaller subgroups of patients in these sub-analyses the baseline levels of P1NP differed in the metformin group compared with the placebo group especially in the metformin naïve subgroup (p-value 0.015). Therefore, the analysis of changes between the groups was compared using ANCOVA with use of baseline levels of the outcome as a covariate in addition to the above mentioned covariates.
IBM SPSS statistics 22 and SAS Enterprise 7.1 were used for the statistical analyses. Values of p < 0.05 (two-sided) were considered significant.
Results
Trial Participants and Baseline Characteristics
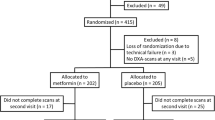
Of the 464 screened patients, 415 were found eligible for participation. Due to technical failure the randomization code was lost for three participants and they were excluded. 206 participants were allocated to metformin in combination with insulin and 206 were allocated to placebo in combination with insulin (Fig. 1). For reasons published elsewhere [13] 31 participants discontinued the intervention during the trial in the metformin group of which 15 participated in the last visit. In the placebo group 49 discontinued the intervention of which 28 returned for the last visit, however, two of these did not have blood samples taken. Thereby, 190 (92%) had blood samples taken at the last follow up in the metformin group and 183 (89%) in the placebo group (Fig. 1).
Baseline characteristics are described in Table 1. 83% had received metformin, 69% had received insulin, 57.5% had received the combination of metformin and insulin before entering the study. The two randomization groups were comparable at baseline and had a mean age of 60.7 years with a predominance of male and post-menopausal female participants. Six had prior fractures and five were treated for osteoporosis with bisphosphonates. At end of trial the insulin doses were 102 vs 138 units per day, increase in body weight 1.6 kg vs. 4.2 kg and HbA1c was reduced to 7.97 vs 8.27% in the metformin and placebo groups, respectively [13].
Changes in P1NP and CTX (Tables 2 and 3)
In the unadjusted analyses (Table 2) the P1NP increase was less pronounced in the metformin + insulin group (3.7 µg/l [95% CI 1.5, 5.9], p < 0.001) compared with the placebo + insulin group (9.0 µg/l [95% CI 6.8, 11.3], p < 0.001) with a group difference of − 5.4 µg/l [95% CI − 8.5, − 2.2], p < 0.001. The CTX also increased in the metformin + insulin group (21 ng/l [95% CI 2, 41], p = 0.033) and placebo + insulin group (44 ng/l [95% CI 25, 64], p < 0.001) but with no significant between group difference (p = 0.11). Adjusting for baseline levels of gender, age, vitamin D, smoking, BMI, type 2 diabetes duration, HbA1c, CRP and insulin dose in the secondary analyses did not change the results significantly (Table 3).
Results of Explorative Analysis (Table 4)
CRP and insulin dosage were significantly associated with P1NP but not CTX levels. For every increase in CRP (mg/l) or insulin dosage (IU) by 1 unit the P1NP decreased by − 0.1 µg/l [95% CI − 0.2, − 0.03], p = 0.012 and 0.03 µg/l [95% CI 0.01, 0.05], p = 0.011, respectively. BMI and HbA1c were associated with CTX, but not P1NP levels. Every increase in BMI (kg/m2) or HbA1c (%) resulted in a decrease in CTX of − 5 ng/l [95% CI − 8, − 2], p = 0.002 and − 10 ng/l [95% CI − 19, − 1], p = 0.037, respectively. Smoking and the duration of T2DM were not associated with any of the two bone markers.
Sex and age were associated with both bone markers. Women had 6.4 µg/l [95% CI 3.5, 9.3] higher P1NP and 69 ng/l [95% CI 41, 97] higher CTX levels than the men (p < 0.001). For every 1-year increase in age P1NP levels decreased by − 0.2 [95% CI − 0.3, − 0.001], p = 0.049, and CTX levels decreased by − 2.3 [95% CI − 4, − 0.7], p = 0.004.
Changes in P1NP and CTX in Metformin or Insulin Naïve Participants (Supplementary Material Tables 5–8)
In total 68 of the participants were metformin naïve before trial entry and 39 of these were randomized to receive metformin + insulin and 29 to receive placebo + insulin. P1NP levels did not change significantly in either group during the 18 months follow up. CTX levels decreased by 69 ng/l [95% CI 23, 114], p < 0.001, in the metformin + insulin group and did not change significantly in the placebo + insulin group, with a between group difference of 90 ng/l [95% CI 20, 160], p = 0.012 (Online Table 5). After adjusting for baseline levels of gender, age, vitamin D, smoking, BMI, duration of T2DM, HbA1c, CRP and insulin dose the observed changes in CTX were no longer significant (Online Table 6).
In total 140 of the participants were insulin naïve prior to trial entry and 72 of these were randomized to metformin + insulin and 68 to placebo + insulin. In the analyses only adjusted for baseline of the outcome (Online Table 7) P1NP and CTX levels increased significantly in the placebo + insulin group (P1NP: 13.3 µg/l [95% CI 10.1, 16.5], p-value < 0.001, CTX: 65 ng/l [95% CI 37, 92], p-value < 0.001) with significant between group difference for both P1NP (11.0 µg/l [95% CI 6.5, 15.5], p-value < 0.001) and CTX (57 ng/l [95% CI 12, 84], p-value < 0.001).
Adjusting further for gender, age, vitamin D, smoking, BMI, duration of T2DM, HbA1c, CRP and insulin dose (Online Table 8) did not change the results significantly except for the difference between the intervention groups difference for CTX (55 ng/l [95% CI 15, 95], p-value 0.007).
Discussion
Both P1NP and CTX increased during the 18 months treatment with metformin vs placebo, both in combination with insulin. The increase in P1NP was significantly more pronounced in the placebo + insulin group compared with the metformin + insulin group, while the group difference in CTX was not significant. After 18 months follow-up the insulin dose, weight gain and HbA1c were lower in the metformin group compared with the placebo group.
Preclinical studies suggest that metformin activates the osteoblast-specific Runx2 (runt-related transcription factor 2) transcription factor via the AMPK/USF-1/SHP regulatory cascade and thereby promotes osteoblast differentiation but has the opposing effect on osteoclast differentiation by decreasing pro-osteoclastic cytokine receptor activator of nuclear factor κB ligand (RANKL) and increasing osteoprotegerin levels [15, 16]. This, combined with a tendency of reduced fracture risk in people treated with metformin [5, 7], would lead us to expect increases in bone formation (P1NP) or/and decreases or no change in bone resorption (CTX). We observed, however, that the increase in P1NP was lower in the metformin + insulin group compared with the placebo + insulin group after 18 months treatment.
A randomized trial comparing short- and long-acting insulin in combination with metformin, rosiglitazone or placebo also found a lower P1NP in the metformin treated group compared with placebo treated group, with no difference in CTX between the groups [17]. Similarly, we could not show a significant group difference regarding CTX. Although approximately 80% of the participants were treated with metformin before trial entry, analysis including only the metformin naïve participants prior to study entry did show a significant between group difference for CTX in the unadjusted model. In the insulin naïve participants prior to the start of trial, we found a significant between group difference for both P1NP and CTX. These analyses including only metformin or insulin naïve participants should be interpreted with caution as they include few participants (especially metformin naïve participants) and represent selected groups and therefore might introduce selection bias and loss of proper randomization. Studies comparing metformin treatment with other oral antidiabetic medications in people with T2DM have found decreases in both P1NP and CTX in their metformin treated groups over time [18, 19]. We found a reduced change from baseline to 18 months in P1NP in the metformin + insulin group as compared with the placebo-insulin group. Although not significant, the difference in baseline to 18 months change in CTX between the metformin + insulin group and the placebo-insulin group was almost of the same magnitude as for P1NP. Thereby, our study does not suggest an anabolic effect of metformin when assessed by P1NP in humans, but it agrees with the mentioned clinical trials and studies showing a lower bone turnover.
The increase in P1NP and CTX in all participants irrespective of randomization group as well as analyses performed in insulin naïve participants prior to study entry might be attributed to the underlying insulin treatment in both groups. Notable, the insulin dose was significantly higher in the participants treated with placebo. A generally lower bone turnover, with reduced bone formation and bone resorption markers, has been found in people with diabetes compared to controls [4, 20]. P1NP levels both at baseline and after 18 months were close to the mean levels found in a Danish reference interval study, while CTX levels at baseline were in the lower end of the normal reference interval and at end of study they were closer to the mean [21]. It is difficult to determine if the increase in P1NP and CTX represents a normalization of bone marker levels with insulin treatment, or an increase in bone turnover, but considering the mentioned reference levels the results could to some extent be interpreted as a move towards normalization of CTX. It is unclear to which degree the increases in markers could be due to insulin treatment in the two groups, but insulin dosage was positively associated with P1NP levels. However, BMI was inversely associated with CTX levels, and it can be speculated that the weight gain associated with insulin treatment may play a role in moderating increases in CTX levels. The negative association of HbA1c with CTX levels could also be interpreted as indirect effects of insulin on bone resorption though improved glycemic control. A recent study in people with T2DM reported that HbA1c was negatively associated with CTX but not P1NP levels suggesting that lowering glucose levels with antidiabetic treatment might affect bone formation differently from bone resorption [17]. The association between HbA1c and P1NP levels in our study did show the same tendency towards a negative association as with CTX levels. In another study, comparing bone turnover markers in people with type 1 and 2 diabetes non-fasting blood glucose levels were negatively associated with both P1NP and CTX levels. HbA1c was also negatively associated with osteocalcin, but not other markers, however, the same tendency was shown for CTX and P1NP levels [22]. How glycemic control affects bone resorption and formation remains to be determined. Some [23, 24] but not all studies [7] have suggested an increased fracture risk associated with the use of insulin. In the present study, all patients were exposed to insulin, and therefore, the present study cannot add new information as to whether insulin may have disadvantageous effects in relation to fracture risk.
A high level of hs-CRP has been found to be predictive of incident fractures. Although the mechanism of actions is not fully understood, it is suggested that cytokines increase the activation of osteoclasts by increasing the expression of RANKL and macrophage colony-stimulating factor (M-CSF) and decreasing osteoprotegerin production [25]. Thus, one would expect markers of inflammation as CRP to be associated with CTX levels, but we find that CRP is negatively associated with P1NP levels, suggesting that inflammation may also affect osteoblast activity.
Strengths
The data and materials used in this sub-study come from the CIMT trial which is a randomized, placebo-controlled blinded design with 18 months follow-up. Recruitment of a substantial number of participants from several hospitals in a defined geographical area is also a strength. Inclusion of gender, age, vitamin D, smoking, BMI, duration of T2DM, HbA1c and insulin treatment made it possible to give a more complete picture of the effects on bone turnover assessed with P1NP and CTX.
Limitations
A limitation is this being a post hoc analyses to a previously performed trial designed to evaluate a different primary outcome. CTX was used to evaluate the effects on bone resorption. But in view of increased collagen glycation in diabetes [3] interpretational differences might exist with use of collagen based turnover markers compared to measures in people without diabetes. About 80% of the participants were treated with metformin and about 65% were treated with insulin before the trial started, and the results could have been different if drug-naive participants had been included. Both randomization groups aimed for an HbA1c ≤ 7% (≤ 53 mmol/mol) which resulted in a difference in insulin dosage (Metformin + Insulin, 102 [95% CI 91–115] IU/day, and Placebo + Insulin, 138 [95% CI 123–155] IU/day and weight gain in the two groups [13]. The study did not regulate supplement use, e.g., calcium and vitamin D, or treatment with other medications which could affect bone remodeling. The participants were predefined as > 30 years and BMI of 25 to 40 kg/m2 and the results can therefore not be extrapolated to metformin treated patients with normal weight.
Conclusion
In conclusion, we find that during 18 months of treatment with metformin or placebo, both in combination with insulin, bone turnover increased in both groups. But the pattern was different, as bone formation (P1NP) increased less during the metformin treatment, while the group difference change in bone resorption (CTX) was not significant, but of a similar magnitude. Insulin combined with metformin may possess bone anabolic properties larger than metformin alone. BMI and HbA1c may influence resorption activity while insulin dosage and CRP may also influence bone formation activity.
References
Janghorbani M, Van Dam RM, Willett WC, Hu FB (2007) Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol 166:495–505
Vestergaard P (2007) Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes–a meta-analysis. Osteoporos Int 18:427–444
Shanbhogue VV, Mitchell DM, Rosen CJ, Bouxsein ML (2016) Type 2 diabetes and the skeleton: new insights into sweet bones. Lancet Diabetes Endocrinol 4:159–173
Starup-Linde J, Vestergaard P (2016) Biochemical bone turnover markers in diabetes mellitus—a systematic review. Bone 82:69–78
Chandran M (2017) Diabetes Drug Effects on the Skeleton. Calcif Tissue Int 100:133–149
Schwartz AV (2017) Diabetes, bone and glucose-lowering agents: clinical outcomes. Diabetologia 60:1170–1179
Vestergaard P, Rejnmark L, Mosekilde L (2005) Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia 48:1292–1299
Campos Pastor MM, Lopez-Ibarra PJ, Escobar-Jimenez F, Serrano Pardo MD, Garcia-Cervigon AG (2000) Intensive insulin therapy and bone mineral density in type 1 diabetes mellitus: a prospective study. Osteoporos Int 11:455–459
Dutta M, Pakhetra R, Garg M (2012) Evaluation of bone mineral density in type 2 diabetes mellitus patients before and after treatment. Med J Armed Forces India 68:48–52
Ruppert K, Cauley J, Lian Y, Zgibor JC, Derby C, Solomon DH (2018) The effect of insulin on bone mineral density among women with type 2 diabetes: a SWAN Pharmacoepidemiology study. Osteoporos Int 29:347–354
Ferrari SL, Abrahamsen B, Napoli N, Akesson K, Chandran M, Eastell R, El-Hajj Fuleihan G, Josse R, Kendler DL, Kraenzlin M, Suzuki A, Pierroz DD, Schwartz AV, Leslie WD, Bone, and Diabetes Working Group of IOF (2018) Diagnosis and management of bone fragility in diabetes: an emerging challenge. Osteoporos Int 29:2585–2596
Lundby Christensen L, Almdal T, Boesgaard T, Breum L, Dunn E, Gade-Rasmussen B, Gluud C, Hedetoft C, Jarloev A, Jensen T, Krarup T, Johansen LB, Lund SS, Madsbad S, Mathiesen E, Moelvig J, Nielsen F, Perrild H, Pedersen O, Roeder M, Sneppen SB, Snorgaard O, Tarnow L, Thorsteinsson B, Vaag A, Vestergaard H, Wetterslev J, Wiinberg N, CIMT Trial Group (2009) Study rationale and design of the CIMT trial: the Copenhagen Insulin and Metformin Therapy trial. Diabetes Obes Metab 11:315–322
Lundby-Christensen L, Tarnow L, Boesgaard TW, Lund SS, Wiinberg N, Perrild H, Krarup T, Snorgaard O, Gade-Rasmussen B, Thorsteinsson B, Roder M, Mathiesen ER, Jensen T, Vestergaard H, Hedetoft C, Breum L, Duun E, Sneppen SB, Pedersen O, Hemmingsen B, Carstensen B, Madsbad S, Gluud C, Wetterslev J, Vaag A, Almdal TP (2016) Metformin versus placebo in combination with insulin analogues in patients with type 2 diabetes mellitus-the randomised, blinded Copenhagen Insulin and Metformin Therapy (CIMT) trial. BMJ Open 6(e008376):2015–008376
Lundby-Christensen L, Vaag A, Tarnow L, Almdal TP, Lund SS, Wetterslev J, Gluud C, Boesgaard TW, Wiinberg N, Perrild H, Krarup T, Snorgaard O, Gade-Rasmussen B, Thorsteinsson B, Roder M, Mathiesen ER, Jensen T, Vestergaard H, Hedetoft C, Breum L, Duun E, Sneppen SB, Pedersen O, Hemmingsen B, Carstensen B, Madsbad S (2016) Effects of biphasic, basal-bolus or basal insulin analogue treatments on carotid intima-media thickness in patients with type 2 diabetes mellitus: the randomised Copenhagen Insulin and Metformin Therapy (CIMT) trial. BMJ Open 6(e008377):2015–008377
McCarthy AD, Cortizo AM, Sedlinsky C (2016) Metformin revisited: Does this regulator of AMP-activated protein kinase secondarily affect bone metabolism and prevent diabetic osteopathy. World J Diabetes 7:122–133
Vianna AGD, Sanches CP, Barreto FC (2017) Review article: effects of type 2 diabetes therapies on bone metabolism. Diabetol Metab Syndr 9:75
Stage TB, Christensen MH, Jorgensen NR, Beck-Nielsen H, Brosen K, Gram J, Frost M (2018) Effects of metformin, rosiglitazone and insulin on bone metabolism in patients with type 2 diabetes. Bone 112:35–41
van Lierop AH, Hamdy NA, van der Meer RW, Jonker JT, Lamb HJ, Rijzewijk LJ, Diamant M, Romijn JA, Smit JW, Papapoulos SE (2012) Distinct effects of pioglitazone and metformin on circulating sclerostin and biochemical markers of bone turnover in men with type 2 diabetes mellitus. Eur J Endocrinol 166:711–716
Zinman B, Haffner SM, Herman WH, Holman RR, Lachin JM, Kravitz BG, Paul G, Jones NP, Aftring RP, Viberti G, Kahn SE, ADOPT Study Group (2010) Effect of rosiglitazone, metformin, and glyburide on bone biomarkers in patients with type 2 diabetes. J Clin Endocrinol Metab 95:134–142
Hygum K, Starup-Linde J, Harslof T, Vestergaard P, Langdahl BL (2017) Mechanisms in endocrinology: diabetes mellitus, a state of low bone turnover - a systematic review and meta-analysis. Eur J Endocrinol 176:R137–R157
Jorgensen NR, Mollehave LT, Hansen YBL, Quardon N, Lylloff L, Linneberg A (2017) Comparison of two automated assays of BTM (CTX and P1NP) and reference intervals in a Danish population. Osteoporos Int 28:2103–2113
Starup-Linde J, Lykkeboe S, Gregersen S, Hauge EM, Langdahl BL, Handberg A, Vestergaard P (2016) Differences in biochemical bone markers by diabetes type and the impact of glucose. Bone 83:149–155
Napoli N, Strotmeyer ES, Ensrud KE, Sellmeyer DE, Bauer DC, Hoffman AR, Dam TT, Barrett-Connor E, Palermo L, Orwoll ES, Cummings SR, Black DM, Schwartz AV (2014) Fracture risk in diabetic elderly men: the MrOS study. Diabetologia 57:2057–2065
Monami M, Cresci B, Colombini A, Pala L, Balzi D, Gori F, Chiasserini V, Marchionni N, Rotella CM, Mannucci E (2008) Bone fractures and hypoglycemic treatment in type 2 diabetic patients: a case-control study. Diabetes Care 31:199–203
Barbour KE, Cauley JA (2013) Measuring inflammatory marker levels to determine risk of bone loss and fractures in older women. MLO Med Lab Obs 45:8
Funding
Funding was obtained from Nordsjællands Hospital, the Jascha foundation, the Osteoporosis Association for patients, the foundation of A & J C Tvergaard, the foundation of Captain Lieutenant Harald Jensen and Wife, Inge and Per Refshalls research grant and Danish Bone Society travel grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Azra Nordklint reports grants from the Jascha foundation, grants from the Osteoporosis Association for patients, grants from the foundation of A & J C Tvergaard, grants from the foundation of Captain Lieutenant Harald Jensen and Wife, grants from Inge and Per Refshalls research grant, grants from Danish Bone Society travel grant, grants from Nordsjællands Hospital, during the conduct of the study, and otherwise has no conflicts of interest to declare. Pia Eiken is an advisory board member for Amgen and Eli Lilly, and on the speakers’ bureau for Amgen and Eli Lilly, and own shares in Novo Nordisk A/S. Louise Lundby Christensen owns shares in Novo Nordisk A/S. Thomas Almdal owns shares in Novo Nordisk A/S. Søren S. Lund has previously published his opinion about the clinical effects of metformin treatment. Søren S. Lund owns shares in Novo Nordisk A/S and shares in dynamically traded investment funds, which may own stocks from pharmaceutical companies. Søren S. Lund has reported former employment at Steno Diabetes Center, which is a diabetes hospital and academic institution previously owned by Novo Nordisk. Søren S. Lund is now employed at Boehringer Ingelheim International GmbH, Ingelheim, Germany. Søren S. Lund’s contribution was his alone and does not necessarily reflect the official position of Boehringer Ingelheim. Trine W. Boesgaard owns shares in Novo Nordisk A/S. Trine W. Boesgaard has reported former employment at Steno Diabetes Center, which is a diabetes hospital and academic institution previously owned by Novo Nordisk. Trine W. Boesgaard is now employed at Novo Nordisk. Trine W Boesgaard’s contribution was hers alone and does not necessarily reflect the official position of Novo Nordisk. Peter Vestergaard, Leif Breum, Birthe Gade-Rasmussen, Simone B Sneppen, Christian Gluud, Bianca Hemmingsen, Thure Krarup, Sten Madsbad, Elisabeth R Mathiesen, Hans Perrild, Lise Tarnow, Birger Thorsteinsson, Niklas Rye Jørgensen and Henrik Vestergaard have no known conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nordklint, A.K., Almdal, T.P., Vestergaard, P. et al. Effect of Metformin vs. Placebo in Combination with Insulin Analogues on Bone Markers P1NP and CTX in Patients with Type 2 Diabetes Mellitus. Calcif Tissue Int 107, 160–169 (2020). https://doi.org/10.1007/s00223-020-00711-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-020-00711-5