Abstract
The effects of nitrogen-containing bisphosphonates (N-BPs) on osteoclasts (Ocs) may differ with dose and regimen. N-BPs reduce Oc bone resorption by inhibiting the enzyme farnesyl diphosphate synthase (FPPS), an effect counteracted by geranylgeraniol (GGOH), which restores geranylgeranylation downstream of FPPS. We assessed GGOH effects on inhibition of bone resorption by the N-BPs alendronate (ALN), ibandronate (IBN), and zoledronate (ZOL) in an assay of rabbit Oc resorption of bovine cortical bone. GGOH blocked inhibition of resorption at low, but not high, N-BP concentrations, with a 14- to 20-fold increase in IC50 values for each N-BP. In vivo, growing male rats were administered doses calculated to mimic bioavailable exposures in daily (ALN, IBN), weekly (ALN), monthly (IBN), and yearly (ZOL) clinical regimens. Tibiae were harvested at 48 h, and metaphyses were analyzed. With lower ALN and IBN doses, Oc numbers rose by 26–48 %, morphology was normal, and there was no increase in apoptotic Ocs. In contrast, with higher IBN and ZOL doses, bone-associated Ocs were generally rounded in appearance and numbers of nuclei/Oc versus vehicle increased 42 and 31 %, respectively (P < 0.05). With ZOL, there was no rise in Oc number, but there was a 6.5-fold increase in apoptotic Ocs versus vehicle and a ≥13.5-fold increase versus lower-dose ALN or IBN (P < 0.05). With higher-dose IBN there was no rise in Oc number but 7- and 14-fold increases in Oc apoptosis versus low-dose ALN and IBN (P < 0.02). These results suggest that different mechanisms may come into play across the dosing spectrum of N-BPs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the mid-1990s, nitrogen-containing bisphosphonates (N-BPs) have been widely used for therapeutic control of bone loss associated with several disease processes characterized by a high rate of bone resorption, the most common of which is postmenopausal osteoporosis. Initially, the typical clinical N-BP dosing regimen, such as that for alendronate (ALN), risedronate (RIS), and ibandronate (IBN), was a single tablet daily. Weekly regimens of ALN and RIS were subsequently introduced using seven times the daily dose [1, 2]. With weekly regimens, stable reductions in bone resorption are observed between doses. Additional regimens, including progressively higher doses administered at proportionally longer intervals between doses, were subsequently developed for monthly (IBN) [3] and yearly (zoledronate, ZOL) [4] dosing. With monthly and yearly regimens, reduction of bone resorption is much greater shortly after dosing and wanes substantially before the next dose.
As structural analogs of pyrophosphate, N-BPs selectively target skeletal surfaces, preferentially binding to sites undergoing active resorption. Newly administered or redistributed N-BP that is bound maintains its pharmacological activity for weeks to months, until it is either resorbed and excreted, by osteoclast (Oc) activation at the site, or rendered inaccessible to Ocs via coverage by new bone [5]. When bone-resorbing Ocs take up N-BP on bone surfaces at remodeling sites and effective intracellular threshold concentrations are achieved, the Ocs undergo morphological changes (notably loss of ruffled border) and become impaired in their ability to further resorb [6].
At clinically used daily or weekly doses, N-BPs such as risedronate, ALN, and IBN act as antiresorptives via inhibition of the mevalonate-pathway enzyme farnesyl diphosphate synthase (FPPS) within the Oc [7, 8]. The functional consequences of this action are inhibited geranylgeranylation and modulation of the activities of small GTPases such as Rac, Rho, and Rab proteins that are thought to play essential roles in the cellular processes required for resorption, such as cytoskeletal remodeling and vesicular trafficking [9, 10]. Within a relatively narrow concentration window, geranylgeraniol (GGOH) restores geranylgeranylation and returns bone resorption to control levels [11]. Though it has long been known that at sufficiently high doses N-BPs can activate osteoclast pathways that trigger programmed cell death (apoptosis), we and others have previously shown that this occurs at higher doses than those required for effective inhibition of resorption in vitro [11, 12].
The pharmacological effects of N-BPs on Ocs are determined by both the concentration of the N-BP in Oc resorption lacunae and the molecular activities of the N-BP following endocytosis. Because the half-life of an N-BP on the resorption surface of bone is ~2–6 weeks, its concentration at these loci is relatively constant over time with once-daily or once-weekly dosing as saturation occurs. In terms of the distribution of N-BP over the bone surface, however, the concentration of new N-BP adherent to actively resorbing surfaces is approximately eightfold higher than that at resting surfaces [13]. From this perspective, daily and weekly dosing may deliver sufficient N-BP to the actively resorbing surfaces to control resorption, while levels of N-BP adherent to resting surfaces may be insufficient to generate an equivalent pharmacological effect. Thus, N-BP at lower doses must be administered frequently (e.g., once-daily or once-weekly) in order to control resorption. By extension, with once-monthly or once-yearly dosing, the higher administered doses may deliver pharmacologically active N-BP concentrations to resting surfaces in advance of resorption cycles initiated between doses. In these cases, while pharmacologically active drug concentrations are delivered to resting surfaces, those localized to resorption surfaces may rise to suprapharmacological levels. It should also be noted that a small pool of N-BP can recirculate in the blood, adding to newly dosed drug available for binding to bone surfaces [14].
In the current study, we hypothesized that the mechanistic basis for inhibition of Oc activity by N-BPs immediately after dosing differs according to dose and that these differences may be discerned from morphological changes in Ocs and the relative induction of Oc apoptosis following short-term N-BP exposure.
Materials and Methods
All animal procedures were carried out according to approved institutional protocols, in accordance with recommendations for the proper care and use of laboratory animals and under the scrutiny and authority of the Institutional Animal Care and Use Committee of Merck Research Laboratories (West Point, PA).
Preparation of Bovine Cortical Bone Slices
Slices (200 μm thick) of 6-mm-diameter bovine cortical bone cylinders were cut using an Isomet low-speed precision saw, after which bones were immersed in 10 % ethanol. Following sonication for 20 min, ethanol was removed and slices were sonicated for an additional 20 min in deionized water. For bone resorption assays, discs were transferred to 96-well plates and then UV light–sterilized for >20 min.
Preparation of Rabbit Ocs
Long bones were aseptically isolated from 10- to 14-day-old New Zealand white rabbits (Covance Research Products, Denver, PA), and the soft tissue was removed. Bones were minced (to ~1-mm pieces) in 5 mL of α-MEM (GIBCO BRL, Gaithersburg, MD) containing penicillin/streptomycin, pH 7.1. The volume of medium was then brought to 25 mL, and the tissue/medium was gently mixed over 6 min, before being allowed to settle. Supernatants were then withdrawn and retained. This process was repeated using an additional 25 mL of medium, after which supernatants were combined and gently mixed. Recovered cells were diluted 1:10 in 2 % acetic acid/PBS and counted using hemocytometer slides. For bone resorption assays, cells were diluted to 5 × 106/mL in α-MEM containing 2 % FBS. The suspension (200 μL) was then used to seed each slice of bovine cortical bone. After 1 h, plating medium was removed and test compounds, diluted in α-MEM containing 2 % FBS and 10 nM 1,25(OH)2D3, were added to triplicate wells. Cultures were then incubated at 37 °C in a 5 % CO2 atmosphere for 3 days, after which medium was removed. Bone resorption was assessed by quantification of C-telopeptide collagen fragments released into the medium (Crosslaps ELISA CTx assay; Osteometer Biotech, Herlev, Denmark).
In Vivo Effects of Bisphosphonates on Ocs
Calculations of N-BP doses for rats that are equivalent to standard human doses were based on a dose of 5 μg daily ALN as the standard dose used to block osteoclastic bone resorption in rats [8] (Table 1). Growing male Sprague–Dawley rats were dosed by subcutaneous bolus injection with either vehicle or the following bisphosphonates:
-
1.
ALN daily: 5 μg/kg on days 1 and 2
-
2.
ALN weekly: 35 μg/kg on day 1, with vehicle on day 2
-
3.
IBN daily: 1.25 μg/kg on days 1 and 2
-
4.
IBN monthly: 150 μg/kg on day 1, with vehicle on day 2
-
5.
ZOL yearly: 333 μg/kg on day 1, with vehicle on day 2
Forty-eight hours after the first injection (i.e., on day 3), animals were killed and proximal tibiae were harvested, formalin-fixed, decalcified, and paraffin-embedded. Sections of proximal tibiae were stained using hematoxylin/eosin. Osteoclast parameters were quantified, in a blinded manner, in regions of secondary spongiosa using Bioquant Osteo software (Bioquant, Nashville, TN) with a Nikon (Melville, NY) Optiphot microscope and an Optronics camera. Nonparametric ANOVA (Kruskal–Wallace, using Crunch Interactive Statistical Package CRISP, San Francisco, CA) was used to analyze apoptosis data. All other ANOVAs were by Fisher’s protected least significant difference (StatView for Windows; SAS Institute, Cary, NC).
Results
GGOH Restores Bone Resorption across a Relatively Narrow N-BP Concentration In Vitro
The effects of a fixed concentration of GGOH (10 μM) on inhibition of rabbit Oc bone resorption by increasing concentrations of N-BPs were examined in cell culture (Fig. 1). In the presence of GGOH, the IC50 values of ALN, IBN, and ZOL shifted 20-, 14-, and 17-fold to the right, from 976, 579, and 6 to 19191, 7,849 and 110 pmol/bone slice, respectively. Whereas at low concentrations of N-BPs GGOH blocked inhibition of bone resorption, at high concentrations of N-BPs GGOH failed to maintain this effect. Higher doses of GGOH are toxic to Ocs and, therefore, could not be evaluated in this system. These results suggest that GGOH can counteract the effects of N-BPs but only within a relatively narrow range of BP concentrations.
Geranylgeraniol (GGOH, 10 μM) blocks low-dose N-BP inhibition of osteoclastic bone resorption. Bone resorption assays were performed as described in “Materials and Methods” section. Statistical analysis of CTX ELISA triplicate readouts were by ANOVA, using Fisher’s PLSD with StatView software. ALN alendronate, IBN ibandronate, ZOL zoledronate
Distinct Effects of Low vs. High Doses of N-BPs on Osteoclasts In Vivo
To examine whether the actions of higher doses of N-BPs (administered monthly or yearly in humans) might differ from those of lower doses (administered daily or weekly) in their effects on Ocs in vivo, we modeled the effects of N-BPs in growing rats treated with doses calculated to mimic clinical dosing associated with daily, weekly, monthly, or yearly administration (Table 1). For these analyses, dosing proceeded for a period of 48 h before tissue samples were harvested, as described in “Materials and Methods” section. Focus on a temporally restricted treatment response was intended to ensure that effects on Ocs were synchronized, favoring linkage to morphological phenotypes from a more homogeneous Oc population than might be expected following extended dosing for weeks or longer.
Effects of High-Dose N-BPs on Oc Morphology
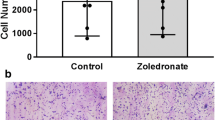
Rats were killed after 2 days of treatment, as noted above. Representative stained sections of decalcified and paraffin-fixed proximal tibiae are shown in Fig. 2a. Osteoclasts (marked with arrows) from rats treated with vehicle or the lower N-BP doses of ALN or IBN were identified as multinucleated cells elongated against the bone surface. Ocs from rats treated with the higher doses of IBN or ZOL were also multinucleated and could be found on the bone surface, although after these treatments cells had a taller, rounded-up, and plump appearance. Blinded measurement of the height of Ocs, using the diameter of the Oc at the center and at an angle perpendicular to the bone surface, is shown in Fig. 3a. The average heights of Ocs after treatment with high-dose IBN or ZOL were 27 and 34 % greater than those for vehicle, respectively (P < 0.01 vs. vehicle for both), whereas cell heights after treatment with lower-dose ALN or IBN were similar to those of vehicle. Lengths of the cells along the bone surface were also measured but did not differ significantly among treatments (data not shown). These data suggest that in the context of acute N-BP treatment morphological responses to low- versus high-dose N-BP differ.
N-BP effects on osteoclast morphology in tibial metaphyses of growing rats. Photomicrographs captured using a Nikon Eclipse E1000 microscope with a ×60 objective (oil immersion) and a Nikon DXM1200X digital camera with Act1 v.2 imaging software. a Arrows indicate osteoclasts. b Osteoclasts of interest are marked with boxes. ALN alendronate, IBN ibandronate, ZOL zoledronate
Quantitation of N-BP effects in tibial metaphyses of growing rats. Photomicrographs were analyzed as described in “Materials and Methods” section. ALN alendronate, IBN ibandronate, ZOL zoledronate
Induction of Apoptosis by High-, but not Low-, Dose N-BPs
In addition to the distinctive morphology after treatment with high-dose N-BPs, these Ocs were more likely to undergo apoptosis, identified by the presence of pyknotic nuclei (Fig. 2b). Blinded quantitation of apoptotic Ocs per microscopic field of tibial metaphyses from growing rats treated with high-dose IBN or ZOL showed increases of 260 and 650 %, respectively, compared with vehicle treatment (Fig. 3b). The numbers of apoptotic Ocs in rats with low-dose ALN and IBN were lower than those observed in bones of animals treated with vehicle. When apoptotic cells were observed after treatment with high-dose N-BPs, they generally were partially or fully detached from bone surfaces (Fig. 2b). These data suggest that the acute apoptotic Oc response is associated with acute high-, but not low-, dose N-BP treatment.
Effects on Oc Number by High- and Low-Dose N-BPs
Treatment with low-dose ALN led to statistically significant increases in Oc number compared with effects of vehicle (P < 0.05, Fig. 3c). While both low- and high-dose IBN treatment resulted in numerical increases in Oc number, the magnitude of increase was less than that achieved with ALN, and the IBN-induced increases were not significant. Treatment with high-dose ZOL induced no change in overall Oc number.
Effects of High- and Low-Dose N-BPs on Numbers of Oc Nuclei
Numbers of nuclei per Oc were quantified, and significant differences between low- and high-dose N-BPs were observed (Fig. 3d). The numbers of nuclei/cell after treatment with high-dose IBN or ZOL were 43 and 31 % higher, respectively, versus vehicle (P < 0.02), whereas the lower doses of N-BPs did not significantly alter nuclei number in these cells.
Discussion
Inhibition of FPPS, the well-characterized mechanism of action for inhibition of bone resorption by N-BPs, can be confirmed by blocking the bisphosphonate effects with the addition of a downstream mevalonate-pathway metabolite, geranylgeranyldiphosphate (GGPP), or by its replenishment using GGOH. Although correlative effects of N-BPs and GGOH on prenylation were not directly examined in these studies due to technical limitations, the findings suggest that at higher N-BP concentrations inhibition of FPPS may not be the sole mechanism of action in play. In vitro, cultured Ocs were protected by GGOH from the FPPS-targeted antiresorptive effects of ALN, IBN, and ZOL at lower concentrations. However, when used at higher concentrations, each N-BP demonstrated what appeared to be an FPPS- or prenylation-independent activity. The N-BPs were not distinguished from each other by responsiveness to GGOH with increasing concentration of drug; the 14- to 20-fold relative increases in N-BP concentration required to see the effect were not related to the magnitude of FPPS inhibition among the N-BPs (ZOL > IBN > ALN) [7].
There are several possible explanations for these findings. First, high-dose N-BPs may act through a mechanism that is independent of FPPS or other enzymes in the mevalonate pathway. Second, FPPS inhibition by high-dose N-BPs might reach levels at which GGOH replenishment of GGPP may be insufficient to completely restore protein geranylgeranylation; GGOH effects are limited to a narrow concentration range, above which the GGOH itself can be cytotoxic (our unpublished observations). Third, more saturating inhibition of FPPS with high-dose N-BP could invoke mevalonate-pathway mechanisms that are prenylation-independent, as discussed below. We undertook in vivo analysis of acute Oc responses to N-BPs to gain clearer insight into the differences observed between the effects of high and low N-BP treatment of Ocs.
Higher doses of N-BPs were associated with a changed Oc morphology in the rat in vivo. Whereas Ocs from rats treated for 2 days with the equivalent of daily or weekly doses of N-BPs in humans exhibited a flattened shape, similar to vehicle-treated Ocs (as previously reported [8]), after treatment with N-BPs at higher doses, Ocs were found to have a taller, rounder appearance, as shown by their significantly greater height from the bone surface. The appearance of larger, rounded Ocs after short-term treatment with suprapharmacological dosing of N-BPs such as ALN and risedronate in rats has been reported previously [6, 8]. This suggests that the morphological responses to acute N-BP treatment are dose-related and cannot effectively serve as points of differentiation between the N-BPs in terms of the intrinsic properties of the individual molecules.
In the rat, the high doses calculated to be equivalent to monthly and yearly clinical N-BP regimens in humans were also associated with increased Oc apoptosis. This was not observed with the lower N-BP doses equivalent to daily or weekly regimens. In fact, there was an increase in Oc number with lower-dose ALN treatment. In contrast, with high-dose IBN and ZOL there was no overall rise in total Oc number. Given the temporally restricted (48-h) coordination of responses, we interpret the absence of an increase in Oc number with high-dose IBN and ZOL as an induction of apoptosis.
While the numbers of apoptotic Ocs for monthly and yearly equivalent doses of N-BPs were greater than those associated with vehicle treatment, with daily or weekly doses numbers were somewhat lower than with vehicle (nonsignificant). This suggests a trend toward reduced Oc apoptosis, which could partially contribute to the overall increase in Oc number observed with ALN treatment. In light of previous in vitro findings [11], this is consistent with our current understanding that N-BPs need not induce Oc apoptosis to inhibit bone resorption. Moreover, it is important to note that treatment with low-dose ALN for 12 weeks causes an even greater rise in Oc number (more than twofold) in the ovariectomized rat [15], consistent with the absence of a substantial apoptotic response to low-dose N-BP after chronic dosing. Interestingly, this earlier study also showed that increased dosing (up to ~500 μg/kg daily equivalent) over 12 weeks results in a gradual attenuation of the rise in Oc number, eventually resetting to levels comparable with those in vehicle-treated controls. This is reminiscent of the spectrum of effects on Oc number in the present study, and it supports the hypothesis that the apoptotic response of Ocs to N-BP treatment is a function of dose.
In examining apoptosis induced by high-dose N-BP treatment, we sometimes observed multinucleated cells with pyknotic nuclei adjacent, but unattached, to bone surfaces. (For purposes of quantification, these cells were included in the total count of apoptotic Ocs, shown in Fig. 3b). The morphology of these apoptotic cells was characteristic of a kind of apoptosis termed “anoikis” [16]. Anoikis is apoptosis associated with reduced adhesion to extracellular matrix components such as the integrins that are involved in attachment of Ocs to bone. In many cell types, detachment from matrix components induces programmed cell death. However, we cannot exclude the possibility that these cells had undergone apoptosis while still on the bone surface, with detachment occurring subsequently. It is notable that the cell death response of human osteogenic sarcoma cells to zoledronic acid in vitro has also been characterized as resembling anoikis [17].
It may be worth noting that dosing frequency itself, and not just the effect of dose per se, could influence the effects of N-BPs on Ocs. N-BPs preferentially bind to resorbing surfaces of bone compared with resting or forming surfaces [13]. Since bone resorption and formation cycles are processive, the character of a given parcel of bone changes over time. As the microenvironment changes, binding of more frequently dosed N-BPs may even out over the total bone surface. In contrast, less frequently dosed N-BPs are likely to concentrate preferentially in regions undergoing resorption at the time of N-BP administration. In those regions exposed to higher concentrations of the less frequently dosed N-BPs, Oc apoptosis may be more likely to occur, whereas in those exposed to lower concentrations, antiresorptive effects may be dominant over apoptosis.
The trend toward a reduction in Oc apoptosis with lower N-BP doses is congruent with findings reported from a phase III trial examining the effects of 3 years of chronic daily ALN dosing [18]. Contrary to the current report, by examination of transilial biopsies these authors observed increases in the numbers of giant multinucleated Ocs with apoptotic morphology to be associated with daily ALN treatment. However, they also saw increases in normal Ocs and total Ocs. Increases in normal and total Ocs after 3 years of ALN dosing in the clinical study could have been due to retardation of apoptosis or to effects of secondary hyperparathyroidism. With regard to the latter, data from a parallel phase III trial show that PTH levels did initially rise upon treatment with ALN but returned to baseline by year 3 [19]. Weinstein et al. [18] conclude that increased Oc numbers after 3 years of clinical treatment were likely due to prolongation of apoptosis by ALN. They attribute the increased Oc numbers to a prolongation both of Oc life span and of the DNA fragmentation phase of Oc apoptosis. The fact that in the current study the giant Oc phenotype occurred acutely after treatment with only high-dose N-BP whereas in the clinical study the phenotype was seen after chronic treatment with low-dose N-BP can be explained by a rapid induction of apoptosis by high N-BP doses versus prolonging of apoptosis triggered with lower dosing, correlating with suppression of and increase in Oc numbers, respectively.
Finally, in considering possible geranylgeranylation (and GGOH)–independent mechanisms, it is notable that the proapoptotic ATP analog ApppI is generated in Ocs and macrophages, among other cells, as a consequence of ZOL-mediated FPPS inhibition and accompanying accumulation of the upstream mevalonate-pathway metabolite isopentenyl pyrophosphate (IPP) [20]. Apoptotic induction by ApppI, like that of the cytotoxic clodronate metabolite AppCCl2p [20], is thought to occur through interaction with the mitochondrial adenine nucleotide translocase, ANT [21]. Given that IPP, and therefore ApppI, accumulation is an upstream response to FPPS inhibition, there is no reason to believe that the combination of an N-BP with GGOH should alter this effect; and accumulation of ApppI could lead to induction of Oc apoptosis irrespective of the presence of GGOH in the system. Moreover, IPP accumulation leading to higher concentrations of ApppI would be predicted to occur with more saturating inhibition of FPPS by the N-BPs when used at higher doses. We previously evaluated the impact of simvastatin treatment on the effects of suprapharmacological doses of N-BP on rat Ocs in vivo [8]. Inhibition of the mevalonate pathway upstream of IPP by simvastatin would be predicted to prevent accumulation of IPP and thus ApppI, although this would have no positive impact on inhibition of geranylgeranylation. Notably, while in vivo high-dose N-BP treatment for 2 days (as with the present study) induced morphological changes similar to those reported here, cotreatment with simvastatin had no discernible effect on preventing changes in Oc morphology. Simvastatin did, however, prevent N-BP-induced downregulation of HMGCoA reductase expression, which we attributed to its effects on blocking N-BP-induced accumulation of upstream metabolites, including IPP. Oc apoptosis was not examined in the presence of simvastatin. While not definitive, the data from this previous work suggest that morphological changes (plump, rounded Ocs) induced by high-dose N-BP may not arise from the formation of ApppI. It remains to be determined if, at sufficiently high doses of N-BPs, ApppI accumulation plays a role in shifting from a low-dose N-BP suppression of Oc resorption that is preferentially associated with blocked isoprenylation to induction of Oc apoptosis that is dependent upon the accumulation of a mevalonate-pathway metabolite.
In summary, we have documented evidence for differential acute responses of Ocs to N-BPs at lower versus higher doses. In vitro evidence suggests an FPPS-mediated and isoprenylation-associated mechanistic window, a 20-fold dosing range within which inhibition of Oc activity can be counteracted by addition of GGOH. In vivo evidence from the current study in growing rats suggests that the higher N-BP doses required for monthly or yearly clinical regimens bring into play additional means of achieving Oc inhibition, including induction of programmed cell death. In this model, acute treatment with high doses of N-BPs induces apoptotic effects resembling anoikis.
With years of clinical treatment, daily ALN at the dose used to treat osteoporosis appears also to induce formation of giant Ocs with apoptotic features [18]. The fact that these apoptotic Ocs arise in the context of an overall increase in Oc number is consistent with the hypothesis that the apoptotic process itself is prolonged. Since lower daily [22] and much higher annual [23] doses of N-BPs reduce the risk of both spine and hip fractures, the clinical implications of different effects on apoptosis remain to be determined.
References
Fosamax [package insert] (2012) Merck Sharpe & Dohme, Whitehouse Station
Actonel [package insert] (2012) Warner Chilcott, Rockaway
Boniva [package insert] (2006) Roche Laboratories, Nutley
Zometa [package insert] (2005) Novartis Pharmaceuticals, East Hanover
Fleisch H (1998) Bisphosphonates: mechanisms of action. Endocr Rev 19:80–100
Sato M, Grasser W, Endo N, Akins R, Simmons H, Thompson DD, Golub E, Rodan GA (1991) Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Invest 88:2095–2105
Dunford JE, Thompson K, Coxon FP, Luckman SP, Hahn FM, Poulter CD, Ebetino FH, Rogers MJ (2001) Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther 296:235–242
Fisher JE, Rodan GA, Reszka AA (2000) In vivo effects of bisphosphonates on the osteoclast mevalonate pathway. Endocrinology 141:4793–4796
Reszka AA, Rodan GA (2004) Nitrogen-containing bisphosphonate mechanism of action. Mini Rev Med Chem 4:711–719
Burridge K, Wennerberg K (2004) Rho and Rac take center stage. Cell 116:167–179
Halasy-Nagy JM, Rodan GA, Reszka AA (2001) Inhibition of bone resorption by alendronate and risedronate does not require osteoclast apoptosis. Bone 29:553–559
Hughes DE, MacDonald BR, Russell RG, Gowen M (1989) Inhibition of osteoclast-like cell formation by bisphosphonates in long-term cultures of human bone marrow. J Clin Invest 83:1930–1935
Masarachia P, Weinreb M, Balena R, Rodan GA (1996) Comparison of the distribution of 3H-alendronate and 3H-etidronate in rat and mouse bones. Bone 19:281–290
Russell RG (2007) Bisphosphonates: mode of action and pharmacology. Pediatrics 119(Suppl 2):S150–S162
Seedor JG, Quartuccio HA, Thompson DD (1991) The bisphosphonate alendronate (MK-217) inhibits bone loss due to ovariectomy in rats. J Bone Miner Res 6:339–346
Frisch SM, Screaton RA (2001) Anoikis mechanisms. Curr Opin Cell Biol 13:555–562
Evdokiou A, Labrinidis A, Bouralexis S, Hay S, Findlay DM (2003) Induction of cell death of human osteogenic sarcoma cells by zoledronic acid resembles anoikis. Bone 33:216–228
Weinstein RS, Roberson PK, Manolagas SC (2009) Giant osteoclast formation and long-term oral bisphosphonate therapy. N Engl J Med 360:53–62
Tucci JR, Tonino RP, Emkey RD, Peverly CA, Kher U, Santora AC (1996) Effect of three years of oral alendronate treatment in postmenopausal women with osteoporosis. Am J Med 101:488–501
Monkkonen H, Auriola S, Lehenkari P, Kellinsalmi M, Hassinen IE, Vepsalainen J, Monkkonen J (2006) A new endogenous ATP analog (ApppI) inhibits the mitochondrial adenine nucleotide translocase (ANT) and is responsible for the apoptosis induced by nitrogen-containing bisphosphonates. Br J Pharmacol 147:437–445
Monkkonen H, Ottewell PD, Kuokkanen J, Monkkonen J, Auriola S, Holen I (2007) Zoledronic acid-induced IPP/ApppI production in vivo. Life Sci 81:1066–1070
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 348:1535–1541
Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356:1809–1822
Gertz BJ, Holland SD, Kline WF, Matuszewski BK, Freeman A, Quan H, Lasseter KC, Mucklow JC, Porras AG (1995) Studies of the oral bioavailability of alendronate. Clin Pharmacol Ther 58:288–298
Ravn P, Neugebauer G, Christiansen C (2002) Association between pharmacokinetics of oral ibandronate and clinical response in bone mass and bone turnover in women with postmenopausal osteoporosis. Bone 30:320–324
Author information
Authors and Affiliations
Corresponding author
Additional information
All authors are employed by Merck Sharp & Dohme, a subsidiary of Merck & Co., the sponsor of the study, and may own stock options in the company.
Rights and permissions
About this article
Cite this article
Fisher, J.E., Rosenberg, E., Santora, A.C. et al. In Vitro and In Vivo Responses to High and Low Doses of Nitrogen-Containing Bisphosphonates Suggest Engagement of Different Mechanisms for Inhibition of Osteoclastic Bone Resorption. Calcif Tissue Int 92, 531–538 (2013). https://doi.org/10.1007/s00223-013-9711-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-013-9711-0