Abstract
Low Intensity Electrical Stimulation (LIES) has been used for bone repair, but little is known about its effects on bone after menopause. Osteocytes probably play a role in mediating this physical stimulus and they could act as transducers through the release of biochemical signals, such as nitric oxide (NO). The aim of the present study was to investigate the effects of LIES on bone structure and remodeling, NOS expression and osteocyte viability in ovariectomized (OVX) rats. Thirty rats (200–220 g) were divided into 3 groups: SHAM, OVX, and OVX subjected to LIES (OVX + LIES) for 12 weeks. Following the protocol, rats were sacrificed and tibias were collected for histomorphometric analysis and immunohistochemical detection of endothelial NO synthase (eNOS), inducible NOS (iNOS), and osteocyte apoptosis (caspase-3 and TUNEL). OVX rats showed significant (p < 0.05 vs. SHAM) decreased bone volume (10% vs. 25%) and trabecular number (1.7 vs. 3.9), and increased eroded surfaces (4.7% vs. 3.2%) and mineralization surfaces (15.9% vs. 7.7%). In contrast, after LIES, all these parameters were significantly different from OVX but not different from SHAM. eNOS and iNOS were similarly expressed in subperiosteal regions of tibiae cortices of SHAM, not expressed in OVX, and similarly expressed in OVX + LIES when compared to SHAM. In OVX, the percentage of apoptotic osteocytes (24%) was significantly increased when compared to SHAM (11%) and OVX + LIES (8%). Our results suggest that LIES counteracts some effects of OVX on bone tissue preserving bone structure and microarchitecture, iNOS and eNOS expression, and osteocyte viability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Bone formation markers are augmented in postmenopausal osteoporotic women submitted to pulsed electrical magnetic fields, as shown by the increase in osteocalcin and pro-collagen type I C-terminal peptide levels [1]. Low-intensity electrical stimulation (LIES) has been used for bone repair for three decades, especially in pseudarthrosis and bone nonunion [2], but little is known about its effects on bone metabolism after menopause. We have previously shown that LIES was able to prevent the effects of ovariectomy (OVX) on bone mineral density (BMD) in rats [3]. Osteocytes are the cells that probably mediate this physical stimulus and control bone turnover. Osteocytes are embedded in bone matrix and are connected to each other, to other bone cells, and to bone surfaces through a network created by their cytoplasmatic processes. They have been suggested to act as mechanosensors and transducers [4] through the release of biochemical signals, which may modulate bone remodeling. These factors remain unknown but nitric oxide (NO) has been hypothesized to play a role in the modification of bone turnover in response to mechanical loading. NO is produced by three distinct isoforms of NO synthase (NOS). Endothelial NOS (eNOS) is the predominant isoform in bone and is strongly expressed in osteocytes and chondrocytes [5–7]. Neuronal NOS (nNOS) is also expressed in osteocytes [5] and inducible NOS (iNOS) has been observed in osteoclasts, osteocytes, and chondrocytes [6, 8] in response to mechanical strain.
In vitro studies have shown that a physical stimulus such as mechanical strain, pulsating fluid flow, or LIES induces the release of NO by osteocytes [7, 9], MC3T3-E1 cells [10], and human osteoblasts [11, 12]. It has also been shown that unloading induces bone loss in iNOS knockout and wild-type animals, but recovery after reloading was only observed in wild-type animals [8].
In addition, NO has been suggested to mediate the effects of estrogen on bone. Estrogen stimulates eNOS activity in osteoblastic cells [13] and the effects of estrogen on bone formation are blunted in eNOS knockout mice [14]. Another study has shown that eNOS may limit ovariectomy-induced increased bone remodeling [15]. Several studies have shown that NO may counteract the effects of OVX [16, 17]. Thus, NO has been suggested to be a regulator of bone metabolism by mediating the effects of estrogen [7, 16, 18], mechanical stimulation [7, 8], pulsating fluid flow [9, 10], and shear stress [11].
Most previous studies have analyzed the effects of mechanical stimulation on either bone structure, NO synthesis, or osteocyte apoptosis. The purpose of the present study was to investigate the effects of LIES on bone structure and remodeling, NOS expression, and osteocyte viability in OVX rats. For that purpose we analyzed the effects of LIES on bone tissue of OVX rats by histomorphometry and on expression of iNOS and eNOS and on osteocyte apoptosis by immunostaining.
Materials and Methods
Animals and Treatments
Thirty 12-week-old Wistar female rats (200–220 g) were subjected to anesthesia (xilazin [Syntec], 20 mg/kg, and ketamine [Agener], 40 mg/kg, IP) for bilateral ovariectomy (n = 20; OVX) or sham operation (n = 10; SHAM) according to the method described by Giardino and colleagues [19]. Ten OVX rats (OVX + LIES) received a low-intensity pulsed electrical field treatment (output power, 30 mW; tension, 40 V peak to peak; frequency, 1.5 MHz; duty cycle, 1:4), 20 min per day, five times a week, starting on the seventh day after surgery, for a total of 12 weeks. Electrical field stimulation equipment was set up in the electronics laboratory of the Bioengineering Department (University of Sao Paulo, Sao Carlos). Metallic electrodes (30 × 21 cm) were positioned on the superior and inferior parts of the cage (16 cm apart from each other) in order to submit the whole body of the rats to this low-intensity electrical field through capacitive coupling, and not cause any discomfort to the animals. The electronic unit and dosage parameters were similar to low-intensity pulsed ultrasound, which is approved by American Food and Drug Administration to accelerate bone healing [2]. Animals were kept in cages under appropriate conditions of light and temperature (alternated cycles of 12 h and temperature around 25°C) and had water and food ad libitum. After 12 weeks of experiments, all animals received 20 mg/kg tetracycline intraperitoneally (Terramicina; Pfizer, Sao Paulo, Brazil), on the 11th and 10th days (first labeling) and 5th and 4th days (second labeling) before sacrifice. Double tetracycline labeling was used for analysis of the dynamic parameters of bone formation. This study was approved by the Committee of Ethics in Animal Research at the School of Medicine of the Federal University of São Paulo, under protocol number 0530/06.
Sample Preparation
On the day of sacrifice, right tibiae were collected for bone histomorphometry. They were dissected and the proximal segment was fixed in 70% ethanol, dehydrated, embedded in methylmetacrylate, and sectioned longitudinally using a Polychrome S microtome (Reichert-Young, Heidelberg, Germany). Five-micrometer-thick sections of the specimens were stained with 0.1% toluidine blue at pH 6.4 and unstained sections were prepared for observation under ultraviolet light. At least two nonconsecutive stained and unstained sections were examined for each sample. The left tibiae were fixed in 4% paraformaldehyde (pH 7.4) for 3 to 4 days before decalcification in 16% EDTA (pH 7.4) for 22 days. Following decalcification, the proximal half of the tibia was embedded in paraffin. Seven-micron-thick sections of the proximal tibiae were cut longitudinally and used for the detection of NOS and apoptosis by immunohistochemistry.
Histomorphometry [20]
Abbreviations of histomorphometric parameters used were derived from the standard nomenclature of the ASBMR Committee [21]. Parameters of bone structure, microarchitecture, and connectivity were measured using an automatic image analyzer (Bone, Explora Nova, La Rochelle, France) equipped with an Optronics Microfre digital camera and were obtained with Bone and MorphoExpert software (Explora Nova) specific for bone histomorphometry: bone volume, which expresses the percentage of cancellous area occupied by bone tissue (BV/TV; %), trabecular thickness (Tb.Th; μm), trabecular separation (Tb.Sp; μm), trabecular number (Tb.N; per mm) derived from area and perimeter measurement according to Parfitt’s formulae [22], and number of nodes (N.Nd/TV). The parameters reflecting bone remodeling were measured using a semiautomatic method (Osteometrics Inc., Atlanta, GA) and include the osteoid volume as a percentage of the bone volume (OV/BV; %), the osteoid surface as a percentage of the bone surface (OS/BS; %), the osteoblast surface as a percentage of the bone surface (Ob.S/BS; %), and the eroded surface as a percentage of the bone surface (ES/BS; %). The dynamic parameters were mineral apposition rate (MAR) and mineralizing surface/bone surface (MS/BS) calculated as double + one-half single labeled surfaces. Bone formation rate/bone surface (BFR/BS) was calculated as (MS/BS) × (MAR).
NOS Immmunostaining
Immunohistochemistry was performed on one paraffin-embedded section using a rabbit polyclonal antibody to eNOS and on another section using rabbit polyclonal antibody to iNOS (Abcam, Cambridge, MA, USA) for each animal. Immunostaining was performed according to the method previously described by Basso and Heersche [23]. Briefly, deparaffinized sections were incubated in glycine for 20 min and in 0.2 g of Trypsin 250, 0.2 g of calcium chloride, and 1 M sodium hydroxide in TBS for 30 min. After that, sections were incubated in peroxidase blocking (Dakocytomation Envision + system HRP, for use with rabbit primary antibody; code K4010; Carpinteria, CA, USA) for 15 min and blocking solution for 20 min. Sections were then incubated in the primary antibody (1:100) in a humidity chamber overnight at +4°C. The next day, sections were washed with TBS containing 0.1% Tween-20 (TBS-Tw) and incubated with a goat anti-rabbit serum at room temperature for 25 min. Negative controls were incubated in buffer without secondary antibody. Sections were washed again with TBS-Tw and peroxidase activity was detected using DAB. Sections were counterstained with Mayer’s hematoxylin, dehydrated, cleared, and mounted with Eukitt. NOS-positive cell regions were evaluated on cortical and trabecular bone of metaphysis and diaphysis using a semiquantitative method with a 3-point scale (absent, +, or ++).
Apoptosis Assays
Both caspase-3 and TUNEL techniques were performed in this study in order to confirm the localization of apoptotic cells. Briefly, caspase technique detects total caspase-3 activity, which is part of a family of molecules that are, among other functions, mediators of apoptosis, and caspase-3 has been shown to be one of the major activated caspases present in apoptotic cells, suggesting that it plays a prominent role in the cell death process [24]. TUNEL, the method of TdT-mediated dUTP-biotin nick end-labeling, detects DNA breaks for in situ identification of programmed cell death [25].
Caspase
The protocol was adapted from Follet et al. [26]. Sections were washed in 100% methanol, 70% methanol, PBS, and PBS + 0.3% Triton X-100. Endogenous peroxidase was inactivated by incubating sections in 3% H2O2 in methanol for 5 min for total caspase-3. After that, incubation in 1.5% goat serum blocking solution (VectaStain ABC Kit) in PBS was performed for 30 min. Primary antibody (caspase-3 [H-277]; sc-7148; Santa Cruz Biotechnology, USA) was diluted in PBS + 0.3% Triton X-100 + 0.1% bovine serum albumin (BSA). Caspase was used at a 1:25 dilution. Sections were incubated overnight at +4°C and washed four or five times in PBS containing 0.1% Tween-20 (PBS-Tw). Sections were then incubated in goat anti-rabbit secondary antibody for 45 min and washed several times in PBS-Tw. Detection of peroxidase activity was performed with DAB. Sections were then counterstained with methyl green, dehydrated, cleared, and mounted with Eukitt.
TUNEL
The Klenow FragEL DNA Fragmentation Detection Kit (Calbiochem, Germany) was used. Sections were incubated in 2 mg/ml proteinase K, 1:100, in 10 mM Tris, pH 8, for 20 min and washed in TBS. Positive control was incubated with 1 μg/ml Dnase I in 1 × TBS/1 mM MgSO4 for 20 min and rinsed with TBS. For inactivation of endogenous peroxidases, specimens were kept in 30% H2O2, 1:10, in methanol for 5 min. Sections were then incubated in Klenow equilibration buffer (1:10 with dH2O) for 20 min, followed by Klenow labeling reaction mixture at 37°C for 1.5 h, Stop Buffer for 5 min, and Blocking Buffer for 10 min, both at room temperature. Detection of peroxidase activity was performed with DAB, for 10 to 15 min. Sections were then washed with dH2O, counterstained with methyl green, dehydrated, cleared, and mounted with Eukitt.
Count of Apoptotic Cells
Two sections per animal (one TUNEL and one caspase) were examined by light microscopy at 400 × magnification. Measurements were performed on three distinct areas: on each cortex in the proximal part of the metaphysis and on the diaphysis cortex in the central area of the tibia (Fig. 1). Each tissue area analyzed was 62.5 mm2. For apoptotic cells, positively stained (apoptotic) osteocytes, negatively stained (alive) osteocytes, empty lacunae, and the total number of lacunae were counted in each field using the Exploranova Morphoexpert (Explora Nova), and their densities are expressed as a percentage of the total number of lacunae. All immunostaining analyses (eNOS, iNOS, TUNEL, and Caspase) were performed by the same investigator, who was blinded to the treatment of all samples.
Statistics
Results are expressed as mean ± SD. Nonparametric tests were performed. Comparisons among the three groups were performed by a Kruskall-Wallis test. If the difference was significant, the comparison between two groups was made by Mann-Whitney U test. Significance was accepted at p < 0.05.
Results
Bone Histomorphometry
Twelve weeks of ovariectomy in rats induced a significant bone loss with a deterioration of trabecular architecture as shown by the decreased BV/TV, NdN/TV, and TbN and increased TbSp (Table 1) compared to SHAM. These alterations resulted from an augmentation of bone resorption and bone turnover as reflected by the increased ES/BS, MS/BS, and BFR/BS (Table 2). In contrast, after LIES, all these parameters (except for NdN/TV) were not significantly different from SHAM, suggesting that LIES was able to prevent most of the effects of OVX.
NOS Expression
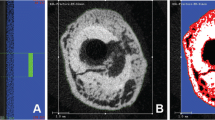
Both eNOS and iNOS were similarly expressed in subperiosteal regions of the cortices of metaphysis and diaphysis of tibiae of the SHAM group. eNOS and iNOS expressions were found in cortical bone, around osteocytes (Fig. 2, I). However, after 12 weeks of ovariectomy, no expression of any of these two NOS isoforms was detected (Table 3 and Fig. 2, I).
Expression of eNOS (I) and detection of osteocyte apoptosis in the tibia of female rats by caspase-3 immunostaining (II) and TUNEL (III). Arrowhead: regions with eNOS positive cells. Similar observations were made with iNOS immunostaining. Detail: sham (I) region with eNOS-positive cells. Bar = 100 μm (I) and 20 μm (II and III)
In the group OVX + LIES, similar expressions of eNOS and iNOS were detected in the cortical bone of tibiae diaphysis, but there were slightly fewer stained regions (with positive cells) compared to SHAM. With this method, we were not able to visualize eNOS or iNOS expression on osteoblasts, osteoclasts, trabecular bone, or medullae in any of the groups.
Apoptosis
After immunostaining for cleaved caspase-3 and labeling of DNA fragmentation by TUNEL staining, strongly positive chondrocytes and osteocytes were present in cortical bone. In OVX rats, the percentage of apoptotic osteocytes was increased, and this difference was significant compared to the OVX + LIES group with caspase technique and compared to SHAM with the TUNEL technique (Table 4). The percentage of viable cells in the OVX group was significantly decreased but the percentage of empty lacunae was significantly increased compared to both the SHAM and the OVX + LIES groups (Table 4 and Fig. 2, II–III). LIES was able to significantly prevent the increase in both apoptotic osteocytes and empty lacunae, which are probably the final result of apoptotic osteocytes that were in them.
Discussion
Osteocytes probably orchestrate skeletal adaptation to mechanical stimuli and NO has been hypothesized to mediate effects of both mechanical loading and estrogens on bone. Osteocytes produce NO in response to shear stress [9] and osteocyte apoptosis can be prevented by NO [27]. In addition, it has been shown that, in the presence of L-NAME - a NOS inhibitor, estrogen is totally ineffective in reversing bone loss in OVX rats, suggesting that the protective effect of estrogens against bone loss may be mediated through NO [16]. So in this study we investigated the influence of LIES on bone structure and remodeling, eNOS and iNOS expression, and osteocyte survival in OVX rats.
Our findings confirm that OVX induced bone loss and microarchitecture deterioration in consequence to an increase in resorption and bone turnover as shown by ES/BS, MS/BS, and BFR/BS. Moreover, OVX rats showed an increased percentage of apoptotic osteocytes (109%) and empty lacunae (506%) compared to SHAM. Others [28] have cited that 3 weeks of ovariectomy may cause a 400% increase in osteocyte apoptosis in tibiae of rats, but no data were found in the literature on the effects of 12 weeks of OVX on osteocyte survival. Aguirre et al. [29] showed that unloading also augments osteocyte apoptosis in mice, and that this change was followed by increased osteoclast number and cortical porosity, reduced trabecular and cortical width, and decreased spinal BMD and vertebral strength. Those authors suggested that dying osteocytes are the signals for osteoclast recruitment in the surrounding area and the resulting increase in bone resorption and bone loss. Besides, it is known that the augmented bone fragility which results from glucocorticoid use [30, 31] and both estrogen [32] and androgen deficiencies [33] is also associated with a higher prevalence of osteocyte apoptosis.
Our study also demonstrates that OVX rats expressed neither eNOS nor iNOS 12 weeks after surgery, while SHAM rats expressed eNOS and iNOS, mainly in the subperiosteal regions of the cortices of tibial metaphysis and diaphysis. These findings suggest that eNOS and iNOS expressions are modulated by estrogen, and are in agreement with previous studies which showed that the effects of OVX depend on NOS expression [15, 16, 34]. In contrast, Cuzzocrea et al. [18] concluded that iNOS could mediate the bone loss induced by OVX in mice. However, in vitro studies corroborated the positive effect of estrogen on eNOS activity [13].
Exposure to LIES prevented most of the effects of OVX on bone. Bone turnover and resorption parameters were similar to those in SHAM animals, and LIES was able to preserve bone structure and microarchitecture, but not bone connectivity (Nd.N/TV),in this bone loss model. This was in agreement with previous results showing that BMD was maintained in OVX rats [3] or increased after fracture in rabbits [35] submitted to LIES or to pulsed electromagnetic fields [36]. These effects of LIES on bone structure and microarchitecture were associated with the maintenance of osteocyte viability and NOS expression.
In the present study, LIES prevented the augmentation of osteocyte apoptosis caused by OVX, keeping the OVX + LIES group with similar levels of osteocyte survival as in the SHAM group. As far as we know, there are no other studies on the effects of LIES on osteocyte survival, but our results are in agreement with the positive effects of mechanical stimulation on osteocyte viability which have already been shown in animals [37] as well as in humans [38]. Mechanical stimulus may augment the expression of Bcl-2 [39], an antiapoptotic gene product, as well as inhibiting osteoclast formation and bone resorption in chicken calvaria cells in vitro [27]. Besides, Mann et al. [38] showed that a mechanical stimulus prevents osteocyte apoptosis and increases the bone formation rate (BFR/BS) and alkaline phosphatase concentration on the surface of human trabecular bone, demonstrating the possible link between the prevention of osteocyte apoptosis and bone formation, which was also observed in our study. The signal transduction pathway between mechanical stimulation and the inhibition of osteocyte apoptosis remains poorly understood but it is likely that the Wnt/β-catenin pathway [40, 41] and NO [27] are involved.
We also showed that LIES can preserve eNOS and iNOS expression on bone of the ovariectomized rats, which is in agreement with in vitro studies demonstrating that electrical stimulation may increase NO synthesis on bone. In vitro studies have shown that NO released under pulsed electromagnetic fields [42] and pulsating fluid flow [43] stimulates osteoblastic differentiation and proliferation. Interestingly, NO was found to increase osteoprotegerin and decrease osteoclastogenesis in bone marrow stromal cells from OVX rats [44, 45]. Hamed et al. [12] have suggested that in human osteoblasts, LIES activated preferentially iNOS and that eNOS was activated by estrogen. However, in our experiments, similar expressions of iNOS and eNOS were observed in cortical bone of OVX rats after 12 weeks of LIES treatment. NO produced by iNOS has also been reported to play a major role in bone formation in response to reloading after tail suspension [8]. In a model of unloading in rats, Basso and Heersche [23] observed an augmentation of osteocyte apoptosis and osteoclast number and a decreased bone mass, which returned to baseline values after reloading, simultaneously with marked augmentations of iNOS and eNOS. Thus we have shown that the bone changes induced by estrogen deficiency and LIES were associated with modifications of NOS expression but we cannot exclude that other factors and pathways may be involved.
Huang et al. [46] recently suggested that osteocytes are the sensory cells that capture electrical stimulation signals and translate them into biochemical signals; these signals would then be transferred through the dendritic processes of osteocytes to the effector cells, such as osteoblasts and osteoclasts, to initiate bone formation. In agreement with this, we hypothesize that LIES preserved osteocyte viability and NOS expression, which could secondarily act on bone remodeling.
In conclusion, our results suggest that LIES may counteract some effects of OVX on bone tissue. It was shown for the first time that LIES is able to preserve bone structure and microarchitecture, iNOS and eNOS expression, and osteocyte viability in bone of OVX rats. Further investigations are now needed to determine whether these factors are all connected in the same mechanism of the response of bone tissue to electrical stimulation.
References
Giordano N, Battisti E, Geraci S, Fortunato M, Santacroce C, Rigato M, Gennari L, Gennari C (2001) Effect of electromagnetic fields on bone mineral density and biochemical markers of bone turnover in osteoporosis: a single-blind, randomized pilot study. Curr Ther Res 62(3):187–193
Lind M, Bünger C (2001) Factors stimulating bone formation. Eur Spine J 10(Suppl 2):S102–S109
Lirani-Galvão APR, Bergamaschi CT, Silva OL, Lazaretti-Castro M (2006) Electrical field stimulation improves bone mineral density in ovariectomized rats. Braz J Med Biol Res 39(11):1501–1505
Burger EH, Klein-Nulend J (1999) Responses of bone cells to biomechanical forces in vitro. Adv Dent Res 13:93–98
Caballero-Alías AM, Loveridge N, Lyon A, Das-Gupta V, Pitsillides A, Reeve J (2004) NOS isoforms in adult human osteocytes: multiple pathways of NO regulation? Calc Tissue Int 75:78–84
Van′t Hof RJ, Ralston SH (2001) Nitric oxide and bone. Immunology 103(3):255–261
Zaman G, Pitsillides AA, Rawlinson SC et al (1999) Mechanical strain stimulates nitric oxide production by rapid activation of endothelial nitric oxide synthase in osteocytes. J Bone Miner Res 14:1123–1131
Watanuki M, Sakai A, Sakata T, Tsurukami H, Miwa M, Uchida Y, Watanabe K, Ikeda K, Nakamura T (2002) Role of inducible nitric oxide synthase in skeletal adaptation to acute increases in mechanical loading. J Bone Miner Res 17(6):1015–1025
Klein-Nulend J, Semeins CM, Ajubi NE, Nijweide PJ, Burger EH (1995) Pulsating fluid flow increases nitric oxide (NO) synthesis by osteocytes but not periosteal fibroblasts–correlation with prostaglandin upregulation. Biochem Biophys Res Commun 217(2):640–648
Mullender MG, Dijcks SJ, Bacabac RG, Semeins CM, Van Loon JJ, Klein-Nulend J (2006) Release of nitric oxide, but not prostaglandin E2, by bone cells depends on fluid flow frequency. J Orthop Res 24(6):1170–1177
Bakker AD, Klein-Nulend J, Tanck E, Albers GH, Lips P, Burger EH (2005) Additive effects of estrogen and mechanical stress on nitric oxide and prostaglandin E2 production by bone cells from osteoporotic donors. Osteoporos Int 16(8):983–989
Hamed A, Kim P, Cho M (2006) Synthesis of nitric oxide in human osteoblasts in response to physiologic stimulation of electrotherapy. Ann Biomed Eng 34(12):1908–1916
Armour KE, Ralston SH (1998) Estrogen upregulates endothelial constitutive nitric oxide synthase expression in human osteoblast-like cells. Endocrinology 139(2):799–802
Armour KE, Armour KJ, Gallagher ME, Gödecke A, Helfrich MH, Reid DM, Ralston SH (2001) Defective bone formation and anabolic response to exogenous estrogen in mice with targeted disruption of endothelial nitric oxide synthase. Endocrinology 142(2):760–766
Grassi F, Fan X, Rahnert J, Weitzmann MN, Pacifici R, Nanes MS, Rubin J (2006) Bone re/modeling is more dynamic in the endothelial nitric oxide synthase(-/-) mouse. Endocrinology 147(9):4392–4399
Wimalawansa SJ, De Marco G, Gangula P, Yallampalli C (1996) Nitric oxide donor alleviates ovariectomy-induced bone loss. Bone 18(4):301–304
Hukkanen M, Platts LA, Lawes T, Girgis SI, Konttinen YT, Goodship AE, MacIntyre I, Polak JM (2003) Effect of nitric oxide donor nitroglycerin on bone mineral density in a rat model of estrogen deficiency-induced osteopenia. Bone 32:142–149
Cuzzocrea S, Mazzon E, Dugo L, Genovese T, Di Paola R, Ruggeri Z, Vegeto E, Caputi AP, Van De Loo FA, Puzzolo D, Maggi A (2003) Inducible nitric oxide synthase mediates bone loss in ovariectomized mice. Endocrinology 144(3):1098–1107
Giardino R, Fini M, Giavaresi G, Mongiorgi R, Gnudi S, Zati A (1993) Experimental surgical model im osteoporosis study. Boll Soc Ital Biol Sper 69(7–8):453–460
Chavassieux P, Arlot M, Meunier P (2001) Clinical use of bone biopsy. In: Marcus R, Feldman D, Kelsey J (eds) Osteoporosis, vol 2, 2nd edn. Academic Press, San Diego, CA, pp 501–509
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry:standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2(6):595–610
Parfitt AM, Mathews CH, Villanueva AR, Kleerekoper M, Frame B, Rao DS (1983) Relationships between surface, volume, and thickness of iliac trabecular bone in aging and in osteoporosis. Implications for the microanatomic and cellular mechanisms of bone loss. J Clin Invest 72(4):1396–1409
Basso N, Heersche JN (2006) Effects of hind limb unloading and reloading on nitric oxide synthase expression and apoptosis of osteocytes and chondrocytes. Bone 39(4):807–814
Faleiro L, Kobayashi R, Fearnhead H, Lazebnik Y (1997) Multiple species of CPP32 and Mch2 are the major active caspases present in apoptotic cells. EMBO 16:2271–2281
Gavrieli Y, Sherman Y, Ben-Sasson SA (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119(3):493–501
Follet H, Li J, Phipps RJ, Hui S, Condon K, Burr DB (2007) Risedronate and alendronate suppress osteocyte apoptosis following cyclic fatigue loading. Bone 40(4):1172–1177
Tan SD, Kuijpers-Jagtman AM, Semeins CM, Bronckers AL, Maltha JC, Von den Hoff JW, Everts V, Klein-Nulend J (2006) Fluid shear stress inhibits TNFalpha-induced osteocyte apoptosis. J Dent Res 85(10):905–909
Tomkinson A, Gevers EF, Wit JM, Reeve J, Noble BS (1998) The role of estrogen in the control of rat osteocyte apoptosis. J Bone Miner Res 13(8):1243–1250
Aguirre JI, Plotkin LI, Stewart SA, Weinstein RS, Parfitt AM, Manolagas SC, Bellido T (2006) Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J Bone Miner Res 21(4):605–615
Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC (1998) Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids: potential mechanisms of their deleterious effects on bone. J Clin Invest 102(2):274–282
Canalis E, Mazziotti G, Giustina A, Bilezikian JP (2007) Glucocorticoid-induced osteoporosis:pathophysiology and therapy. Osteoporos Int 18(10):1319–1328
Tomkinson A, Reeve J, Shaw RW, Noble BS (1997) The death of osteocytes via apoptosis accompanies estrogen withdrawal in human bone. J Clin Endocrinol Metab 82(9):3128–3135
Kousteni S, Bellido T, Plotkin LI, O’Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC (2001) Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 104(5):719–730
Samuels A, Perry MJ, Gibson RL, Colley S, Tobias JH (2001) Role of endothelial nitric oxide synthase in estrogen-induced osteogenesis. Bone 29(1):24–29
Wang CJ, Yang KD, Wang FS, Hsu CC, Chen HH (2004) Shock wave treatment shows dose-dependent enhancement of bone mass and bone strength after fracture of the femur. Bone 34(1):225–230
Chang K, Chang WH (2003) Pulsed electromagnetic fields prevent osteoporosis in an ovariectomized female rat model: a prostaglandin E2-associated process. Bioelectromagnetics 24(3):189–198
Noble BS, Peet N, Stevens HY, Brabbs A, Mosley JR, Reilly GC, Reeve J, Skerry TM, Lanyon LE (2003) Mechanical loading: biphasic osteocyte survival and targeting of osteoclasts for bone destruction in rat cortical bone. Am J Physiol Cell Physiol 284(4):C934–C943
Mann V, Huber C, Kogianni G, Jones D, Noble B (2006) The influence of mechanical stimulation on osteocyte apoptosis and bone viability in human trabecular bone. J Musculoskelet Neuronal Interact 6(4):408–417
Bakker A, Klein-Nulend J, Burger E (2004) Shear stress inhibits while disuse promotes osteocyte apoptosis. Biochem Biophys Res Commun 320:1163–1168
Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, Kharode Y, Sauter L, Babij P, Brown EL, Hill AA, Akhter MP, Johnson ML, Recker RR, Komm BS, Bex FJ (2006) Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem 281(42):31720–31728
Bonewald LF, Johnson ML (2008) Osteocytes, mechanosensing and Wnt signaling. Bone 42(4):606–615
Diniz P, Soejima K, Ito G (2002) Nitric oxide mediates the effects of pulsed electromagnetic field stimulation on the osteoblast proliferation and differentiation. Nitric Oxide 7:18–23
Vezeridis PS, Semeins CM, Chen Q, Klein-Nulend J (2006) Osteocytes subjected to pulsating fluid flow regulate osteoblast proliferation and differentiation. Biochem Biophys Res Commun 348(3):1082–1088
Fan X, Roy E, Zhu L, Murphy TC, Ackert-Bicknell C, Hart CM, Rosen C, Nanes MS, Rubin J (2004) Nitric oxide regulates receptor activator of nuclear factor-kappaB ligand and osteoprotegerin expression in bone marrow stromal cells. Endocrinology 145(2):751–759
Wang FS, Wang CJ, Chen YJ, Huang YT, Huang HC, Chang PR, Sun YC, Yang KD (2004) Nitric oxide donor increases osteoprotegerin production and osteoclastogenesis inhibitory activity in bone marrow stromal cells from ovariectomized rats. Endocrinology 145(5):2148–2156
Huang CP, Chen XM, Chen ZQ (2008) Osteocyte: the impresario in the electrical stimulation for bone fracture healing. Med Hypotheses 70(2):287–290
Acknowledgments
The authors gratefully acknowledge the technical assistance of Helene Follet for caspase technique, Delphine Goehrig for immunohistochemistry, and Vanda Jorgetti for histomorphometry support. This study was supported by CAPES and CNPq (Brazil).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lirani-Galvão, A.P.R., Chavassieux, P., Portero-Muzy, N. et al. Low-Intensity Electrical Stimulation Counteracts the Effects of Ovariectomy on Bone Tissue of Rats: Effects on Bone Microarchitecture, Viability of Osteocytes, and Nitric Oxide Expression. Calcif Tissue Int 84, 502–509 (2009). https://doi.org/10.1007/s00223-009-9227-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-009-9227-9