Abstract
Bisphosphonates are used in treating patients with breast cancer. In vitro studies have shown that bisphosphonates act directly on tumour cells, inhibiting cell proliferation and inducing apoptosis. In most such studies, drugs were added to culture media exposing cells to high bisphosphonate concentrations in solution. However, since bisphosphonates bind to bone hydroxyapatite with high affinity and remain bound for very long periods of time, these experimental systems are not an optimal model for the action of the drugs in vivo. The aim of this study was to determine whether bone-bound zoledronate has direct effects on adjacent breast cancer cells. Bone slices were pre-incubated with bisphosphonate solutions, washed, and seeded with cells of the breast cancer cell lines, MCF7 or MDA-MB-231. Proliferation was assessed by cell counts and thymidine incorporation for up to 72 h. Inhibition of the mevalonate pathway was tested by measuring the levels of unprenylated Rap1A, and apoptosis was examined by the presence of cleaved caspase-8 on western blots. The proliferation rate of breast cancer cells on zoledronate-treated bone was significantly lower compared to cells on control bone. Other bisphosphonates showed a similar inhibitory effect, with an order of potency similar to their clinical potencies. Unprenylated Rap1A accumulated in MCF7 cells on zoledronate-treated bone, suggesting zoledronate acted through the inhibition of the mevalonate pathway. Accumulation of cleaved caspase-8 in MDA-MB-231 cells on bisphosphonate-treated bone indicated increased apoptosis in the cells. In conclusion, bone-bound zoledronate inhibits breast cancer cell proliferation, an activity that may contribute to its clinical anti-tumour effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone is a common site of metastasis in patients with breast cancer, with approximately 80% of advanced breast cancer metastasising into bone tissue [1, 2]. Dissemination to bone is a crucial step for metastasis of tumour cells [3]. It involves initial adhesion of tumour cells at the metastatic site, extravasation, colonisation and growth on this site [4]. Morbidity associated with bone metastases remains a major clinical problem even with advances in treatment options [5].
Bisphosphonates are potent inhibitors of bone resorption and are used as a standard treatment for breast cancer patients, aiming to mitigate the risk of skeletal complications in metastatic disease [6]. Bisphosphonates have been shown to inhibit multiple steps in the metastatic process, including tumour cell adhesion and proliferation, and in addition they inhibit cell invasion by modifying the bone microenvironment [7]. Bisphosphonates have a high affinity for the hydroxyapatite in the bone extracellular matrix and once administered into the body they quickly bind to bone hydroxyapatite, where they remain bound for a very long time, with a half-life of up to 10 years [8]. The most commonly used bisphosphonate in the treatment of metastatic breast cancers is the nitrogen-containing bisphosphonate zoledronate. Other clinically used nitrogen-containing bisphosphonates are alendronate, ibandronate and pamidronate, and non-nitrogen-containing bisphosphonates that have been used clinically include clodronate, etidronate and tiludronate. The two groups of bisphosphonate have different mechanisms of action; the nitrogen-containing compounds inhibit the enzyme farnesyl pyrophosphate synthase (FPPS) in the mevalonate pathway, whereas the non-nitrogen-containing bisphosphonates inhibit the mitochondrial ADP/ATP transporter and are pro-apoptotic [9,10,11]. Interestingly, some clinical studies have found that bisphosphonate treatment not only reduces skeletal complications but also affects cancer progression. Gnant et al. [12] found that adjuvant zoledronate treatment decreased the relative risk of cancer progression by 36%, and reduced the number of breast cancer metastases. A number of clinical studies have demonstrated that adjuvant zoledronate treatment increases cancer-free survival [12,13,14,15], decreases recurrence of breast cancer [12, 16,17,18] and decreases the incidence of cancer in normal older women [19].
In vitro studies have shown that bisphosphonates can act directly on tumour cells, inducing apoptosis and inhibiting cell proliferation, adhesion and migration [20,21,22,23,24]. Most in vitro studies investigating the effect of bisphosphonates on tumour cells were performed by adding the drugs to tumour cell culture media, directly exposing cells to bisphosphonates in solution [24,25,26]. Studies were carried out with high concentrations of bisphosphonates and prolonged incubation periods [27, 28]. However, since bisphosphonates remain bound to bone for a very long time, experimental systems where cells are directly treated with bisphosphonates in the culture solution are not an optimal model for the action of the drugs in vivo.
Thus, the aim of the present study was to determine whether bone-bound zoledronate has direct effects on adjacent breast cancer cells, thus investigating the possible mechanisms of the beneficial effects of this drug on breast cancer in vivo.
Methods
Preparation of Bisphosphonate-Coated Bovine Bone Slices
Zoledronate (Novartis), pamidronate (Mayne Pharma, Melbourne), clodronate (Boehringer Mannheim), ibandronate (Roche) and alendronate (Sigma-Aldrich, St. Louis) were prepared as stock solutions of 100 μM in phosphate buffered saline (PBS; pH 7.4) and filter sterilised. Cortical bovine bone slices were prepared as previously described [29]. Briefly, bovine cortical bone pieces cut to a thickness of 70 μm and to a size of 4 × 4 mm were sonicated, sterilised in 70% ethanol and pre-incubated in either PBS or different concentrations of bisphosphonate solutions (1, 10, 30, 60 or 100 μM) overnight at room temperature. At the end of the incubation, the bone slices were washed with PBS and transferred to 96-well plates (1 bone slice/well) with cell culture media.
Cell lines and Cell Culture Conditions
The human breast cancer MCF7 cell line was used. MCF7 cells were maintained and cultured in Minimum Essential Medium Alpha (αMEM; Gibco, Life Technologies) containing 5% fetal bovine serum (FBS; Gibco, Life Technologies), 0.01 mg/mL recombinant human insulin (Sigma-Aldrich, St. Louis, MO) and 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco, Life Technologies). Cell cultures were maintained and cultured in T75 flasks (Corning) at 37 °C in a humidified atmosphere containing 5% CO2 until sub-confluent. Cells were seeded drop-wise onto bisphosphonate pre-treated bovine bone slices in 96-well plates (20,000, 40,000 or 60,000 cells/bone). Cells were allowed to adhere for 18–22 h and then bone slices with adherent cells were transferred to new wells containing fresh culture media, then visualised or used for analysis of cell proliferation or protein expression.
MDA-MB-231 cells, a human breast cancer cell line, were maintained and cultured in αMEM containing 5% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin. The cells were seeded onto bisphosphonate pre-treated bovine bone slices in 96-well plates at the densities of 5000 and 20,000 cells/slice for proliferation and apoptosis assays, respectively. The other culture conditions were the same as for MCF7 cells above.
Visualisation of Bone Slices
For visualisation of adherent cells on bone, the bone slices were fixed with 10% neutral buffered formalin (LabServ Pronalys) and stained with toluidine blue dye for 30 s at the end of each culture. Cells were visualised on an Olympus SZ61 stereo microscope.
Analysis of Cell Proliferation
Cell Numbers
At the end of the culture period, bone slices with adherent cells were incubated with 10 mg/mL collagenase (Sigma-Aldrich) to disaggregate cell clumps. Bone slices were then incubated with trypsin/EDTA (Gibco, Invitrogen) at 37 °C until cells detached. An aliquot of the cell suspension was counted in a hemocytometer and results were expressed as numbers of cells/bone.
3H-Thymidine Incorporation Assay
Six hours before the end of each culture period, 3H-thymidine (0.1 μCi/well; PerkinElmer, Waltham, MA) was added into the culture media. At the end of the culture period, thymidine incorporation was terminated by the addition of 10% trichloroacetic acid. Bone slices with adherent cells were washed with ethanol:ether mix, dried, dissolved in 2 M potassium hydroxide at room temperature for 1 h, neutralized with 1 M hydrochloric acid and the entire dissolved sample was counted for radioactivity.
Western Blots
Following removal of culture media, the bone slices with the adherent cells were washed twice with PBS then protein expression was assessed using Western blot methodology. For each treatment group, cells from 11 bone slices were directly lysed with RIPA buffer (ThermoScientific, Rockford, IL) containing protease inhibitors (Roche Diagnostics, Mannheim, Germany) and pooled together. Samples were resolved on 4–15% stain-free precast gel (Bio-Rad, Hercules, CA) and transferred to PVDF membranes (Bio-Rad, Hercules, CA). The membranes were blocked with 5% (w/v) non-fat milk powder in TBS-Tween buffer. Membranes were then incubated overnight at 4 °C with either goat polyclonal anti-Rap1A antibody (SC-1482, Santa Cruz Biotechnology, Santa Cruz, CA, 1:200) which reacts with the unprenylated form of Rap1A or rabbit monoclonal anti-caspase-8 antibody (MA5-15054, Invitrogen, Thermo Fischer Scientific, MA, 1:300) which reacts with the cleaved forms of caspase-8. Mouse anti-α-tubulin monoclonal antibody (T-5168, Sigma-Aldrich, 1:500) and mouse anti-β-actin monoclonal antibody (SC-69879, Santa Cruz Biotechnology, Santa Cruz, CA, 1:1000) were used for internal loading controls. After 1-h incubation with the secondary antibody of anti-goat peroxidase-conjugated IgG (A5420, Sigma-Aldrich, 1:8000) or anti-mouse IgG (A9044, Sigma-Aldrich, 1:10,000; ab97046, Abcam, 1:10,000), chemiluminescence was visualised using Amersham ECL Plus Western Blotting Detection Reagents (GE Healthcare, Buckinghamshire, UK).
Statistical Analysis
The statistical tests used are indicated in the figure legends. All tests were two-tailed and a 5% significance level was maintained throughout. Data were analysed using GraphPad Prism 5.0 (GraphPad, Inc., San Diego, CA, USA). Representative graphs from two or three repeated experiments are shown.
Results
Initial adhesion of MCF7 cells to bone slices was assessed by microscopy and by counting the cells 4 h after seeding (Fig. 1). The cell numbers on control and zoledronate-treated bone were comparable. MCF7 cells were incubated on bones slices for up to 72 h in order to investigate the effect of bone-bound zoledronate on cell number. Cell numbers were similar on control and zoledronate-treated bones at 24 h but, at 48 and 72 h, significantly fewer cells were seen on the zoledronate-treated bones (Fig. 2). Analysis of 3H-thymidine incorporation produced similar results, demonstrating significant inhibition of DNA synthesis at 48 and 72 h of incubation on the zoledronate-treated bones compared to controls.
Adhesion of MCF7 cells to control and zoledronate-treated bone slices. a Four hours after seeding MCF7 cells on bone slices, cells were harvested and counted. Zoledronate used for pre-treatment was 100 µM, bars represent mean ± 95% CI, n = 6. Data were analysed by Student’s t test and the groups were not significantly different. b Representative micrographs of toluidine blue stained MCF7 cells on control (left panel) and zoledronate-treated (right panel) bone slices at 4 h. Scale bar represents 500 µm
Growth of MCF7 cells on control and zoledronate-treated bone slices. a MCF7 cells seeded on the bone slices were harvested and counted at the indicated time points (upper panel). Incorporation of 3H-thymidine added 6 h prior to the end of each culture period was determined (lower panel). Concentration of zoledronate used for pre-treatment of bone slices was 100 µM, bars represent mean ± 95% CI, n = 6. Data were analysed by two-way ANOVA with Bonferroni’s post hoc test. **P < 0.01, ***P < 0.001, ****P < 0.0001 versus control at the same time point. b Representative micrographs of MCF7 cells on control (left panels) and zoledronate-coated (right panels) bone slices at 24, 48 and 72 h. Scale bar represents 500 µm
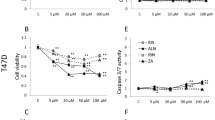
We next determined whether the inhibition of cell proliferation was dependent on the concentration of zoledronate used in the pre-incubation of the bone slices (Fig. 3). A dose-dependent inhibitory effect was seen over a range of zoledronate pre-treatment concentrations, from 30 to 100 μM. In order to determine whether the inhibitory effect on cell proliferation could also be induced by other clinically used bisphosphonates, cell counts and 3H-thymidine incorporation were determined in MCF7 cells cultured for 72 h on bone slices pre-incubated with either zoledronate, alendronate, ibandronate, pamidronate or clodronate (Fig. 4). Cell numbers were similar on control bone slices and bones pre-incubated with clodronate, whereas all the other bisphosphonates significantly reduced the number of cells. Incorporation of 3H-thymidine was significantly reduced with zoledronate and alendronate, while it was not with ibandronate, pamidronate or clodronate.
Dose-response of MCF7 cell proliferation on control and zoledronate-treated bone slices. The bone slices were pre-treated with different concentrations of zoledronate overnight, and then washed with PBS before being used in the cell culture. No zoledronate was added to the culture medium. Bars represents mean ± 95% CI, n = 6. Data were analysed by two-way ANOVA with Dunnett’s post hoc test. *P < 0.05, **P < 0.01, ****P < 0.0001, versus control
Comparison of MCF7 cell proliferation on bone slices pre-incubated with different bisphosphonates. MCF7 cells seeded on bone slices were harvested and counted (upper panel). Incorporation of 3H-thymidine added 6 h prior to the end of the 72-h culture period was determined (lower panel). Concentration of the bisphosphonates used in the pre-incubation was 100 µM, bars represent mean ± 95% CI, n = 5 or 6. Data were analysed by one-way ANOVA with Dunnett’s post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 versus control. ZOL zoledronate, ALN alendronate, IBN ibandronate, PAM pamidronate, CLO clodronate
To determine whether these findings were generalizable to other breast cancer cell lines, we repeated the thymidine incorporation experiment in MDA-MB-231 cells, which have a more invasive phenotype [30]. Very similar results were found, with substantial reductions in thymidine incorporation on bones pre-treated with zoledronate, alendronate and ibandronate, but no significant effects with pamidronate or clodronate (Fig. 5).
Comparison of MDA-MB-231 cell proliferation on bone slices pre-incubated with different bisphosphonates. Incorporation of 3H-thymidine added 6 h prior to the end of the 72-h culture period was determined. Concentration of the bisphosphonates used in the pre-incubation were 100 µM, bars represent mean ± 95% CI, n = 6. Data were analysed by one-way ANOVA with Dunnett’s post hoc test. ****P < 0.0001 versus control. ZOL zoledronate, ALN alendronate, IBN ibandronate, PAM pamidronate, CLO clodronate
While the substantial reductions in thymidine incorporation with bisphosphonates shown here indicate that inhibition of cell proliferation is an important contributor to the reduction in cell numbers, it is also possible that this is contributed by bisphosphonate-induced apoptosis. We were unable to study apoptosis of MCF7s on the bisphosphonate-treated bone slices, as caspases, one of the most prominent players in apoptosis signalling, were difficult to detect in the cell line. However, these studies were successfully undertaken in the MDA-MB-231 cells (Fig. 6). Cleaved caspase-8 was just detectable in control cells grown on untreated bone, and these levels were significantly increased by the addition of etoposide to the media (a positive control), or by pre-treatment of bones with either alendronate or zoledronate.
Nitrogen-containing bisphosphonates have been shown to act on osteoclasts through the inhibition of the enzyme FPPS [10]. The downstream products of this enzyme are involved in the prenylation of small GTPases, including Rap1A. In order to determine whether bisphosphonates act through a similar mechanism in MCF7 cells, we compared the levels of unprenylated Rap1A in MCF7 cells cultured on plastic, bone slices, and bone slices pre-incubated with alendronate or zoledronate (Fig. 7). Unprenylated Rap1A could not be detected in MCF7 cells cultured on plastic or bone slices, but was present in the cells cultured on bone slices pre-incubated with the bisphosphonates. This finding suggests that similar mechanisms are involved in the effects of alendronate and zoledronate in tumour cells and in osteoclasts.
Discussion
The present studies demonstrate that bone-bound zoledronate and other bisphosphonates can inhibit the growth of adjacent breast cancer cells. These results suggest that when zoledronate is used clinically in patients with breast cancer, the cells become exposed to the drug as they adhere to bone surfaces and their growth on bone is inhibited. In this model system, similar number of cells adhered to untreated bone slices and to those pre-treated with zoledronate, but at 48 and 72 h the number of cells and their growth on the pre-treated bones were significantly lower than the controls.
We observed dose-dependent effects of zoledronate used for pre-treatment of bone slices. There was no effect of zoledronate concentration ≤ 10 μM, whereas the effects were seen at ≥ 30 μM. Similar observation was made by Tamura et al. [31] that 10 to 100 μM of zoledronate in the culture media reduced proliferation of four oral carcinoma cell lines. After intravenous injection in humans, peak serum levels of zoledronate after 4 mg reach approximately 2 μM [32], and considering the concentration of zoledronate in plasma after infusion rapidly drops due to the drug binding to bone, the concentration of bone-bound zoledronate is expected to be higher than the serum level [33]. Previously, Cornish et al. [29] have calculated the amount of zoledronate on the bone to be 150 nmol per gram of bone, for an average cancer patient administered 4 mg per month for 4 years. Also, Kuiper et al. [34] estimated the concentration of zoledronate in bone marrow to be between 20 and 100 μM in mice which had been administered a dose equivalent to that of human receiving 4 mg per month. Thus, the zoledronate concentrations used in our study appear to be within the clinically used range and the inhibitory effect on breast cancer cells is likely to be clinically relevant.
The results seen here from comparisons of clinically available bisphosphonates were consistent with the potency and affinity for hydroxyapatite of those drugs. The significant inhibitory effect of zoledronate and alendronate on MCF7 proliferation compared to less effective ibandronate and pamidronate is also seen from other studies which tested the binding affinity of bisphosphonates. The studies showed that zoledronate had the highest binding affinity to hydroxyapatite of bone, followed by alendronate and other bisphosphonates including ibandronate and clodronate [35, 36]. Clodronate, a non-nitrogen-containing bisphosphonate, did not show evidence of growth inhibition in breast cancer cells, and both cell count and thymidine incorporation results suggested clodronate on the bone surface did not have any effect on the proliferation of the cancer cells. These results are consistent with the nitrogen-containing bisphosphonates being more potent than non-nitrogen-containing bisphosphonates, which are observed in pre-clinical as well as clinical studies [37,38,39].
The nitrogen-containing bisphosphonates including zoledronate and alendronate inhibit osteoclasts and myeloma cells by inhibiting enzymes in the mevalonate pathway [37]. It has been established that the bisphosphonates induce apoptosis of osteoclasts by inhibiting prenylation of small GTP proteins including Rap, Ras and Rho [40]. The present results show that bisphosphonates have the same effect in breast cancer cells, and this is likely to mediate the growth inhibition shown here. This inference is supported by zoledronate having a greater effect than alendronate on both cell growth and Rap1A levels, whereas clodronate, a bisphosphonate that does not act on farnesyl pyrophosphate synthase, had no real effects on either. We have previously demonstrated similar effects of alendronate and zoledronate pre-treatment on Rap1A in Chinese hamster ovary (CHO) cells and in the Caco-2 human colorectal adenocarcinoma epithelial cell line grown on bone slices [29], so this is not specific to breast cancer cells. The mevalonate pathway is a crucial metabolic pathway for tumour growth and progression and therefore a potential therapeutic target [41]. Cancer cells are characterised by aberrant cell growth and metabolism and they upregulate the mevalonate pathway to support their proliferation [41]. The inhibitory effect of bone-bound bisphosphonate on the mevalonate pathway of adjacent breast cancer cells would be an explanation for the observations that bisphosphonates suppress the tumour burden in breast cancer patients. Zoledronate has anti-tumour effects in pre-clinical studies [26, 31, 42,43,44], and clinical studies found that zoledronate reduced solid tumour bone metastases [3, 45]. One study with patients with recurrent solid tumours also showed that the zoledronate-treated group had a higher proportion of bone metastases-free patients compared to the control group [46]. Also, studies show that zoledronate treatment reduces the incidence of micro-metastases in bone marrow in breast cancer [3, 47] which could explain its possible role in increasing cancer-free survival seen in these patients.
In our previous study, we have shown that bone-bound bisphosphonates directly inhibit the proliferation of primary osteoblast cells, as well as other cells including human colorectal adenocarcinoma epithelial cells and Chinese hamster ovary cells [29]. In our current study, we showed that bone-bound bisphosphonates also directly affect the proliferation of a breast cancer cell line. Our 3H-thymidine results show that cell proliferation was significantly inhibited compared to the control group at the same time point. The cell count results show that, while the cell numbers on control bones increased, there was no increase in cell numbers on the zoledronate-treated bone over the culture period of 72 h. One possible explanation for these results could be that the cell proliferation in the zoledronate group was compensated for by apoptosis of the cells exposed to the bone-bound zoledronate, as shown with the presence of cleaved caspase-8 in MDA-MB-231 cells. Zoledronate-induced apoptosis of breast cancer cells was also observed in the other studies with MCF7 and MDA-MB-231, which examined the effect of zoledronate in solution [25, 48,49,50]. Also, there is evidence that bisphosphonates induce apoptosis in animal models inoculated with human breast cancer cells. Hiraga et al. [51] showed ibandronate-induced apoptosis in MDA-MB-231 tumour xenograft in mammary fat pads in mice. There are several studies that have looked into the mechanism of bisphosphonates on apoptosis of human cancer cells. These studies suggest inhibition of mevalonate pathway by bisphosphonate leads to disruption of downstream signalling and failure of prenylation of small GTPases, resulting in apoptosis in human cancer cells [48, 52].
The other possible explanation for the inhibition of the cell proliferation in zoledronate-treated group compared to the control group in our study is cell cycle arrest. There is in vitro evidence that bisphosphonates, including zoledronate, induce MCF7 cell cycle arrest and hence reduce cell proliferation [53,54,55,56]. This cell cycle arrest and reduction in cell proliferation by zoledronate was also seen in other breast cancer cell line treated with zoledronate [57, 58]. Zoledronate was also found to arrest or prolong the cell cycle progression of other cancer cell lines including osteosarcoma, melanoma, myeloma, small- and non-small-cell lung cancer, mesothelioma, and cholangiocarcinoma cells, [59,60,61,62,63,64,65,66,67], as well as non-cancer cells including human primary oral mucosal keratinocytes [68]. However, these studies were assessing the effects of bisphosphonates in culture media, which is not an appropriate model for its clinical use where its presence in biological fluids after administration is measured in hours.
In conclusion, our study demonstrates that bone-bound zoledronate effectively reduces the growth and number of breast cancer cells, suggesting a direct anti-tumour effect of the drug. Since clinical use of zoledronate results in bone-bound drug being present for many years after dosing, and since this bone is adjacent to the marrow space where micro-metastases from breast cancer are known to reside, this toxicity may contribute to zoledronate’s anti-tumour effects found in clinical studies.
References
Eilon G, Mundy GR (1978) Direct resorption of bone by human breast cancer cells in vitro. Nature 276:726–728
Galasko CS (1969) The detection of skeletal metastases from mammary cancer by gamma camera scintigraphy. Br J Surg 56:757–764
Aft R, Naughton M, Trinkaus K, Watson M, Ylagan L, Chavez-MacGregor M, Zhai J, Kuo S, Shannon W, Diemer K, Herrmann V, Dietz J, Ali A, Ellis M, Weiss P, Eberlein T, Ma C, Fracasso PM, Zoberi I, Taylor M, Gillanders W, Pluard T, Mortimer J, Weilbaecher K (2010) Effect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: an open label, randomised, phase 2 trial. Lancet Oncol 11:421–428
Suva LJ, Griffin RJ, Makhoul I (2009) Mechanisms of bone metastases of breast cancer. Endocr Relat Cancer 16:703–713
Brown JE, Cook RJ, Major P, Lipton A, Saad F, Smith M, Lee KA, Zheng M, Hei YJ, Coleman RE (2005) Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J Natl Cancer Inst 97:59–69
Chlebowski RT, Col N (2011) Bisphosphonates and breast cancer incidence and recurrence. Breast Dis 33:93–101
Liu J, Huang W, Zhou R, Jia S, Tang W, Luo Y, Zhang J (2015) Bisphosphonates in the treatment of patients with metastatic breast, lung, and prostate cancer: a meta-analysis. Medicine 94:e2014
Lin JH (1996) Bisphosphonates: a review of their pharmacokinetic properties. Bone 18:75–85
Zhang Y, Leon A, Song Y, Studer D, Haase C, Koscielski LA, Oldfield E (2006) Activity of nitrogen-containing and non-nitrogen-containing bisphosphonates on tumor cell lines. J Med Chem 49:5804–5814
van Beek E, Pieterman E, Cohen L, Lowik C, Papapoulos S (1999) Farnesyl pyrophosphate synthase is the molecular target of nitrogen-containing bisphosphonates. Biochem Biophys Res Commun 264:108–111
Lehenkari PP, Kellinsalmi M, Napankangas JP, Ylitalo KV, Monkkonen J, Rogers MJ, Azhayev A, Vaananen HK, Hassinen IE (2002) Further insight into mechanism of action of clodronate: inhibition of mitochondrial ADP/ATP translocase by a nonhydrolyzable, adenine-containing metabolite. Mol Pharmacol 61:1255–1262
Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Postlberger S, Menzel C, Jakesz R, Seifert M, Hubalek M, Bjelic-Radisic V, Samonigg H, Tausch C, Eidtmann H, Steger G, Kwasny W, Dubsky P, Fridrik M, Fitzal F, Stierer M, Rucklinger E, Greil R, Marth C (2009) Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med 360:679–691
Valachis A, Polyzos NP, Coleman RE, Gnant M, Eidtmann H, Brufsky AM, Aft R, Tevaarwerk AJ, Swenson K, Lind P, Mauri D (2013) Adjuvant therapy with zoledronic acid in patients with breast cancer: a systematic review and meta-analysis. Oncologist 18:353–361
Eidtmann H, de Boer R, Bundred N, Llombart-Cussac A, Davidson N, Neven P, von Minckwitz G, Miller J, Schenk N, Coleman R (2010) Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36-month results of the ZO-FAST study. Ann Oncol 21:2188–2194
de Boer R, Bundred N, Eidtmann H (2010) The effect of zoledronic acid on aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole: the ZO-FAST study 5-year final follow-up. In: 33rd annual San Antonio breast cancer symposium, San Antonio, TX
Coleman R, Powles T, Paterson A, Gnant M, Anderson S, Diel I, Gralow J, von Minckwitz G, Moebus V, Bergh J, Pritchard KI, Bliss J, Cameron D, Evans V, Pan H, Peto R, Bradley R, Gray R (2015) Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet 386:1353–1361
Tevaarwerk A, Stewart JA, Love R, Binkley NC, Black S, Eickhoff J, Mulkerin DL (2007) Randomized trial to assess bone mineral density effects of zoledronic acid in postmenopausal women with breast cancer. J Clin Oncol 25:19558
Mauri D, Valachis A, Polyzos NP, Tsali L, Mavroudis D, Georgoulias V, Casazza G (2010) Does adjuvant bisphosphonate in early breast cancer modify the natural course of the disease? A meta-analysis of randomized controlled trials. J Natl Compr Cancer Netw JNCCN 8:279–286
Reid IR, Horne AM, Mihov B, Stewart A, Garratt E, Wong S, Wiessing KR, Bolland MJ, Bastin S, Gamble GD (2018) Fracture prevention with zoledronate in older women with osteopenia. N Engl J Med 379:2407–2416
Coxon JP, Oades GM, Kirby RS, Colston KW (2004) Zoledronic acid induces apoptosis and inhibits adhesion to mineralized matrix in prostate cancer cells via inhibition of protein prenylation. BJU Int 94:164–170
Miwa S, Mizokami A, Keller ET, Taichman R, Zhang J, Namiki M (2005) The bisphosphonate YM529 inhibits osteolytic and osteoblastic changes and CXCR-4-induced invasion in prostate cancer. Can Res 65:8818–8825
Muller S, Migianu E, Lecouvey M, Kraemer M, Oudar O (2005) Alendronate inhibits proliferation and invasion of human epidermoid carcinoma cells in vitro. Anticancer Res 25:2655–2660
van der Pluijm G, Vloedgraven H, van Beek E, van der Wee-Pals L, Lowik C, Papapoulos S (1996) Bisphosphonates inhibit the adhesion of breast cancer cells to bone matrices in vitro. J Clin Investig 98:698–705
Boissier S, Magnetto S, Frappart L, Cuzin B, Ebetino FH, Delmas PD, Clezardin P (1997) Bisphosphonates inhibit prostate and breast carcinoma cell adhesion to unmineralized and mineralized bone extracellular matrices. Can Res 57:3890–3894
Fromigue O, Lagneaux L, Body JJ (2000) Bisphosphonates induce breast cancer cell death in vitro. J Bone Miner Res 15:2211–2221
Jiang P, Zhang P, Mukthavaram R, Nomura N, Pingle SC, Teng D, Chien S, Guo F, Kesari S (2016) Anti-cancer effects of nitrogen-containing bisphosphonates on human cancer cells. Oncotarget 7:57932–57942
Holen I, Coleman RE (2010) Bisphosphonates as treatment of bone metastases. Curr Pharm Des 16:1262–1271
Stresing V, Daubine F, Benzaid I, Monkkonen H, Clezardin P (2007) Bisphosphonates in cancer therapy. Cancer Lett 257:16–35
Cornish J, Bava U, Callon KE, Bai J, Naot D, Reid IR (2011) Bone-bound bisphosphonate inhibits growth of adjacent non-bone cells. Bone 49:710–716
Iorns E, Drews-Elger K, Ward TM, Dean S, Clarke J, Berry D, Ashry DE, Lippman M (2012) A new mouse model for the study of human breast cancer metastasis. PLoS ONE 7:e47995
Tamura T, Shomori K, Nakabayashi M, Fujii N, Ryoke K, Ito H (2011) Zoledronic acid, a third-generation bisphosphonate, inhibits cellular growth and induces apoptosis in oral carcinoma cell lines. Oncol Rep 25:1139–1143
Reid IR, Brown JP, Burckhardt P, Horowitz Z, Richardson P, Trechsel U, Widmer A, Devogelaer JP, Kaufman JM, Jaeger P, Body JJ, Brandi ML, Broell J, Di Micco R, Genazzani AR, Felsenberg D, Happ J, Hooper MJ, Ittner J, Leb G, Mallmin H, Murray T, Ortolani S, Rubinacci A, Saaf M, Samsioe G, Verbruggen L, Meunier PJ (2002) Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N Engl J Med 346:653–661
Lambrinoudaki I, Vlachou S, Galapi F, Papadimitriou D, Papadias K (2008) Once-yearly zoledronic acid in the prevention of osteoporotic bone fractures in postmenopausal women. Clin Interv Aging 3:445–451
Kuiper JWP, Forster C, Sun C, Peel S, Glogauer M (2012) Zoledronate and pamidronate depress neutrophil functions and survival in mice. Br J Pharmacol 165:532–539
Nancollas GH, Tang R, Phipps RJ, Henneman Z, Gulde S, Wu W, Mangood A, Russell RG, Ebetino FH (2006) Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone 38:617–627
Rizzoli R (2011) Bisphosphonates for post-menopausal osteoporosis: are they all the same? QJM 104:281–300
Zekri J, Mansour M, Karim SM (2014) The anti-tumour effects of zoledronic acid. J Bone Oncol 3:25–35
Boissier S, Ferreras M, Peyruchaud O, Magnetto S, Ebetino FH, Colombel M, Delmas P, Delaisse JM, Clezardin P (2000) Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases. Can Res 60:2949–2954
Grey A, Reid IR (2006) Differences between the bisphosphonates for the prevention and treatment of osteoporosis. Ther Clin Risk Manag 2:77–86
Guo R-T, Cao R, Liang P-H, Ko T-P, Chang T-H, Hudock MP, Jeng W-Y, Chen CKM, Zhang Y, Song Y, Kuo C-J, Yin F, Oldfield E, Wang AHJ (2007) Bisphosphonates target multiple sites in both cis- and trans-prenyltransferases. Proc Natl Acad Sci USA 104:10022–10027
Mullen PJ, Yu R, Longo J, Archer MC, Penn LZ (2016) The interplay between cell signalling and the mevalonate pathway in cancer. Nat Rev Cancer 16:718–731
Koto K, Horie N, Kimura S, Murata H, Sakabe T, Matsui T, Watanabe M, Adachi S, Maekawa T, Fushiki S, Kubo T (2009) Clinically relevant dose of zoledronic acid inhibits spontaneous lung metastasis in a murine osteosarcoma model. Cancer Lett 274:271–278
Koto K, Murata H, Kimura S, Horie N, Matsui T, Nishigaki Y, Ryu K, Sakabe T, Itoi M, Ashihara E, Maekawa T, Fushiki S, Kubo T (2010) Zoledronic acid inhibits proliferation of human fibrosarcoma cells with induction of apoptosis, and shows combined effects with other anticancer agents. Oncol Rep 24:233–239
Di Salvatore M, Orlandi A, Bagala C, Quirino M, Cassano A, Astone A, Barone C (2011) Anti-tumour and anti-angiogenetic effects of zoledronic acid on human non-small-cell lung cancer cell line. Cell Prolif 44:139–146
Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, Chin JL, Vinholes JJ, Goas JA, Chen B (2002) A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst 94:1458–1468
Mystakidou K, Katsouda E, Parpa E, Kelekis A, Galanos A, Vlahos L (2005) Randomized, open label, prospective study on the effect of zoledronic acid on the prevention of bone metastases in patients with recurrent solid tumors that did not present with bone metastases at baseline. Med Oncol 22:195–201
Rack B, Juckstock J, Genss EM, Schoberth A, Schindlbeck C, Strobl B, Heinrigs M, Rammel G, Zwingers T, Sommer H, Friese K, Janni W (2010) Effect of zoledronate on persisting isolated tumour cells in patients with early breast cancer. Anticancer Res 30:1807–1813
Jagdev SP, Coleman RE, Shipman CM, Rostami HA, Croucher PI (2001) The bisphosphonate, zoledronic acid, induces apoptosis of breast cancer cells: evidence for synergy with paclitaxel. Br J Cancer 84:1126–1134
Neville-Webbe HL, Coleman RE, Holen I (2010) Combined effects of the bisphosphonate, zoledronic acid and the aromatase inhibitor letrozole on breast cancer cells in vitro: evidence of synergistic interaction. Br J Cancer 102:1010
Senaratne SG, Pirianov G, Mansi JL, Arnett TR, Colston KW (2000) Bisphosphonates induce apoptosis in human breast cancer cell lines. Br J Cancer 82:1459–1468
Hiraga T, Williams PJ, Mundy GR, Yoneda T (2001) The bisphosphonate ibandronate promotes apoptosis in MDA-MB-231 human breast cancer cells in bone metastases. Can Res 61:4418–4424
Senaratne SG, Mansi JL, Colston KW (2002) The bisphosphonate zoledronic acid impairs Ras membrane [correction of impairs membrane] localisation and induces cytochrome c release in breast cancer cells. Br J Cancer 86:1479–1486
Ibrahim T, Mercatali L, Sacanna E, Tesei A, Carloni S, Ulivi P, Liverani C, Fabbri F, Zanoni M, Zoli W, Amadori D (2012) Inhibition of breast cancer cell proliferation in repeated and non-repeated treatment with zoledronic acid. Cancer Cell Int 12:48
Dhar S, Chiplunkar SV (2010) Lysis of aminobisphosphonate-sensitized MCF-7 breast tumor cells by Vγ9 Vδ2 T cells. Cancer Immun 10:10
Lan YC, Chang CL, Sung MT, Yin PH, Hsu CC, Wang KC, Lee HC, Tseng LM, Chi CW (2013) Zoledronic acid-induced cytotoxicity through endoplasmic reticulum stress triggered REDD1-mTOR pathway in breast cancer cells. Anticancer Res 33:3807–3814
Gschwantler-Kaulich D, Weingartshofer S, Grunt TW, Mairhofer M, Tan Y, Gamper J, Singer CF (2017) Estradiol impairs the antiproliferative and proapoptotic effect of zoledronic acid in hormone sensitive breast cancer cells in vitro. PLoS ONE 12:e0185566
Merrell MA, Wakchoure S, Lehenkari PP, Harris KW, Selander KS (2007) Inhibition of the mevalonate pathway and activation of p38 MAP kinase are independently regulated by nitrogen-containing bisphosphonates in breast cancer cells. Eur J Pharmacol 570:27–37
Mansouri M, Mirzaei SA, Lage H, Mousavi SS, Elahian F (2014) The cell cycle arrest and the anti-invasive effects of nitrogen-containing bisphosphonates are not mediated by DBF4 in breast cancer cells. Biochimie 99:71–76
Romani AA, Desenzani S, Morganti MM, La Monica S, Borghetti AF, Soliani P (2009) Zoledronic acid determines S-phase arrest but fails to induce apoptosis in cholangiocarcinoma cells. Biochem Pharmacol 78:133–141
Okamoto S, Jiang Y, Kawamura K, Shingyoji M, Tada Y, Sekine I, Takiguchi Y, Tatsumi K, Kobayashi H, Shimada H, Hiroshima K, Tagawa M (2014) Zoledronic acid induces apoptosis and S-phase arrest in mesothelioma through inhibiting Rab family proteins and topoisomerase II actions. Cell Death Dis 5:1517
Iguchi T, Miyakawa Y, Yamamoto K, Kizaki M, Ikeda Y (2003) Nitrogen-containing bisphosphonates induce S-phase cell cycle arrest and apoptosis of myeloma cells by activating MAPK pathway and inhibiting mevalonate pathway. Cell Signal 15:719–727
Chang JUN, Wang WEI, Zhang HUI, Hu Y, Yin Z (2012) Bisphosphonates regulate cell proliferation, apoptosis and pro-osteoclastic expression in MG-63 human osteosarcoma cells. Oncol Lett 4:299–304
Manzano-Moreno FJ, Ramos-Torrecillas J, De Luna-Bertos E, Ruiz C, Garcia-Martinez O (2015) High doses of bisphosphonates reduce osteoblast-like cell proliferation by arresting the cell cycle and inducing apoptosis. J Cranio-Maxillo-Fac Surg 43:396–401
Forsea AM, Müller C, Riebeling C, Orfanos CE, Geilen CC (2004) Nitrogen-containing bisphosphonates inhibit cell cycle progression in human melanoma cells. Br J Cancer 91:803–810
Ory B, Blanchard F, Battaglia S, Gouin F, Redini F, Heymann D (2007) Zoledronic acid activates the DNA S-phase checkpoint and induces osteosarcoma cell death characterized by apoptosis-inducing factor and endonuclease-G translocation independently of p53 and retinoblastoma status. Mol Pharmacol 71:333–343
Matsumoto S, Kimura S, Segawa H, Kuroda J, Yuasa T, Sato K, Nogawa M, Tanaka F, Maekawa T, Wada H (2005) Efficacy of the third-generation bisphosphonate, zoledronic acid alone and combined with anti-cancer agents against small cell lung cancer cell lines. Lung Cancer 47:31–39
Li YY, Chang JW, Chou WC, Liaw CC, Wang HM, Huang JS, Wang CH, Yeh KY (2008) Zoledronic acid is unable to induce apoptosis, but slows tumor growth and prolongs survival for non-small-cell lung cancers. Lung Cancer 59:180–191
Ohnuki H, Izumi K, Terada M, Saito T, Kato H, Suzuki A, Kawano Y, Nozawa-Inoue K, Takagi R, Maeda T (2012) Zoledronic acid induces S-phase arrest via a DNA damage response in normal human oral keratinocytes. Arch Oral Biol 57:906–917
Acknowledgements
The authors acknowledge Mr. Greg Gamble for providing statistical advice and Dr. Yi Lance Xu for providing antibodies for Western blots. This study was funded by the Health Research Council of New Zealand (Grant 15/576 – Reid Mechanisms and Management of Musculoskeletal Disease).
Funding
This study was funded by the Health Research Council of New Zealand (Grant 15/576 – Reid Mechanisms and Management of Musculoskeletal Disease).
Author information
Authors and Affiliations
Contributions
YEP and JML performed the experimental research and analysis. UB contributed to the experimental work. JC, DN and IRR designed the study and the experimental plan. YEP prepared the first draft of the manuscript, DN, JC, UB, JML and IRR provided critical feedback and contributed to the final version.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Reid reports grants and personal fees from Amgen, personal fees and non-financial support from Novartis, outside the submitted work. Young-Eun Park, Usha Bava, Jian-ming Lin, Jillian Cornish and Dorit Naot declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Park, YE., Bava, U., Lin, Jm. et al. Bone-Bound Bisphosphonates Inhibit Proliferation of Breast Cancer Cells. Calcif Tissue Int 105, 497–505 (2019). https://doi.org/10.1007/s00223-019-00590-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-019-00590-5