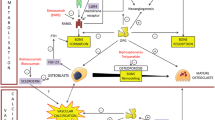
Abstract
Low circulating levels of undercarboxylated osteocalcin (ucOC) is associated with a higher risk of cardiovascular disease, yet whether ucOC has a direct effect on endothelium-dependent vasorelaxation, or in proximity to its postulated receptor, the class CG protein-coupled receptor (GPCR6A), in blood vessels remains unclear. Immunohistochemistry and proximity ligation assays were used to localize the presence of ucOC and GPRC6A and to determine the physical proximity (< 40 nm) in radial artery segments collected from patients undergoing coronary artery bypass surgery (n = 6) which exhibited calcification (determined by Von Kossa) and aorta from New Zealand white rabbits exhibiting atherosclerotic plaques. Endothelium-dependent vasorelaxation was assessed using cumulative doses of acetylcholine in vitro on abdominal aorta of rabbits fed a normal chow diet (n = 10) and a 4-week atherogenic diet (n = 9) pre-incubated with ucOC (10 ng/mL) or vehicle. Both ucOC and GPRC6A were localized in human and rabbit diseased-blood vessels. Proximity ligation assay staining demonstrated physical proximity of ucOC with GPRC6A only within plaques in rabbit arteries and the endothelium layer of rabbit arterioles. Endothelium-dependent vasorelaxation was impaired in atherogenic abdominal aorta compared to healthy aorta and ucOC attenuated this impairment. ucOC attenuated impaired endothelium-dependent vasorelaxation in rabbit abdominal aorta following an atherogenic diet, however, this effect may be independent of GPRC6A. It is important that future studies determine the underlying cellular mechanisms by which ucOC effects blood vessels as well as whether it can be used as a therapeutic agent against the progression of atherosclerosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Deterioration of endothelium-dependent vasorelaxation, also known as endothelial dysfunction, is the underlying cause of atherosclerosis, a risk factor for cardiovascular disease (CVD) [1]. CVD is the leading cause of death in the United States and Australia and the incidence continues to increase [2, 3]. Generally, the focus of CVD research is on the pathophysiological aspect of atherosclerosis by treating patients with anti-hyperlipidaemia and anti-inflammatory agents with slight improvements in reducing CVD over the years [4]. As such, novel therapeutic agents targeting endothelial dysfunction are required to further reduce CVD.
The skeleton is an endocrine organ that participates in energy metabolism and glucose homeostasis [5,6,7]. Osteocalcin (OC) in its undercarboxylated form (ucOC), regulates energy metabolism and glucose homeostasis in rodents [7, 8]. Most likely via it’s postulated receptor, the a class CG protein-coupled receptor 6A (GPCR6A) [9,10,11].
In humans, high serum ucOC levels correlate with lower glucose levels, HbA1c and higher insulin sensitivity [12,13,14,15]. In addition, patients undergoing coronary artery bypass grafting (CABG) have significantly lower ucOC compared to matched controls before and after adjusting for metabolic risk factors and renal function [16]. Furthermore, lower serum ucOC is correlated with higher common carotid artery intimal medial thickness in patients with carotid atherosclerotic plaques [17]. There might also be a possible beneficial effect of ucOC on reducing the progression of calcification. Several studies have determined that higher total osteocalcin (including ucOC) concentrations are associated with lower abdominal aortic calcification progression in elderly men [18] and in hypertensive patients with carotid artery calcification [19].
Taken together, these studies suggest that ucOC may have a role in atherosclerosis, however, the presence of ucOC and GPRC6A as well as their proximity interaction in diseased blood vessels is not clear. Likewise, whether ucOC has an impact on endothelium-dependent vasorelaxation of diseased and healthy blood vessels has not been elucidated. The aim of the current study was to test the following hypotheses (a) ucOC and GPRC6A are present in human and rabbit following atherogenic diet and, (b) ucOC administration ex vivo improves endothelial-dependent vasorelaxation.
Methods
Specimen Preparation
Human Arteries
Archived, paraffin-embedded, discarded segments of human radial arteries (n = 6) from patients undergoing CABG [20] were used in the current study. Only non-traumatized vessel segments were included in the studies. Harvesting of radial arteries was performed using techniques previously established in the operating theatres [21].
Rabbit Arteries
Male New Zealand white rabbits (3 months of age) were fed an atherogenic diet consisting of normal rabbit chow diet supplemented with 0.5% cholesterol plus 1% methionine plus 5% peanut oil (n = 12) for 4 weeks as approved by Victoria University Animal Ethics Committee AEC03/11 [22] that conforms with NIH guidelines for use of laboratory animals. The animals were housed in individual cages and maintained at a constant temperature of approximately 21 °C. Food and water were supplied ad libitum. The animals were euthanized by exsanguination during anaesthesia with 4% isoflurane. Coronary artery dysfunction is rare and difficult to induce in various animal models including rabbits, thus the abdominal aorta was utilized in this study [23]. The abdominal aorta from n = 3 rabbits fed an atherogenic diet that did not undergo pharmacological assessment of vascular function was cleaned of connective tissue and fat, cut into 3 mm rings, and fixed in 4% paraformaldehyde in phosphate-buffered saline overnight. The rings were then processed for paraffin, mounted in the same paraffin block as radial arteries to keep uniform cutting thickness and immunohistochemistry procedures consistent. The abdominal aorta from (n = 9) atherogenic rabbits as well as healthy rabbits (n = 10), fed a normal chow diet, were excised and cleaned of connective tissues for isometric tension studies in determining the effect of ucOC on endothelium-dependent vasorelaxation ex vivo.
Von Kossa (Calcification)
To determine calcium deposition in human and rabbit-diseased artery, tissue sections were deparaffinised, rehydrated and stained with an aqueous solution of 1% silver nitrate (Catalogue # 209139, Sigma, Australia) for 2 h under 100-W lamp. Sections were rinsed in 5% sodium thiosulfate (remove unreactive silver) for 5 min followed by standard hematoxylin and eosin stain (HES) protocol. The protocol was repeated in healthy rabbit artery (as calcification does not occur in healthy aorta) followed by HES protocol for a negative control.
Briefly, a standard HES was conducted in only rabbit artery where haematoxylin was incubated for 5 min, rinsed in Scott’s tap water followed by 1% eosin (Catalogue # 230251, Sigma) and then dehydrated and mounted with DPX mounting media.
Immunohistochemistry
To determine whether ucOC and GPRC6A are expressed in human and rabbit-diseased arteries, immunohistochemistry was used. Immunohistochemistry was performed in triplicates. Sections were cut at 5 µm, deparaffinised, rehydrated and incubated with 1% goat serum in 10 mm TrisCl (pH 7.4) for 20 min before incubating with the primary antibody diluted (1:50 concentration) in 1% goat serum in 10 mm TrisCl (pH 7.4). Mouse monoclonal IgG against ucOC (GLUOC4-5, Enzo Lifescience, USA) which corresponds to amino acid 14–30 human osteocalcin decarboxylated glutamic acid residues 21 and 24, or goat polyclonal IgG against GPRC6A (Catalogue # 55950, diluted 1:150, Santa Cruz, USA) was incubated overnight. Mouse monoclonal IgG against HHF-35 (Catalogue #201M-9, Sigma, Australia) for smooth muscle cell detection in vascular layer was also incubated on human-diseased artery only overnight [24]. Immunohistochemistry was performed using the ‘Vector Impress’ commercially available kit following the manufacturers’ directions. Antigenic sites were developed with diaminobenzidine (DAB), counterstained with haematoxylin, dehydrated and mounted with DPX mounting media. The expression of ucOC and GPRC6A were depicted by dark brown (from DAB staining) precipitate.
It should be noted that the tissue sections stained with HHF-35 (VSMC detection) underwent an HES not just a hematoxylin stain.
In situ Proximity ligation Assay (PLA)
PLA (Sigma-Aldrich, USA) was used to verify signalling interaction in close proximity of < 40 nm [25] between ucOC and the GPRC6A receptor. After dewaxing of rabbit abdominal aorta and human radial artery tissue, blocking solution was added to vessels and then placed into a pre-heated humidity chamber at 37 °C for 30 min. Slides were incubated overnight with anti-ucOC (GLUOC4-5 Enzo Lifescience, USA) antibody and anti-GPRC6A receptor (Catalogue#55950, diluted 1:150, Santa Cruz, USA) in antibody diluent (1:50 concentration). Tissues were incubated with PLA probes (PLA MINUS anti-mouse and PLA PLUS anti-goat) in a humidity chamber for 60 min at 37 °C. Ligation-ligase (ligation stock diluted in distilled H2O with ligase at 1:40 concentration) for 30 min followed by amplification-polymerase (amplification stock diluted in H2O with polymerase at 1:80 dilution) for 90 min were performed. Brightfield detection stock was then added to the reaction for 60 min at room temperature. DAB-chromagen was diluted in DAB-buffer and was added to the tissues for 10 min at room temperature. Following washing, haematoxylin was applied to the slide (10 min) to stain the nucleus followed by a wash in Scott’s tap water to blue nuclei. Slides were then dehydrated, and mounted with DPX mounting media. Protein interactions are observed as brown (DAB) areas in the tissue and were captured with Leica DFC450 digital camera (at 100 × and 400 × magnification).
Isometric Tension Studies for Endothelium-Dependent Vasorelaxation Assessment
Abdominal aortic rings were cut into ~ 3 mm aortic rings and mounted onto 2 metal hooks attached to force displacement transducers of organ baths which were filled with KREBS (118 mmol/L NaCl, 4.7 mmol/L KCl, 1.2 mmol/L MgSO4·7H2O, 1.2 mmol/L KH2PO4, 25 mmol/L NaHCO3, 11.7 mmol/L glucose and 1.25 mmol/L CaCl2, all chemicals from Sigma-Aldrich, St. Louis, MO, USA). Endothelium function was assessed by vasorelaxation responses of aortic rings. Aortic rings were first pre-constricted with phenylephrine (3 × 10−7 M; Sigma-Aldrich, Melbourne VIC Australia) until plateau was reached and then incubated for 5 min with either recombinant ucOC (10 ng/mL; Glu13,17,20, osteocalcin (1–46) (mouse) trifluoroacetate salt, catalogue # H-6552.0500 purchased from Auspep, Melbourne VIC, Australia) or control (KREBS). This was followed by a cumulative dose response curve to acetylcholine (ACh from − 8 to − 5 M; Sigma-Aldrich) in 2-min time intervals per dose.
We considered endothelial dysfunction when there was a significant reduction of vasorelaxation in atherogenic blood vessels compared to healthy blood vessels (control) measured by either EC50, Emax or total area under the curve (AUC) for entire acetylcholine dose response curve. Please refer to Fig. 1 for experimental design.
Experimental design for in vitro studies. Diet duration was 4 weeks after acclimatization period. ucOC (10 ng/mL) was pre-incubated for 5 min prior to cumulative doses of acetylcholine concentrations. Aortic rings were cut from abdominal aorta. IHC immunohistochemistry, PLA proximity ligation assay, Ach acetylcholine
Statistics
GraphPad prism was used to analyse data (Version 6.07, GraphPad Software Inc, La Jolla, CA, USA). A two-way ANOVA-repeated measures by both factors were performed followed by a Sidak’s multiple comparisons post hoc test, to determine significances between doses of ACh. p < .05 was considered significant. A one-way ANOVA followed by a post hoc test was used to determine significances for EC50 and Emax and an unpaired t test was also determined for area under the curve. Data are presented as mean ± SEM.
Results
HHF-35 & Von Kossa Staining on Human and Rabbit-Diseased Artery
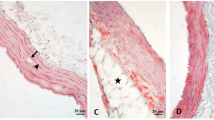
VSMC actin-positive cells (determined by HHF35, brown stain) were identified in human-diseased artery within the endothelium layer, suggesting infiltration of VSMC (Fig. 2a, b). A standard HES coloration on diseased rabbit artery (Fig. 2c) was also conducted and served as a control for the HHF-35-stained tissues in Fig. 2. Calcified areas were histologically detectable by Von Kossa staining. Human diseased artery exhibited calcification within the media layer (Fig. 3a) and the adventitia layer (Fig. 3b). Unlike human-diseased artery, calcification was not observed in diseased rabbit artery (Fig. 3c) compared to control rabbit artery (Fig. 3d).
IHC detection of HHF-35 (brown) for VSMC staining in diseased human artery and a standard HES stain in diseased rabbit artery. Both images of human diseased artery (a, b) clearly demonstrate VSMC localised with the media layer as well as VSMC infiltration into the endothelium layer (below internal elastic lumina). Control for HHF-35 staining is the standard HES coloration in diseased rabbit artery (c). Typical representation of triplicate experimentation. VSMC vascular smooth muscle cell
Von Kossa staining for calcification in both diseased human and rabbit artery. Calcification was detected in the media (a) and adventitia (b) layer of diseased human artery. There was no visible calcification in both diseased (c) and healthy rabbit artery (d). Calcification is represented in dark brown to black. Pink and blue stain represents HES in all panels. Typical representation of triplicate experimentation
Immunohistochemistry Detection of ucOC and GPRC6A
Both ucOC and GPRC6A are present (Fig. 4a, b, respectively) in the endothelial layer, adventitia, and cells immediately below the internal elastic laminae (at the vascular smooth muscle cell (VSMC region) of human-diseased arteries. However, ucOC and GPRC6A were not in close proximity in human-diseased arteries (Fig. 4d).
Normal and PLA immunohistolocalization of ucOC and GPRC6A. Immunohistochemical localization of ucOC (a) and GPRC6A (b) is expressed in human diseased artery. c negative control for IHC. However, PLA staining demonstrates that there is no signalling interaction between ucOC and GPRC6A (d). e negative control for PLA. Typical representation of triplicate experimentation. VSMC vascular smooth muscle cell
Similarly, both ucOC and GPRC6A were observed in healthy rabbit arteries (Fig. 5a, b, respectively), diseased rabbit artery exhibiting plaques (Fig. 6a, b) and diseased rabbit arterioles (Fig. 7a, b). However, there was a close proximity (< 40 nm) between ucOC and GPRC6A within the developed plaques exhibited on rabbit-diseased arteries (Fig. 6d, arrows). ucOC and GPRC6A were also in close proximity (< 40 nm) within diseased rabbit arteriole, specifically at the endothelium and adventitia layer (Fig. 7d), but not at the endothelium or adventitia layer of the healthy rabbit abdominal aorta (Fig. 5d). As the arteriole is from diseased rabbits, it is possible that ucOC may act on the GPRC6A only during diseased conditions.
Normal and PLA immunohistolocalization of ucOC and GPRC6A in healthy rabbit abdominal artery. ucOC (a) and GPRC6A (b) expression in the endothelial wall and adventitial layer of rabbit arteries. c Negative control for IHC. No PLA staining demonstrates that there is no signalling interaction between ucOC and GPRC6A in any layer of the blood vessel (d). e Negative control for PLA. Typical representation of triplicate experimentation
Normal and PLA immunohistolocalization of ucOC and GPRC6A in plaques of rabbit abdominal aorta. ucOC (a) and GPRC6A (b) expression in rabbit plaque. Plaque is shown by dotted semi-circle arrows. c Negative control for IHC. PLA staining demonstrated by dark brown spots show that there is signalling interaction between ucOC and GPRC6A (d). e Negative control for PLA. Typical representation of triplicate experimentation
Normal and PLA immunohistolocalization of ucOC and GPRC6A in rabbit diseased arterioles. ucOC (a) and GPRC6A (b) expression in rabbit arteriole of adventitia (dotted arrow) and even in endothelium layer (solid black arrow (a) and orange arrow (b)). c Negative control for IHC. PLA staining demonstrated by dark brown spots show that there is signalling interaction between ucOC and GPRC6A (d) at the endothelial layer (orange arrow). e Negative control for PLA. Typical representation of triplicate experimentation
The Effects of ucOC on Endothelial Dysfunction
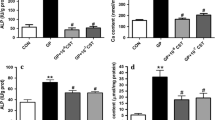
After establishing the presence of ucOC in diseased blood vessels, it was assessed whether ucOC has an effect on endothelium function in normal and atherogenic blood vessels. Endothelial dysfunction was exhibited in the aorta of atherogenic rabbits as Emax was markedly reduced compared to healthy aortic rings (Table 1). Vasorelaxation (− 31.03 ± 6.43% to − 63.94 ± 4.60%, n = 9) from atherogenic aortic rings was significantly reduced, p < 0.01, compared to healthy aortic rings (− 61.341 ± 5.84% to − 86.551 ± 5.03%, n = 10) in a dose-dependent manner (− 7 M to − 6 M, Fig. 8a). AUC also showed that total dilation of atherogenic arteries markedly reduced compared to healthy, p = <0.05, respectively (Fig. 8a).
Effects of ucOC on endothelial dysfunction in response to ACh vasorelaxation in rabbit atherogenic aortic rings compared to healthy aortic rings. Endothelial dysfunction is evident in atherogenic aortic rings compared to healthy aortic rings (a, **p < 0.01for atherogenic n = 9 vs. healthy n = 10), ucOC (10 ng/mL) has no positive or negative impact on healthy aortic rings (b). ucOC improved endothelial dysfunction in atherogenic aortic rings (c; *p < 0.05, **p < 0.01, ***p < 0.0001). Data represented as mean ± SEM shown in the table above (d)
Normal endothelial function was not altered by ucOC (10 ng/mL) pre-incubation in healthy aortic rings (Fig. 8b). However, in atherogenic aortic rings, ucOC significantly attenuated the impaired endothelium-dependent vasorelaxation (− 26.06 ± 3.59% to − 76.06 ± 3.17%, p < 0.05) compared to atherogenic aortic rings pre-incubated with control (− 10.56 ± 3.23% to − 61.68 ± 7.27%) at log ACh concentrations − 7.5 M to − 5.5 M (Fig. 8c). AUC also showed a trend (p = 0.06) for ucOC to improve overall vasorelaxation compared to control (Fig. 8c).
ucOC tend to improve Emax, − 64.48 ± 5.4%, compared to control − 53.61 ± 2.46% in atherogenic aortic rings (p < 0.08, Table 1). Finally, there was no significant differences between ucOC incubation in atherogenic aortic rings compared to healthy aortic rings (shown in Table of Fig. 6).
No significant differences between all groups for the EC50 were shown (Table 2).
Discussion
We report that ucOC and GPRC6A are expressed in human and rabbit-diseased artery. Moreover, it appears that GPRC6A, the postulated receptor of ucOC in several target tissues, may not be the main ucOC receptor in blood vessels such as the aorta, but is likely to be in cells with developed plaque and arterioles. ucOC treatment, in vitro, attenuates impaired endothelium-dependent vasorelaxation following atherogenic diet.
OC is the most abundant non-collagenous protein in bone. It is synthesized and secreted by mature osteoblasts [26]. OC binds to the hydroxyapatite mineral of bone with high affinity and may promote bone mineralization [27], and in this context, it is reported that OC is involved in calcifying VSMCs [28, 29]. In addition, higher circulating total OC levels are related to an increased risk of atherosclerosis in patients with type-2 diabetes [30] and calcification in patients with coronary artery disease [31]. This is in contrast to the possible beneficial effects that ucOC has on glucose regulation and insulin sensitivity [9, 32,33,34] and a potential role in atherosclerosis and blood vessel disease [35]. A higher circulating level of ucOC is also related to a reduced risk of developing carotid plaques [17]. Another study has shown that total osteocalcin (which include ucOC) is associated with lower abdominal aortic calcification progression and longer 10-year survival in elderly men from the MINOS cohort [18]. Here, we report that ucOC and GPRC6A are expressed in the endothelial layer, adventitia, and in cells that lay right below the internal elastic laminae of diseased arteries from both human (where calcification and VSMC infiltration into plaque also occurred) and rabbit models. This may indicate a potential impact of ucOC in blood vessel function, an impact that we explored in our isometric tension studies.
Besides the bone marrow, adipose tissue or peripheral blood, a previous study found that the adventitia layer of human arteries is another source of mesenchymal stem cells (MSC’s) production [36]. As mentioned above, OC is produced from osteoblasts which are MSC’s derivatives [37] and may explain the presence of IHC localisation of ucOC in the adventitia layer of diseased human arteries. This is the first study that demonstrates the expression of ucOC on the endothelium layer of diseased human and rabbit arteries and healthy rabbit artery. A recent study published interesting hypothesis with supporting evidence which demonstrated the ability of endothelial cells to generate osteoblasts in prostate cancer metastasis cell line [38], it is, therefore, important for further studies to be conducted in determining whether the endothelium of diseased human arteries also generates osteoblasts thus expressing ucOC.
Using the PLA method, ucOC did not signal closely with GPRC6A in the majority of diseased blood vessels, which may indicate that GPRC6A is not the ucOC receptor in this tissue. Positive PLA signals were observed sporadically in the endothelial layer of arterioles and within plaques only, suggesting that the ucOC/GPRC6A interaction may be cell specific as it is in the Leydig cells of the testes, pancreatic β-cells [39, 40] and perhaps skeletal muscle [7, 10]. However, our findings do not rule out the possibility that, under some conditions, such as exercise, as higher circulating levels of ucOC are observed [12, 41], ucOC may indeed bind to GPRC6A in blood vessels, a hypothesis which requires further research.
An important finding of the current study is that ucOC and GPRC6A are within approximately 40 nm in close physical proximity in specific cells atherosclerotic plaques. GPR’s play crucial roles in atherosclerosis in that they can worsen inflammation but also play a role in lessening inflammation [42]. It was reported that a higher serum ucOC is related to a reduced risk of developing plaque within the carotid humans [17]. Future studies will need to determine the role of ucOC and its interaction with GPRC6A in abrogating plaque formation and whether the interaction is crucial in the plaque stage of atherosclerosis.
After determining the localisation of ucOC in blood vessels, it was important to investigate if ucOC impacts endothelial function assessed as vasorelaxation in response to ACh ex vivo. It is well established that nitric oxide is released from the endothelium to cause dilation of VSMC [43]. However, during endothelial dysfunction nitric oxides production and, therefore, its release is reduced [44], a process that is generally assessed in ex vivo isometric studies and dose response curves to acetylcholine (a neurotransmitter that signals endothelial nitric oxide synthase, eNOS, to produce nitric oxide for its release to target VSMC). Furthermore, it has been reported that recombinant ucOC (daily 30 µg/kg for 5 weeks) via intraperitoneal injections markedly increased serum nitric oxide in atherogenic mice [45]. The same investigators also reported an increase of eNOS activity in human aortic rings after ucOC (25 and 100 ng/mL) administration in vitro, leading to the hypothesis that ucOC may exert protective role on CVD by improving endothelial-dependent vasorelaxation. Indeed, our results confirm that ucOC may improve endothelial-dependent vasorelaxation in atherogenic aortic rings. This suggests that ucOC plays an interactive role with the endothelium, whether through activating the GPRC6A or stimulating other signalling pathways that improve nitric oxide production and, therefore, vasorelaxation is yet to be determined. It is also possible to state that ucOC may even restore endothelial dysfunction in atherogenic aortic rings as there were no significant differences between ucOC incubation in atherogenic aortic rings compared to healthy aortic rings. While ucOC may have a role blood vessel function under pathophysiological conditions such as an atherogenic diet, it may not have any effect under physiological conditions as determined in this study in the healthy aortic rings. A tight control of solute permeability between each neighbouring endothelial cells exist under physiological conditions [46]. Previously, it has been shown that 10 ng/mL ucOC incubation in human aortic endothelial cells does not affect endothelial cell permeability under physiological conditions [47], as such we propose that cellular signalling cascades cannot be manipulated by ucOC under normal conditions. This may suggest why ucOC (at 10 ng/mL) in this study showed no effect on vascular function in normal conditions.
There are several limitations to our study. First, although other studies use either calindol or Nps2143 as GPRC6A antagonist [48], these compounds are more potent on the calcium-sensing GPRC receptors [49]. As such, we could not determine whether the GPRC6A receptor was signalled by ucOC in attenuating endothelial dysfunction. In addition, we cannot exclude the possibility that VSMC’s were altered by ucOC and may have influenced our results, however, acetylcholine mediated vasorelaxation in aortic rings is well accepted as a standard measure of endothelial function in isometric studies, suggesting that in our study ucOC exerts its effect at the endothelium. Another potential limitation of the current study is that the PLA and IHC method cannot distinguish whether ucOC is from local production or from the circulation. However, since osteocalcin is mainly produced by osteoblasts [50], it is likely that the ucOC observed in the healthy rabbit artery is derived from the circulation. On the contrary, the idea of VSMC differentiation into osteoblast-like phenotype during calcification has been formed previously [51,52,53]. Although this hypothesis has not been fully elucidated, a cell culture study determined that VSMC from rats and inbred mice aorta exposed to high phosphate calcium-containing media (to induce calcification) for 10 days induced osteogenic phenotype of VSMCs [54]. Osteogenic markers such as increased osteocalcin, BMP-2, BMP-4 and BMP7 were all increased in VSMC’s grown in calcification media compared to control. As VSMC produce osteocalcin during disease (i.e., calcification), it may suggest that the ucOC observed in the diseased human and rabbit arteries from our study may perhaps come from local production. This hypothesis should be tested in future studies.
In conclusion, ucOC and GPRC6A are expressed in human and rabbit-diseased arteries and ucOC signalling through GPRC6A may potentially be a target of interest in the treatment of plaque calcification. ucOC attenuated impaired endothelium-dependent vasorelaxation following atherogenic diet. Future studies should explore whether ucOC can be used as a therapeutic avenue for the progression of atherosclerosis.
References
Creager MA et al (2003) Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Circulation 108(12):1527–1532
AIHW (2014) Cardiovascular disease, diabetes and chronic kidney disease: Australian facts: prevalence and incidence. Cardiovascular, diabetes and chronic kidney disease series no. 2. Cat. no. CDK 2. AIHW, Canberra
Heidenreich PA et al (2011) Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 123(8):933–944
Prashar Y et al (2017) Emerging role of various signaling pathways in the pathogenesis and therapeutics of atherosclerosis. Rev Vasc Med 10–11:1–12
Rached MT et al (2010) FoxO1 expression in osteoblasts regulates glucose homeostasis through regulation of osteocalcin in mice. J Clin Invest 120(1):357–368
Ferron M et al (2010) Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 142(2):296–308
Levinger I et al (2016) The effects of muscle contraction and recombinant osteocalcin on insulin sensitivity ex vivo. Osteoporos Int 27(2):653–663
Lee NK et al (2007) Endocrine regulation of energy metabolism by the skeleton. Cell 130(3):456–469
Oury F et al (2011) Endocrine regulation of male fertility by the skeleton. Cell 144(5):796–809
Pi M, Quarles LD (2012) Multiligand specificity and wide tissue expression of GPRC6A reveals new endocrine networks. Endocrinology 153(5):2062–2069
Lin X et al (2016) Hindlimb immobilization, but not castration, induces reduction of undercarboxylated osteocalcin associated with muscle atrophy in rats. J Bone Miner Res 31(11):1967–1978
Levinger I et al (2014) The effect of acute exercise on undercarboxylated osteocalcin and insulin sensitivity in obese men. J Bone Miner Res 29(12):2571–2576
Levinger I et al (2011) The effect of acute exercise on undercarboxylated osteocalcin in obese men. Osteoporos Int 22(5):1621–1626
Hwang YC et al (2009) The uncarboxylated form of osteocalcin is associated with improved glucose tolerance and enhanced beta-cell function in middle-aged male subjects. Diabetes Metab Res Rev 25(8):768–772
Kanazawa I et al (2011) Serum undercarboxylated osteocalcin was inversely associated with plasma glucose level and fat mass in type 2 diabetes mellitus. Osteoporos Int 22(1):187–194
Kim KM et al (2016) Lower uncarboxylated osteocalcin and higher sclerostin levels are significantly associated with coronary artery disease. Bone 83:178–183
Zhang M et al (2015) Undercarboxylated osteocalcin as a biomarker of subclinical atherosclerosis in non-dialysis patients with chronic kidney disease. J Biomed Sci 22:75
Confavreux CB et al (2013) Higher serum osteocalcin is associated with lower abdominal aortic calcification progression and longer 10-year survival in elderly men of the MINOS cohort. J Clin Endocrinol Metab 98(3):1084–1092
Okura T et al (2010) Undercarboxylated osteocalcin is a biomarker of carotid calcification in patients with essential hypertension. Kidney Blood Press Res 33(1):66–71
Zulli A et al (2008) Human diseased arteries contain cells expressing leukocytic and embryonic stem cell markers. Hum Pathol 39(5):657–665
Zulli A et al (2003) The resistance of the IMA to atherosclerosis might be associated with its higher eNOS, ACE and ET-A receptor immunoreactivity. Arterioscler Thromb Vasc Biol 23(7):1308
Zulli A et al (2008) Co-localization of angiotensin-converting enzyme 2-, octomer-4- and CD34-positive cells in rabbit atherosclerotic plaques. Exp Physiol 93(5):564–569
Liao J, Huang W, Liu G (2015) Animal models of coronary heart disease. J Biomed Res 30(1):3–10
Tsukada T et al (1987) HHF35, a muscle-actin-specific monoclonal antibody. I. Immunocytochemical and biochemical characterization. Am J Pathol 126(1):51–60
Bagchi S, Fredriksson R, Wallén-Mackenzie Å (2015) In situ proximity ligation assay (PLA). In: Hnasko R (ed) ELISA: methods and protocols. Springer, New York, NY, pp 149–159
Karsenty G (1998) Transcriptional regulation of osteoblast differentiation during development. Front Biosci 3:d834–d837
Ducy P (2011) The role of osteocalcin in the endocrine cross-talk between bone remodelling and energy metabolism. Diabetologia 54(6):1291–1297
Kapustin AN, Shanahan CM (2011) Osteocalcin: a novel vascular metabolic and osteoinductive factor? Arterioscler Thromb Vasc Biol 31(10):2169–2171
Idelevich A, Rais Y, Monsonego-Ornan E (2011) Bone Gla protein increases HIF-1alpha-dependent glucose metabolism and induces cartilage and vascular calcification. Arterioscler Thromb Vasc Biol 31(9):e55–e71
Sheng L et al (2013) Serum osteocalcin level and its association with carotid atherosclerosis in patients with type 2 diabetes. Cardiovasc Diabetol 12:22
Zhang H et al (2015) Correlation between osteocalcin-positive endothelial progenitor cells and spotty calcification in patients with coronary artery disease. Clin Exp Pharmacol Physiol 42(7):734–739
Ferron M et al (2008) Osteocalcin differentially regulates β cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci 105(13):5266–5270
Ferron M et al (2012) Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone 50:568–575
Levinger I et al (2017) Multifaceted interaction of bone, muscle, lifestyle interventions and metabolic and cardiovascular disease: role of osteocalcin. Osteoporos Int 28(8):2265–2273
Tacey A et al (2018) Potential role for osteocalcin in the development of atherosclerosis and blood vessel disease. Nutrients 10(10):1426
Corselli M et al (2012) The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev 21(8):1299–1308
Hass R et al (2011) Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal: CCS 9:12
Paiva AE et al (2017) Endothelial cells as precursors for osteoblasts in the metastatic prostate cancer bone. Neoplasia (New York) 19(11):928–931
Pi M et al (2005) Identification of a novel extracellular cation-sensing G-protein-coupled receptor. J Biol Chem 280(48):40201–40209
Pi M, Wu Y, Quarles LD (2011) GPRC6A mediates responses to osteocalcin in β-cells in vitro and pancreas in vivo. J Bone Miner Res 26(7):1680–1683
Lin X et al (2017) Recombinant uncarboxylated osteocalcin per se enhances mouse skeletal muscle glucose uptake in both extensor digitorum longus and soleus muscles. Front Endocrinol (Lausanne) 8:330
Sun L, Ye RD (2012) Role of G protein-coupled receptors in inflammation. Acta Pharmacol Sin 33(3):342–350
Tousoulis D et al (2012) The role of nitric oxide on endothelial function. Curr Vasc Pharmacol 10(1):4–18
Mudau M et al (2012) Endothelial dysfunction: the early predictor of atherosclerosis. Cardiovasc J Africa 23(4):222–231
Kondo A, Kawakubo-Yasukochi TK, Mizokami A, Chishaki S, Takeuchi H, Hirata H (2016) Uncarboxylated osteocalcin increases serum nitric oxide levels and ameliorates hypercholesterolemia in mice fed an atherogenic diet. Electron J Biol 13(1):22–28
Chistiakov DA, Orekhov AN, Bobryshev YV (2015) Endothelial barrier and its abnormalities in cardiovascular disease. Front Physiol 6:365
Millar SA, Anderson SI, O’Sullivan E (2019) Human vascular cell responses to the circulating bone hormone osteocalcin. J Cell Physiol 234(11):21039–21048
Faure H et al (2009) Molecular determinants of non-competitive antagonist binding to the mouse GPRC6A receptor. Cell Calcium 46(5–6):323–332
Clemmensen C et al (2014) The GPCR, class C, group 6, subtype A (GPRC6A) receptor: from cloning to physiological function. Br J Pharmacol 171(5):1129–1141
O’Connor EM, Durack E (2017) Osteocalcin: the extra-skeletal role of a vitamin K-dependent protein in glucose metabolism. J Nutr Intermed Metab 7:8–13
Schurgers LJ et al (2018) Initiation and propagation of vascular calcification is regulated by a concert of platelet- and smooth muscle cell-derived extracellular vesicles. Front Cardiovasc Med 5:36
Durham AL et al (2018) Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness. Cardiovasc Res 114(4):590–600
Bostrom K et al (1993) Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest 91(4):1800–1809
Montezano AC et al (2010) Vascular smooth muscle cell differentiation to an osteogenic phenotype involves TRPM7 modulation by magnesium. Hypertension 56(3):453–462
Acknowledgements
Associate Professor Itamar Levinger was supported by a Heart Foundation Future Leader Fellow (ID: 100040). This study was funded by The Rebecca L Cooper Medical Research Foundation.
Author information
Authors and Affiliations
Contributions
TQ, LKG: data curation, formal analysis, investigation & writing drafts. LKG and ABT: writing-review & editing. DLH: resources & writing-review & editing. BFB: resources & writing-review & editing. VA: writing-review & editing and supervision. IL: funding acquisition, resources, supervision and writing-review & editing. AZ: conceptualization, investigation, methodology, resources, software, supervision and writing-review and editing.
Corresponding author
Ethics declarations
Conflict of interest
Tawar Qaradakhi, Laura K. Gadanec, Alex B. Tacey, David L. Hare, Brian F. Buxton, Vasso Apostoloppoulos, Itamar Levinger and Anthony Zulli declare that they have no conflict of interest. These authors also declare no competing financial interests.
Ethical Approval
The study was approved by the Austin Hospital Medical Research Ethics Committee and followed institutional guidelines that conform with the Declaration of Helsinki.
Human and Animal Rights and Informed Consent
Patients gave informed consent to collect discarded arteries.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qaradakhi, T., Gadanec, L.K., Tacey, A.B. et al. The Effect of Recombinant Undercarboxylated Osteocalcin on Endothelial Dysfunction. Calcif Tissue Int 105, 546–556 (2019). https://doi.org/10.1007/s00223-019-00600-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-019-00600-6