Abstract
Summary
Ovariectomized mice were used to assess the ability of low-intensity vibrations to protect bone microarchitecture and marrow composition. Results indicate that low-intensity vibrations (LIV), introduced 2 weeks postsurgery, slows marrow adipogenesis in OVX mice but does not restore the bone within the period studied. However, immediate application of LIV partially protects quality.
Introduction
The aim of this study was to evaluate consequences of estrogen depletion on bone marrow (BM) phenotype and bone microarchitecture, and effects of mechanical signals delivered as LIV on modulating these changes.
Methods
LIV (0.3 g, 90 Hz) was applied to C57BL/6 mice immediately following ovariectomy or 2 weeks postestrogen withdrawal for 2 (ST-LIV) or 6 weeks (LT-LIV), respectively. Sham-operated age-matched controls (ST-AC, LT-AC) and ovariectomized controls (ST-OVX, LT-OVX) received sham LIV treatment. Bone microstructure was evaluated through μCT and BM adipogenesis through histomorphometry, serum markers, and genes expression analysis.
Results
LT-OVX increased BM adipogenesis relative to LT-AC (+136 %, p ≤ 0.05), while LT-LIV introduced for 6w suppressed this adipose encroachment (−55 %, p ≤ 0.05). In parallel with the fatty marrow, LT-OVX showed a marked loss of trabecular bone, −40 % (p ≤ 0.05) in the first 2 weeks following ovariectomy compared to LT-AC. Application of LT-LIV for 6w following this initial 2w bone loss failed to restore the lost trabeculae but did initiate an anabolic response as indicated by increased serum alkaline phosphatase (+26 %, p ≤ 0.05). In contrast, application of LIV immediately following ovariectomy was more efficacious in the protection of trabecular bone, with a +29 % (p > 0.05) greater BV/TV compared to ST-OVX at the 2w time period.
Conclusions
LIV can mitigate adipocyte accumulation in OVX marrow and protect it by favoring osteoblastogenesis over adipogenesis. These data also emphasize the rapidity of bone loss with OVX and provide perspective in the timing of treatments for postmenopausal osteoporosis where sooner is better than later.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent data collected in the USA indicates that 10.2 million adults have osteoporosis and another 43.4 million have low bone mass [1]. The majority of osteoporosis sufferers are women past menopause, who lose almost 20 % of their bone density within the first 5 to 7 years following the decline in estrogen levels [2, 3]. Since the early studies of Fuller Albright, estrogen has been recognized as a major systemic regulator of bone metabolism [4], with its withdrawal leading to accelerated bone loss [5]. Recent research has expanded our understanding of estrogen’s regulation of bone remodeling, providing the foundation for treatment strategies for postmenopausal bone loss, including estrogen replacement, anti-resorptive (i.e., bisphosphonates) or anabolic (i.e., parathyroid hormone) therapy, each intended to reestablish the balance between bone formation and resorption [6, 7]. Non-drug approaches, including exercise, are geared toward reducing the risk of fractures by preventing loss of bone, may not be feasible for all subjects, and have not been sufficient to combat the severity of bone loss following menopause [7, 8].
In addition to the loss of bone, menopause is often followed by significant gains in adipose tissue, and a concomitant increase in inflammatory cytokines, insulin sensitivity [9–11], and other comorbidities related to obesity [12]. In parallel with an increase in visceral adipose depots, estrogen deficiency is permissive to a marked increase in adipocyte encroachment into the bone marrow [13, 14], altering the bone marrow phenotype and ultimately disrupting functions of hematopoietic and mesenchymal progenitors that reside there.
Considering that both osteoblasts and adipocytes arise from a common progenitor, the mesenchymal stem cell (MSC) [15], there is a possibility that the increased presence of one terminally differentiated cell type (e.g., adipocyte) is inversely correlated to an available population to recruit the other (osteoblast) [16, 17]. In that context, it is important to recognize that exercise in general and mechanical signals in particular, including the low impact signals engendered by low intensity vibrations (LIV), have been shown to be anabolic to the skeleton while simultaneously suppressing fat accumulation [18, 17, 19, 16].
In the study reported here, an ovariectomized mouse model was used to evaluate the consequences of estrogen depletion on marrow phenotype and bone quality, as well as the effects of mechanical signals, delivered in the form of LIV, on these outcomes. Two time points are considered, the introduction of LIV after significant bone had already been lost and the capacity of LIV to protect bone quality at the time of estrogen withdrawal.
Materials and methods
Animals and experimental design
All animal procedures were reviewed and approved by the Stony Brook University’s Institutional Animal Care and Use Committee. In the first phase of the study (8w OVX + 6w LIV), which tested the ability of LIV to reverse OVX induced bone loss, 8w old female C57BL/6 mice underwent an ovariectomy (n = 20) or sham surgery (n = 10) at The Jackson Laboratory (Bar Harbor, ME, USA). Animals were delivered to Stony Brook University 1w after surgery, where they acclimated for an additional week. The LIV intervention began 2w post-OVX surgery, for a total of 6w. The ovariectomized mice were divided into two groups, long-term ovariectomized + sham LIV (LT-OVX, n = 10) and long-term ovariectomized + LIV treatment (LT-LIV, n = 10). Intact sham surgery animals served as the long-term age-matched controls (LT-AC, n = 10) and also received sham LIV.
After the conclusion of the phase 1 study, the second phase (2w OVX + 2w LIV) was designed to determine the ability of LIV to preserve bone quality rather than restore it. In this protocol, 8w old female C57BL/6 mice underwent an ovariectomy or sham surgery and received either LIV or sham LIV beginning 1 day post-op for a total of 2w. The groups were defined as follows: short-term ovariectomized + sham LIV (ST-OVX, n = 7), short-term ovariectomized + LIV treatment (ST-LIV, n = 8), and short-term age-matched controls (ST-AC, n = 6). The mice were euthanized at 2w following the procedure. All mice in both phases were single housed and fed a standard rodent chow diet (LabDiet Prolab RMH 3000, Purina Mills LLC, St. Louis, MO, USA) with food and water ad libitum. Facility conditions were maintained under a 12:12-h light/dark photoperiod at 21 °C.
In vivo mechanical stimulation
The LT-LIV and ST-LIV groups were subject to vertically oscillating low intensity vibration (0.3 g at 90 Hz, where g is earth’s gravitational force = 9.81 m/s2). Mice received LIV for 15 min/day for 5 days/week for a total of 6 weeks (phase 1: 8w OVX + 6w LIV) or 7 days/week for a total of 2 weeks (phase 2: 2w OVX + 2w LIV). Mice were placed into a partitioned container (one mouse per partition), centered on the oscillating LIV system. AC and OVX were sham vibrated with the same handling and container but without activating the LIV device.
μCT assessment of skeletal phenotype
The phase 1 8w OVX + 6w LIV group was imaged in vivo pre- and post-LIV treatment, as well as ex vivo post-LIV treatment. In vivo parameters of bone microarchitecture were measured before (t = 0w) and after (t = 6w) LIV treatment, corresponding to 2w and 8w post-OVX, respectively. Animals were anesthetized (2 % isoflurane) and secured in a custom-made bed while scanning in the vivaCT 40 (Scanco Medical, Basserdorf, Switzerland). Transverse sections of the tibia were measured at a voxel size of 17.5 μm. The phase 2 2w OVX + 2w LIV group was only imaged ex vivo posttreatment. At the conclusion of both phase 1 and 2 protocols, ex vivo high-resolution μCT was performed postsacrifice, on cortical and trabecular bone at the tibial mid-diaphysis and proximal metaphysis (490 μm distal to the growth plate), respectively. Data were collected using the ex vivo μCT40 (Scanco Medical, Basserdorf, Switzerland) at a voxel size of 12 μm. A well-established script [20] was used to measure bone volume fraction (BV/TV), trabecular thickness/separation/number (Tb.Th., Tb.Sp., Tb.N.), connectivity density (Conn.D), and cortical thickness (Cort.Th.).
Adipocyte encroachment into the bone marrow
Postsacrifice, tissues were extracted from all animals and the right tibia fixed in 10 % formalin, dehydrated in serial concentrations of 70, 95, and 100 % isopropyl alcohol and embedded in 85 % poly(methyl methacrylate) (PMMA), 15 % N-butyl phthalate and 2 % benzoyl peroxide. Lateral, sagittal sections of the proximal metaphysis were cut at 8 μm with a sledge microtome (Leica, Bannockburn, IL, USA), sections stained with Wright Giemsa and imaged at 10× on a fluorescent microscope (Carl Zeiss Microscope). Adipocytes, which appeared as white circular ghosts on a blue and purple background (marrow), were quantified using a semi-automated program (NIH ImageJ). All samples were blinded and analyzed by a sole researcher.
Bone marrow cell differentiation assessment via RT-PCR
Immediately upon sacrifice, bone marrow from the left femur was flushed and preserved in RNA later (Qiagen, Valencia, CA, USA) until analysis. RNA was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and quantified with a spectrophotometer (NanoDrop; Thermo Scientific, Wilmington, DE, USA). A high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA) was used to convert to cDNA. Real-time PCR (StepOnePlus, Applied Biosystems) and the TaqMan gene expression assays (Applied Biosystems) were used to evaluate gene expression levels involved in osteoblastogenesis (RUNX2) [21] and adipogenesis (PPARγ) [22]. Beta-Actin was used as the housekeeping gene for normalization of all samples and the delta CT method for calculating relative expressions of the genes of interest.
Biochemical characterization of bone turnover
Serum from mice that were fasted overnight but given free access to water was collected at euthanasia for measurement of biochemical markers. Total Alkaline Phosphatase (ALP; Biovision, Inc., Milpitas, CA, USA) measurements were performed using ELISA kits according to the manufacturer’s recommendations. Serum tartrate-resistant acid phosphatase (TRAP5b) was measured with a TRAP ELISA kit (MouseTRAP™ Assay, Immunodiagnostic Systems LTD, Boldon, Tyne & Wear, UK). Both ELISAs were evaluated with a standard curve according to the manufacturer’s protocol.
Dynamic histomorphometry
Calcein was administered to animals in phase 1 twice within 24 h via intra-peritoneal injection at 10-mg/kg body weight at two time points, the first at 12–13 days prior to sacrifice and the second 5–6 days prior. The right tibia was extracted, dehydrated, and embedded in PMMA as described above. Eight micrometer sections of the proximal metaphysis were de-plasticized in acetone prior to imaging at 10× on a fluorescent microscope (Carl Zeiss, NY, USA). Images were analyzed using Osteomeasure software (OsteoMetrics, GA, USA) to quantify single- and double-labeled bone surfaces to obtain mineral apposition rates (MAR) and mineralizing surfaces (MS).
Statistics
All data are presented as mean ± SD. Analyses between groups were done with a one-way analysis of variance (ANOVA) and Tukey post hoc test. To analyze data between time points, a paired Student’s t test was used. All data were considered significant with a p ≤ 0.05 and analyzed with SPSS 14.0 (SPSS, Inc.).
Results
Ovariectomy increased body mass
Ovariectomy was confirmed at euthanasia by uterine hypoplasia in both phase 1 and 2 protocols (data not shown). Uterine mass was −82 ± 6 % lower in LT-OVX mice (p ≤ 0.05) compared to intact controls, LT-AC (Fig. 1a). Ovariectomy induced a rapid gain in body weight. In the 2w following the surgery, ST-OVX mice had a mean +10 % increase in body weight compared to their baseline presurgery weight, while ST-AC had a −2 % decrease in body weight. LIV did not mitigate the increase in body weight, as ST-LIV also experienced a +8 % increase and was not significantly different from that of ST-OVX (Fig. 1b). Lastly, there were no significant differences in food consumption between the three groups in either phase of the experiment (data not shown).
a Uterus mass was significantly reduced at the conclusion of the phase 1 study (8w OVX + 6w LIV) evidencing the success of ovariectomy. LIV treatment did not have a significant affect on uterus mass. b The percent change in body weight in the 2w period of the phase 2 protocol shows significant weight gain in both ST-OVX and ST-LIV but not in ST-AC. *p ≤ 0.05 vs. AC within same time point
LIV protects against rapid and severe bone loss following OVX when applied immediately upon cessation of estrogen
In the phase 1 protocol baseline, in vivo measurements at t = 0w (i.e., 2w post-OVX, prior to LIV treatment) showed a dramatic −40 % (p ≤ 0.05) reduction in trabecular BV/TV in LT-OVX compared to LT-AC. Cortical BV/TV was slightly altered with a −3 % (p ≤ 0.05) reduction in LT-OVX compared to controls. Longitudinal in vivo μCT measurements between the baseline (t = 0w) and end point (t = 6w) time-points within each group showed that animals experienced natural, age-related changes in trabecular bone parameters, with a −5, −9, and −9 % (all p > 0.05) decrease in BV/TV in LT-AC, LT-OVX, and LT-LIV mice, respectively. Trabecular architectural parameters showed similar age-related changes. Changes in the cortical bone during the 6w treatment period were subtler, with a +0.6 (p > 0.05), +3, and +2 % (p ≤ 0.05) increase in cortical BV/TV in LT-AC, LT-OVX, and LT-LIV, respectively. Data are given in Online Resource 1.
Higher resolution ex vivo μCT measurements of those tissues harvested in phase 2, 2w OVX + 2w LIV, corroborated the in vivo baseline measures from the phase 1 study. Significant trabecular bone loss had already occurred in just 2w, with bone volume fraction (BV/TV) falling by −38 ± 6 % (p ≤ 0.05) in ST-OVX compared to intact controls (Fig. 2a). Trabecular microarchitecture was also compromised by OVX within this 2w period, as evidenced by a +19 ± 6 % (p ≤ 0.05) increase in trabecular spacing (Tb. Sp., Fig. 2b) and a −18 ± 4 % (p ≤ 0.05) decrease in trabecular number (Tb. N., Fig. 2c) compared to ST-AC. In contrast, the ST-LIV group shows +29 % (p > 0.05) greater trabecular BV/TV after 2w of LIV administration immediately following the ovariectomy compared to ST-OVX receiving sham LIV, with no statistical significance to ST-AC. Trabecular spacing and number decreased by −3 % and increased by +6 % in ST-LIV mice as compared to ST-OVX. Neither trabecular thickness (Fig. 2d) nor cortical bone parameters (data not shown) showed significant differences between any groups 2w post-OVX through ex vivo scans.
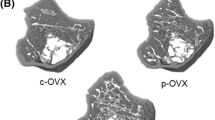
Ex vivo μ-CT analysis for phase 2 protocol (2w OVX + 2w LIV) showed that within 2 weeks of OVX, mice had a severe loss of a trabecular bone volume fraction with a further deterioration of trabecular architecture. b Trabecular spacing was increased in both ST-OVX and ST-LIV animals compared to ST-AC. LIV application immediately following ovariectomy partially protected trabecular bone volume fraction and c number. No differences were shown in d trabecular thickness. e Ex vivo μ-CT 3D reconstructed representative images of proximal tibia scans showing trabecular architecture and cortical bone in ST-AC, ST-OVX, and ST-LIV post-2w OVX + 2w LIV. *p ≤ 0.05 vs. AC groups
LIV suppresses adipose encroachment in OVX marrow
After the phase 1 study, 8w OVX + 6w LIV, total adiposity in the proximal marrow space was +136 % (p ≤ 0.05) greater in LT-OVX compared to LT-AC (Fig. 3a). In some contrast, adiposity in the marrow of LT-LIV mice was −55 % (p ≤ 0.05) lower than that measured in LT-OVX with no statistical significance to LT-AC. Further, individual adipocyte size was +107 % (p ≤ 0.001) greater in LT-OVX over LT-AC, while adipocytes in the marrow of LT-LIV were only +39 % (p > 0.05) greater than control (−33 %, p ≤ 0.05, smaller than LT-OVX, Fig. 3b). Number of adipocytes per marrow space showed similar trends to that of adipocyte size with +24 % increase in LT-OVX compared to LT-AC and −38 % decrease in LT-LIV compared to LT-OVX (p > 0.05, Fig. 3c).
Histomorphometry and staining with wright-giemsa of the bone marrow in the tibial metaphysis showed a significantly increased total adipocyte area and b individual adipocyte area in LT-OVX compared to LT-AC after 8w OVX + 6w LIV. c Number of adipocytes showed increasing trends in LT-OVX compared to the LT-AC group. LT-LIV was able to significantly reduce total adipocyte area by reducing the size of individual adipocytes as well as trending toward normalized numbers of adipocytes in the bone marrow cavity. d Representative proximal tibia histological sections, where the arrow points toward an adipocyte. *p ≤ 0.05 vs. AC groups; #p ≤ 0.05 vs. OVX
LIV promotes osteoblast but not osteoclast activity
Biochemical analysis of bone turnover as measured in the serum of ovariectomized animals in the phase 1 long-term study showed deficiencies in both ALP and TRAP5b, a marker of bone formation and resorption, respectively, in LT-OVX animals compared to LT-AC (−15 %, p > 0.05 and −31 %, p ≤ 0.05, respectively). While TRAP5b levels were similar between LT-LIV and LT-OVX, circulating ALP levels of LT-LIV animals were +26 % (p ≤ 0.05) greater than that measured in LT-OVX. The 8 % greater value of ALP in LT-LIV compared to LT-AC was not significantly different. However, in the phase 2 study, 2w post-OVX showed +21 % (p > 0.05) increase in ALP with no differences with application of LIV for the first 2w following surgery (Fig. 4c). TRAP5b levels in both ST-OVX and ST-LIV animals show no significant differences from ST-AC after 2w (Fig. 4d).
Serum alkaline phosphatase (ALP) was a slightly reduced in LT-OVX compared to LT-AC, after the phase 1 study, 8w OVX + 6w LIV, while LT-LIV had a significant increase in ALP levels compared to LT-OVX. b Serum tartrate-resistant acid phosphatase 5b (TRAP5b) was significantly reduced in both ovariectomized groups compared to LT-AC. LIV treatment did not have an affect on TRAP5b concentrations. c ALP was increased in both ST-OVX and ST-LIV mice compared to ST-AC after 2w OVX + 2w LIV. d TRAP5b was not significantly different after 2w in either ST-OVX or ST-LIV compared to ST-AC. *p ≤ 0.05 vs. AC groups; #p ≤ 0.05 vs. OVX
Mineral apposition rates of both cortical and trabecular bone were unchanged even 8w post-OVX (Fig. 5a, b). The application of LIV also had no significant impact on the apposition rate during this time. However, cortical mineralizing surfaces were significantly decreased in LT-OVX (−37 ± 17 %, p ≤ 0.05) but not in LT-LIV (−32 ± 22 %, p > 0.05) compared to LT-AC (Fig. 5c). Trabecular mineralizing surfaces showed decreasing but not significant trends in LT-OVX and LT-LIV animals compared to LT-AC with a −33 and −11 % decrease (p > 0.05, Fig. 5d).
Dynamic bone histomorphometry for the phase 1 protocol, 8w OVX + 6w LIV, showed a no differences in cortical or b trabecular mineral apposition rate (MAR). c Cortical bone showed significantly lower cortical mineralizing surfaces in LT-OVX compared to LT-AC, while LT-LIV was not significantly different from either LT-AC or LT-OVX. d Trabecular mineralizing surfaces were reduced in LT-OVX, while LT-LIV showed an increasing trend in mineralizing surfaces. *p ≤ 0.05 vs. AC groups
Expression of RUNX2 in bone marrow showed a −7 % (p > 0.05) decrease in LT-OVX animals compared to controls with a +12 % (p > 0.05) increase in LT-LIV animals compared to LT-OVX (Fig. 6a). Although not significant, the trends on the transcriptional level indicate increased osteoblastic activity, whereas PPARγ, an indication of adipogenesis, expression showed a +28 % (p > 0.05) increase in LT-OVX compared to LT-AC and only a −5 % (p > 0.05) decrease in LT-LIV compared to LT-OVX (Fig. 6b).
Real-time PCR for phase 1 protocol (8w OVX + 6w LIV) showed no significant differences in gene expression of RUNX2 and PPARγ in the bone marrow between any groups. However, trends support increased osteoblast formation (RUNX2) in LT-LIV-treated animals compared to LT-OVX and increased adipocyte formation (PPARγ) in LT-OVX animals compared to LT-AC
Discussion
Bone loss is not only a major consequence of menopause, aging, immobilization, and microgravity but is also associated with excess accretion of adipocytes in the marrow [13, 23–26]. Here, we show that OVX causes both marked deterioration of bone quality as well as the transformation of bone marrow toward a fat phenotype. While the bone loss is not recovered by low magnitude mechanical signals applied for 6w, there is evidence that these stimuli can mitigate adipocyte accumulation in the marrow, suggesting that LIV can protect a marrow away from adipogenesis. Further, this study emphasizes that the introduction of an intervention, whether chemical or physical, should be as early as possible with the goal of protecting existing bone, rather than later, as an effort to recover it, as evidenced by the application of LIV immediately upon estrogen cessation.
Here, using a murine model of estrogen deficiency, OVX mice showed marked trabecular bone loss of 38 % (p ≤ 0.05) within just 2w after ovarian removal as compared to intact animals, emphasizing that even a short absence of estrogen can have profound consequences on the quality and quantity of the bone. Following the rapid bone loss measured at 2w, the rate of loss slowed, as reflected by only a slight further reduction of 6 % measured in the subsequent 6w in OVX compared to controls. This reinforces findings by other groups [27] that indicate that OVX causes a rapid imbalance in bone remodeling, one that elevates resorption and suppresses formation.
Exercise has been shown to be anabolic to bone and help slow osteopenia following menopause [7, 28]. In the current study, LIV was introduced as a surrogate for exercise to combat OVX-induced bone loss. However, LIV, which was applied following 2w of bone loss for a total duration of 6w, failed to recover trabecular bone from the marked loss that had abruptly occurred following surgery. Scientists considering OVX as a model to evaluate countermeasures for osteopenia must take into account the possibility that the rapid rate of bone loss may severely challenge the ability of any given therapy to restore the compromised architecture. Perhaps the outcome of this study would have been different with longer trials [19, 28, 29], earlier interventions [30, 31], or refractory periods [32, 33] to augment the responsiveness of the skeleton.
The abrupt cessation of estrogen negatively impacted not only the bone but also the bone marrow, with OVX permissive to encroachment of excess adipocytes into the marrow [13, 23, 25]. By 8w post-OVX, the marrow compartment in the proximal tibial metaphysis of OVX showed a 136 % (p ≤ 0.05) increase in total adipocyte area and a 107 % (p ≤ 0.05) increase in size of individual adipocytes as compared to controls. That the number of adipocytes per unit area showed only an increasing trend of 24 % (p > 0.05) suggests that this encroachment is achieved through an aggregate of size and number of fat cells. Considering that homeostasis of the bone marrow is maintained by constant interaction amongst resident cells including osteoblasts, adipocytes, and hematopoietic and mesenchymal stem cells [34], it is not surprising that disruptions in this balance can be detrimental to many processes and organ systems beyond the bone.
Relative to the skeleton, a biased commitment of mesenchymal stem cell fate selection toward adipogenesis comes at the expense of osteoblastogenesis, leading to an altered ratio of fat to bone cells [35] and contributing to the inability of bone formation to keep pace with resorption. Although the osteoblast-adipocyte system is believed to be a net-closed system, it is plausible for systemic stresses (i.e., estrogen deficiency, radiation, diet induced obesity, etc.) to suppress the stem cell (i.e., MSC) and progenitor pool ultimately altering the marrow microenvironment and the representative population of cells that inhabit it. While not studied here, identifying the effects of estrogen deficiency on MSC populations could shed light to the above and corroborate the hypothesis that retaining the marrow phenotype will help protect the bone structure by maintaining the balance between the stem/progenitor pools.
Interestingly, LIV mitigated the encroachment of adipose tissue into the marrow space of OVX animals, with 55 % (p ≤ 0.05) smaller total adipose area, 33 % (p ≤ 0.05) smaller adipocytes, and 38 % (p > 0.05) fewer adipocytes per area of marrow space compared to that of OVX. An upward trend of gene expression of PPARγ, a master regulator gene of adipogenesis, in the bone marrow supports the measured increase in adiposity observed in OVX marrow. However, the small differences between OVX and LIV in PPARγ expression would not seem to explain the large differences in adipose encroachment between these groups. That only the size of adipocytes, and not the number, was statistically significant between OVX and LIV that might partially explain this finding. Identifying factors that regulate adipocyte size may represent a viable strategy to retain the marrow and adipose phenotype [12].
Alkaline phosphatase, a protein secreted by osteoblasts and associated with osteoid formation and mineralization [36], has been shown to increase 1 week after ovariectomy. However, these transient elevations are overwhelmed by the increase in TRAP5b levels, which increase immediately after OVX and persist for longer [37] before normalizing to baseline levels by 2w [37, 27, 38, 39]. After prolonged estrogen deficiency, both ALP and TRAP5b progressively decline to the point that bone turnover approaches “normal” levels, even though the severe loss of the bone remains [38]. The rapid increase in serum ALP (21 %) following 2w of OVX had decreased to 15 % below controls by 8w [27, 38]. Mice receiving LIV for 6w showed significantly higher levels of ALP (26 and 7 %, p ≤ 0.05) compared to OVX, suggesting increased osteoblastic activity with the application of the mechanical signals, data which also suggests that recovering bone quality requires much longer periods than the time to lose it. Originally, the LIV protocol was determined from previous studies, primarily [40], which showed that 6w of LIV significantly affected both trabecular and cortical bone in mice. However, since OVX-induced changes to cortical bone are much slower than that seen in trabeculae [41], alterations to the LIV protocol in future work with longer durations of intervention or incorporation of refractory periods [33] may be necessary in order to see changes on the tissue level.
TRAP5b levels, a phosphatase specifically secreted by osteoclasts, are reduced by 32 % in OVX animals compared to controls 8w post-OVX and 6w postsham LIV. This decline in TRAP5b secretion may be due in part to decreased resorptive activity as well as the substantial loss of bone surfaces, reducing the areas available for resorption to even occur. The application of LIV did not have any significant impact on osteoclast activity as TRAP5b levels remained similar to that of OVX animals. These data indicate that although at 8w post-OVX and 6w post-LIV, bone-remodeling markers remain suppressed, and bone lost in the first 2w was not recovered by LIV; these mechanical signals promote aspects of the osteogenic response, simultaneously suppressing an adipogenic response. The ultimate benefit, if any, of suppressing the adipose phenotype in the bone marrow is not yet known, but it is suspected of protecting critical elements of hematopoiesis, lymphopoiesis, and the balance of the immune system [34].
Dynamic histomorphometry showed that mineralizing surfaces of both trabecular and cortical bone were reduced in OVX, reaching a 37 % reduction in cortical bone, with LIV elevating formative activity on the surfaces toward control. Paralleling these changes, gene expression of RUNX2, a key regulator of osteoblast differentiation and bone formation, showed similar decreasing trends in OVX [14], while LIV again trended to normalize this activity. These data together with the increased ALP levels indicate a promotion of osteoblastogenesis and suggest that mechanical signals may ultimately help restore the bone lost by OVX if continued over much longer periods.
In contrast, when LIV was applied immediately after ovariectomy, as done in phase 2 (2w OVX + 2w LIV), mice showed +29 % (p > 0.05) greater trabecular bone volume fraction compared to OVX, with no significant difference to bone morphology in age-matched control animals. This finding suggests that treatment—of any modality—should be introduced as early as possible relative to endocrine shifts, to curb the abrupt degradation that rapidly follows insult. While not studied here, presumably, this strategy would also hold true in cases of injury, paralysis, or bed rest: the sooner, the better [42]. As micro-CT analysis shows the protection of trabecular BV/TV stems from preservation of the number of trabeculae rather than thickness or shape, such critical scaffolds may provide the key surfaces to restoring skeletal structure as remodeling returns toward normal.
It is important to note that the results we see here are in young growing mice who are metabolically active and in which changes occur faster than those that would be expected to be seen in postmenopausal women. Moreover, estrogen deprivation is experienced gradually over several years in postmenopausal women rather than the abrupt cessation by ovariectomy, potentially affecting the response to mechanical signals. While ovariectomy is a standard to study the menopause in animals, these points should be considered when translating findings to humans [41].
Additionally, there are certain methodological limitations that need to be considered in future experiments to better understand the results such as the gene analysis of RUNX2 and PPAR-γ done on an amalgam of bone marrow from the femur. While not significantly different between groups, the gene expression trends may show a greater correlation to the μ-CT and histomorphometry data of the tibia if they had been assessed on the tibial bone marrow, as well as considering region site-specificity (proximal versus distal shaft). The difficulty of extracting marrow only from trabecular regions also limits the ability to compare specific regions of the bone with the corresponding marrow phenotype. The differences in marrow phenotype have also been shown in different skeletal regions based on results from a recent collaboration with Wallace et al. [43], which showed that differential skeletal sensitivity to exercise (humerus versus femur) correlates closely to MSC population. Thus, evaluation of different sites such as the humerus might reveal greater changes in trabecular as well as cortical bone while bringing light to site-specific responsiveness.
Additionally, adipose assessment was only performed on the tibial metaphysis in which Styner et al. [44] have shown a greater amount of adipocytes to reside compared to other regions. However, it would be beneficial for a more complete picture of bone marrow adiposity to include an evaluation of other anatomical sites within the tibia (i.e., epiphysis and diaphysis) as well as implementation of newer techniques as described by Styner et al. These data would be helpful for further understanding whether the protection of the marrow leads to restoration of the bone. Whether correlative or causal, preservation of the bone marrow phenotype might be a key strategy of treatment, retaining the micro niche as home to progenitor populations that maintain our musculoskeletal and immune functions.
In conclusion, LIV mitigates bone marrow adipose encroachment but cannot restore bone devastation caused by OVX. Conversely, LIV shows some evidence of protecting the bone from early devastation caused by estrogen cessation. These studies shed light on the importance of a balanced bone marrow micro milieu for bone health as well as early treatment approaches with the intention of protecting bone lost due to estrogen deficiency rather than those that aim to restore the bone devastation.
References
Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, Dawson-Hughes B (2014) The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res 29(11):2520–2526. doi:10.1002/jbmr.2269
Gallagher JC, Goldgar D, Moy A (1987) Total bone calcium in normal women: effect of age and menopause status. J Bone Miner Res 2(6):491–496. doi:10.1002/jbmr.5650020605
Endicott RD (2013) Knowledge, health beliefs, and self-efficacy regarding osteoporosis in perimenopausal women. J Osteoporos 2013:853531. doi:10.1155/2013/853531
Khosla S, Oursler MJ, Monroe DG (2012) Estrogen and the skeleton. Trends Endocrinol Metab 23(11):576–581. doi:10.1016/j.tem.2012.03.008
Lerner UH (2006) Bone remodeling in post-menopausal osteoporosis. J Dent Res 85(7):584–595
Palacios S, Mejia A (2015) Antiresorptives and anabolic therapy in sequence or combination for postmenopausal osteoporosis. Climacteric:1–3. doi:10.3109/13697137.2015.1016378
Delmas PD (2002) Treatment of postmenopausal osteoporosis. Lancet 359(9322):2018–2026. doi:10.1016/S0140-6736(02)08827-X
Body JJ, Bergmann P, Boonen S, Boutsen Y, Bruyere O, Devogelaer JP, Goemaere S, Hollevoet N, Kaufman JM, Milisen K, Rozenberg S, Reginster JY (2011) Non-pharmacological management of osteoporosis: a consensus of the Belgian Bone Club. Osteoporos Int 22(11):2769–2788. doi:10.1007/s00198-011-1545-x
Nadal-Casellas A, Proenza AM, Llado I, Gianotti M (2011) Effects of ovariectomy and 17-beta estradiol replacement on rat brown adipose tissue mitochondrial function. Steroids 76(10–11):1051–1056. doi:10.1016/j.steroids.2011.04.009
Teede HJ, Lombard C, Deeks AA (2010) Obesity, metabolic complications and the menopause: an opportunity for prevention. Climacteric 13(3):203–209. doi:10.3109/13697130903296909
Gaspard U (2009) Hyperinsulinaemia, a key factor of the metabolic syndrome in postmenopausal women. Maturitas 62(4):362–365. doi:10.1016/j.maturitas.2008.11.026
Ludgero-Correia A Jr, Aguila MB, Mandarim-de-Lacerda CA, Faria TS (2011) Effects of high-fat diet on plasma lipids, adiposity, and inflammatory markers in ovariectomized C57BL/6 mice. Nutrition. doi:10.1016/j.nut.2011.07.014
Benayahu D, Shur I, Ben-Eliyahu S (2000) Hormonal changes affect the bone and bone marrow cells in a rat model. J Cell Biochem 79(3):407–415
Chen TY, Zhang ZM, Zheng XC, Wang L, Huang MJ, Qin S, Chen J, Lai PL, Yang CL, Liu J, Dai YF, Jin DD, Bai XC (2013) Endogenous n-3 polyunsaturated fatty acids (PUFAs) mitigate ovariectomy-induced bone loss by attenuating bone marrow adipogenesis in FAT1 transgenic mice. Drug Des Devel Ther 7:545–552. doi:10.2147/DDDT.S45263
Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM (2009) Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr 19(2):109–124
Luu YK, Capilla E, Rosen CJ, Gilsanz V, Pessin JE, Judex S, Rubin CT (2009) Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J Bone Miner Res 24(1):50–61. doi:10.1359/jbmr.080817
Rubin CT, Capilla E, Luu YK, Busa B, Crawford H, Nolan DJ, Mittal V, Rosen CJ, Pessin JE, Judex S (2007) Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc Natl Acad Sci U S A 104(45):17879–17884. doi:10.1073/Pnas.0708467104
Judex S, Lei X, Han D, Rubin C (2007) Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J Biomech 40(6):1333–1339. doi:10.1016/j.jbiomech.2006.05.014
Chan ME, Adler BJ, Green DE, Rubin CT (2012) Bone structure and B-cell populations, crippled by obesity, are partially rescued by brief daily exposure to low-magnitude mechanical signals. Faseb J 26(12):4855–4863. doi:10.1096/fj.12-209841
Lublinsky S, Ozcivici E, Judex S (2007) An automated algorithm to detect the trabecular-cortical bone interface in micro-computed tomographic images. Calcif Tissue Int 81(4):285–293. doi:10.1007/s00223-007-9063-8
Carola Krause DJJdG, Marcel Karperien, Peter ten Dijke (2008) Signal transduction cascades controlling osteoblast differentiation. In: Rosen CJ (ed) Primer on the metabolic bone diseases and disorders of mineral metabolism. American Society for Bone and Mineral Research. John Wiley & Sons, pp 10–16
Brun RP, Spiegelman BM (1997) PPAR gamma and the molecular control of adipogenesis. J Endocrinol 155(2):217–218
Elbaz A, Rivas D, Duque G (2009) Effect of estrogens on bone marrow adipogenesis and Sirt1 in aging C57BL/6J mice. Biogerontology 10(6):747–755. doi:10.1007/s10522-009-9221-7
Gevers EF, Loveridge N, Robinson IC (2002) Bone marrow adipocytes: a neglected target tissue for growth hormone. Endocrinology 143(10):4065–4073
Martin RB, Zissimos SL (1991) Relationships between marrow fat and bone turnover in ovariectomized and intact rats. Bone 12(2):123–131
Ozcivici E, Luu YK, Rubin CT, Judex S (2010) Low-level vibrations retain bone marrow’s osteogenic potential and augment recovery of trabecular bone during reambulation. PLoS One 5(6), e11178. doi:10.1371/journal.pone.0011178
Tanizawa T, Yamaguchi A, Uchiyama Y, Miyaura C, Ikeda T, Ejiri S, Nagal Y, Yamato H, Murayama H, Sato M, Nakamura T (2000) Reduction in bone formation and elevated bone resorption in ovariectomized rats with special reference to acute inflammation. Bone 26(1):43–53
Sakakura Y, Shide N, Tsuruga E, Irie K, Yajima T (2001) Effects of running exercise on the mandible and tibia of ovariectomized rats. J Bone Miner Metab 19(3):159–167
Flieger J, Karachalios T, Khaldi L, Raptou P, Lyritis G (1998) Mechanical stimulation in the form of vibration prevents postmenopausal bone loss in ovariectomized rats. Calcif Tissue Int 63(6):510–514
Ohlsson C, Engdahl C, Fak F, Andersson A, Windahl SH, Farman HH, Moverare-Skrtic S, Islander U, Sjogren K (2014) Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS One 9(3), e92368. doi:10.1371/journal.pone.0092368
Lane NE, Haupt D, Kimmel DB, Modin G, Kinney JH (1999) Early estrogen replacement therapy reverses the rapid loss of trabecular bone volume and prevents further deterioration of connectivity in the rat. J Bone Miner Res 14(2):206–214. doi:10.1359/jbmr.1999.14.2.206
Zhang R, Gong H, Zhu D, Gao J, Fang J, Fan Y (2014) Seven day insertion rest in whole body vibration improves multi-level bone quality in tail suspension rats. PLoS One 9(3), e92312. doi:10.1371/journal.pone.0092312
Sen B, Xie Z, Case N, Styner M, Rubin CT, Rubin J (2011) Mechanical signal influence on mesenchymal stem cell fate is enhanced by incorporation of refractory periods into the loading regimen. J Biomech 44(4):593–599. doi:10.1016/j.jbiomech.2010.11.022
Adler BJ, Kaushansky K, Rubin CT (2014) Obesity-driven disruption of haematopoiesis and the bone marrow niche. Nat Rev Endocrinol 10(12):737–748. doi:10.1038/nrendo.2014.169
Pino AM, Rosen CJ, Rodriguez JP (2012) In osteoporosis, differentiation of mesenchymal stem cells (MSCs) improves bone marrow adipogenesis. Biol Res 45(3):279–287. doi:10.1590/S0716-97602012000300009
Kai-Chiang Yang K-CY, Huang J-Y, Wu C-C, Lin F-H (2011) Sintered dicalcium pyrophosphate decreases bone turnover rate in osteoporotic rat: a study on serum biochemical bone turnover markers. Biomed Aging Pathol 1:45–51. doi:10.1016/j.biomag.2010.10.004
Miyazaki T, Matsunaga T, Miyazaki S, Hokari S, Komoda T (2004) Changes in receptor activator of nuclear factor-kappaB, and its ligand, osteoprotegerin, bone-type alkaline phosphatase, and tartrate-resistant acid phosphatase in ovariectomized rats. J Cell Biochem 93(3):503–512. doi:10.1002/jcb.20201
Garcia-Perez MA, Noguera I, Hermenegildo C, Martinez-Romero A, Tarin JJ, Cano A (2006) Alterations in the phenotype and function of immune cells in ovariectomy-induced osteopenic mice. Hum Reprod 21(4):880–887. doi:10.1093/humrep/dei413
Surve VV, Andersson N, Alatalo S, Lehto-Axtelius D, Halleen J, Vaananen K, Hakanson R (2001) Does combined gastrectomy and ovariectomy induce greater osteopenia in young female rats than gastrectomy alone? Calcif Tissue Int 69(5):274–280
Xie L, Rubin C, Judex S (2008) Enhancement of the adolescent murine musculoskeletal system using low-level mechanical vibrations. J Appl Physiol (1985) 104(4):1056–1062. doi:10.1152/japplphysiol.00764.2007
Jee WS, Yao W (2001) Overview: animal models of osteopenia and osteoporosis. J Musculoskelet Neuronal Interact 1(3):193–207
Giangregorio L, McCartney N (2006) Bone loss and muscle atrophy in spinal cord injury: epidemiology, fracture prediction, and rehabilitation strategies. J Spinal Cord Med 29(5):489–500
Wallace I, Pagnotti G, Rubin-Sigler J, Naeher M, Judex S, Rubin CT, Demes B (2015) Focal enhancement of the skeleton to exercise correlates to Mesenchymal stem cell responsivity rather than peak forces. J Exp Biol
Styner M, Thompson WR, Galior K, Uzer G, Wu X, Kadari S, Case N, Xie Z, Sen B, Romaine A, Pagnotti GM, Rubin CT, Styner MA, Horowitz MC, Rubin J (2014) Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise. Bone 64:39–46. doi:10.1016/j.bone.2014.03.044
Acknowledgments
This study was supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) EB014351 and National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) AR043498. The authors thank Alyssa Tuthill, Gabriel Pagnotti, Andrea Trinward, Tee Pamon, Jeyantt Srinivas Sankaran, Vihitaben Patel, and Shipra Arjun for their laboratory assistance.
Conflicts of interest
CTR is a founder of Marodyne and the holder of several patents related to low intensity vibration. Divya Krishnamoorthy, Danielle M Frechette, Benjamin J Adler, Danielle E Green, and M. Ete Chan declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 214 kb)
Rights and permissions
About this article
Cite this article
Krishnamoorthy, D., Frechette, D.M., Adler, B.J. et al. Marrow adipogenesis and bone loss that parallels estrogen deficiency is slowed by low-intensity mechanical signals. Osteoporos Int 27, 747–756 (2016). https://doi.org/10.1007/s00198-015-3289-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-015-3289-5