Abstract
Recent studies have suggested an increased fracture risk with acid-suppressive medication use. We studied two cohorts of men and women over age 65 who were enrolled in the Osteoporotic Fractures in Men Study (MrOS) and the Study of Osteoporotic Fractures (SOF), respectively. We used dual-energy X-ray absorptiometry and assessed baseline use of proton pump inhibitors (PPIs) and/or H2 receptor antagonists (H2RAs) in 5,755 men and 5,339 women. Medication use and bone mineral density (BMD) were assessed, and hip and other nonspine fractures were documented. On multivariate analysis, men using either PPIs or H2RAs had lower cross-sectional bone mass. No significant BMD differences were observed among women. However, there was an increased risk of nonspine fracture among women using PPIs (relative hazard [RH] = 1.34, 95% confidence interval [CI] 1.10–1.64). PPI use was also associated with an increased risk of nonspine fracture in men but only among those who were not taking calcium supplements (RH = 1.49, 95% CI 1.04–2.14). H2RA use was not associated with nonspine fractures, and neither H2RA use nor PPI use was associated with incident hip fractures in men or women. The use of PPIs in older women, and perhaps older men with low calcium intake, may be associated with a modestly increased risk of nonspine fracture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Acid-suppressive medications, such as H2 receptor antagonists (H2RAs) and proton pump inhibitors (PPIs), are among the most widely prescribed medications worldwide. Over 82 million PPI prescriptions and 19 million H2RA prescriptions are written each year, such that global pharmaceutical sales for antiulcer agents exceed $26 billion annually [1]. These potent acid-suppressing agents are used for the treatment of various disorders, ranging from gastroesophageal reflux to noncardiac chest pain. In addition, indications for long-term “maintenance” therapy with this drug class continue to expand [2].
Studies are now reporting unexpected potential adverse consequences of chronic acid suppression due to H2RAs and PPIs, such as impaired absorption of vitamin B12 [3] and an increased risk of community-acquired pneumonia [4]. Several recent studies in human volunteers have suggested that short-term use of acid-suppressive medications can decrease calcium absorption [5–7], perhaps leading to calcium deficiency. Reduced fractional calcium absorption has been linked to an increased risk of fracture, especially among older women with low calcium intake [8, 9]; and thus, it is possible that acid-suppressive medications may potentiate fracture risk. Furthermore, a study of long-term omeprazole administration in rats found decreased bone mineral density (BMD) in the treatment compared to the placebo group [10]. In a small study of men, Grisso et al. [11] reported an increase in hip fracture with the use of cimetidine. In addition, two large case-control studies from Denmark and the United Kingdom, respectively, have recently reported a 22–60% increase in the odds of hip fracture for users of PPIs [12, 13]. Neither of these studies reported the effects of PPI or H2RA use on bone mass or rates of bone loss.
We performed a secondary data analysis to examine the association between acid-suppressive medication use and bone density, rates of hip bone loss, and fracture risk in prospective cohorts of men and women over the age of 65 who were enrolled in the Osteoporotic Fractures in Men Study (MrOS) and the Study of Osteoporotic Fractures (SOF), respectively. We further hypothesized that the deleterious effects of acid suppression would be most apparent among participants with lower calcium intakes.
Subjects and Methods
The design of the MrOS was derived from that of the SOF, to allow parallel analyses in men and women. Both are ongoing prospective studies of large cohorts recruited from several communities in the United States.
SOF Participants (Women)
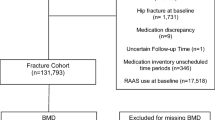
Study participants were community-dwelling women 65 years of age and older who were enrolled in the SOF. Caucasian women were recruited from 1986 to 1988 through population-based listings from four clinical centers in the United States: the Kaiser-Permanente Center for Health Research (Portland, OR), the University of Minnesota (Minneapolis, MN), the University of Maryland (Baltimore, MD), and the University of Pittsburgh (Pittsburgh, PA). Women who were unable to walk without help and women who had a history of bilateral hip replacement were excluded [14]. Starting in 1996, an additional cohort of black women was recruited to the study, and they are included in our study population [15]. The appropriate institutional review boards approved the study, and written informed consent was obtained from all participants.
Our analysis was limited to active surviving women who participated in the sixth clinic visit (1997–1999), when a detailed listing of medication use was obtained, and those who had technically adequate hip BMD measurements (n = 5,339). Participants completed follow-up measurements at an eighth clinical exam an average of 4.9 years later, at which time medication history (n = 3,283) and hip BMD measurement (n = 2,856) were repeated. Of note, 916 of these 5,339 participants died before visit 8 was completed. Fracture data continued to be collected after the last clinical visit.
MrOS Participants (Men)
From March 2000 through April 2002, a total of 5,995 men 65 years of age and older were recruited for participation in MrOS from six areas of the United States: Birmingham, AL; Minneapolis, MN; Palo Alto, CA; Pittsburgh, PA; Portland, OR; and San Diego, CA [16, 17]. As in SOF, men with a history of bilateral hip replacement and those who were unable to walk without assistance were excluded. Our analysis is limited to those 5,755 men with information on medication use and technically adequate hip BMD measurements at baseline. Participants completed follow-up measurements at the second clinic visit an average of 4.6 years later, at which time medication history and hip BMD assessment were repeated (n = 4,230). Fracture data continued to be collected after the last clinical visit. The institutional review board at each center approved the study protocol, and written informed consent was obtained from all participants.
Use of Acid-Suppressive Medications
In both the MrOS and SOF cohorts, participants were asked to bring all current prescription medications, defined as any use in the previous 4 weeks, to the clinic visit. In the SOF, women were also asked to bring nonprescription medications to all visits. In the MrOS, nonprescription medications were not documented until the follow-up visit. Interviewers completed a medication history for each participant and, in the SOF, also included information on duration of usage. A computerized medication coding dictionary was used to categorize these medications [18]. We performed our primary analysis by classifying participants in mutually exclusive medication use categories of users of PPIs, users of H2RAs, and nonusers of either PPIs or H2RAs. We also pooled use of PPIs and H2RAs in a secondary predictor variable. For the fracture analysis, we used time-dependent analysis to allow for crossover and discontinuous PPI or H2RA medication use as documented over subsequent visits.
BMD and Ascertainment of Fractures
BMD at the hip was measured using dual-energy X-ray absorptiometry with the QDR-1000, QDR-2000 (SOF), or QDR-4500 (MrOS) scanners (Hologic, Waltham, MA). Repeat BMD measurements were performed on the same instruments used for the initial measurements. Detailed descriptions of the BMD measurements have been previously published [17, 19, 20]. The rate of change in BMD was expressed as an annualized percentage of difference between follow-up BMD and initial BMD divided by initial BMD.
In both the MrOS and SOF cohorts, participants were contacted every 4 months by postcard or telephone to determine the occurrence of fractures. Data continued to be collected after the clinic visits were completed. Both studies completed 95% or more of these follow-up contacts. All fractures were confirmed by review of radiographic reports or, in some cases, by review of the radiographs by a study radiologist. In the SOF, fractures that occurred after visit 6 but before August 2007 were included in the analysis. In the MrOS, all confirmed fractures up until August 2007 were included in the analysis. We excluded self-reported vertebral fractures. Based on recent literature indicating the predictive value of traumatic fractures [21], we included fractures that occurred due to major trauma in our primary analysis. However, we also performed secondary analysis, which excluded traumatic fractures. We excluded participants who had had a previous hip fracture in the analysis of incident hip fractures. The average follow-up for fractures was 7.6 years in the SOF and 5.6 years in the MrOS.
Other Measurements
In both studies, participants completed a questionnaire and were interviewed at the initial visit. They were asked about selected medical history, smoking status, alcohol intake, physical activity, and self-reported health. In the SOF, physical activity was assessed by asking women if they walked for exercise. In the MrOS, physical activity was assessed using the Physical Activity Scale of the Elderly (PASE) [22]. A full current medication history was obtained as described above. Body weight and height were measured using a balance-beam scale and a stadiometer.
In both studies, dietary calcium intake was assessed by a food-frequency questionnaire [23]. In the MrOS, dietary caffeine intake was assessed with the food-frequency questionnaire, while in the SOF it was assessed through specific questions. In the SOF cohort, we assessed use of calcium supplements similarly to the assessment of medication use [8]. In the MrOS, it was assessed as part of the food-frequency questionnaire.
Statistical Analysis
Each study was analyzed separately but in parallel fashion. Differences in characteristics at the initial visit between users of acid-suppressive medications in the two categories compared to nonusers of any acid-suppressive medications were analyzed using chi-squared tests for categorical variables, t-tests for normally distributed continuous variables, and Wilcoxon’s rank sum tests for continuous variables with skewed distributions.
Linear regression models were used to examine the difference between acid-suppressive medication users and nonusers at the initial visit for both cross-sectional BMD and annualized percent change in BMD. The adjusted means per group were calculated using the least square means procedure.
Cox regression models were used to examine the association between use of acid-suppressive medications and risk of subsequent fracture. Because the follow-up for fractures extends over multiple time points where medication use data were gathered, acid-suppressive medication use was used as a time-dependent covariate. This allowed participants to switch between the user and nonuser groups over time within the model [24, 25]. For the association of PPI use to incident hip fracture outcomes, we had 80% power to detect unadjusted relative hazards (RHs) of 1.50 in the SOF cohort and 2.04 in the MrOS cohort.
All models were minimally adjusted for clinic and age, then further for those characteristics related to either PPI or H2RA use at the P < 0.10 level. Cross-sectional BMD models were performed at the initial visit and, therefore, were not adjusted for weight change (follow-up visit weight—initial visit weight). Weight change data were missing on those men or women who did not return for the follow-up visit. For fracture models, multiple imputation was used for these missing data. The imputation model included all other covariates in the multivariate model. A total of five imputed data sets were used in the fracture analyses [26].
Secondary analyses were performed on the subset of participants who were not taking hormone replacement therapy or a medication for osteoporosis, defined as use of bisphosphonates, raloxifene, calcitonin, fluoride, or teriparatide. We also performed secondary analyses which excluded traumatic fractures. The interaction of the use of calcium supplements and acid-suppressive medications in these multivariate models was also examined, using P < 0.10 to indicate potential interactions due to the low power of such tests. Models stratified by calcium supplement use (yes/no) were performed.
Finally, analyses were performed in which propensity scores were calculated indicating the likelihood of acid-suppressive medication use (e.g., PPI, H2RA) based on logistic regression with the use variable as the outcome and the covariates in the multivariate model [27]. This propensity score was then used in place of the covariates in the models. Because use of propensity scores did not substantially alter findings regarding the association between PPI or H2RA use and rates of hip bone loss, models adjusted for multiple covariates are presented.
Results
Baseline Characteristics
The baseline characteristics for users and nonusers of acid-suppressive medications within the SOF and MrOS cohorts are shown in Table 1. The women in the SOF cohort were older (average 79 years) compared to the men in the MrOS cohort (average 74 years) due to the different enrollment periods that were considered. There were also more African American participants in the SOF due to targeted recruitment. In general, participants using PPIs and H2RAs tended to have slightly higher body mass indexes (BMIs), to report more inactivity, and to have poorer self-reported health compared to nonusers. In addition, use of prescribed drugs was higher among users of PPIs and H2RAs, including a significantly higher usage of corticosteroids and non-steroidal anti-inflammatory drugs (NSAIDS).
PPI and H2RA Use
Within the SOF cohort, mean duration of H2RA and PPI use at the initial visit was 3.6 years and 1.8 years, respectively. Of note, there was a large increase in the proportion of participants taking PPIs at the follow-up visits (5% at the SOF initial visit vs. 16% at the follow-up visit). In addition, over time a greater proportion of participants were taking any form of acid-suppressive therapy (14.3% at the SOF initial visit vs. 22.8% at the follow-up visit). Similar trends were observed within the MrOS cohort, with an almost 50% increase in the use of PPIs between the initial and follow-up visits (data not shown).
Cross-Sectional BMD and Bone Loss Analyses
After adjustment for multiple covariates, men in the MrOS who were PPI users had slightly lower total hip BMD at the initial visit compared to nonusers of these drugs (Table 2, P = 0.05). Similar results were obtained using femoral neck BMD in the MrOS cohort (data not shown). Among women in the SOF, minimally adjusted analyses of cross-sectional total hip (Table 2) and femoral neck BMD (data not shown) showed a slight increase in BMD for those on either H2RA or PPI medication, but this association did not remain after adjustment for multiple confounders.
Annualized percent change of total hip BMD was calculated as a measure of rate of bone loss. Minimally adjusted models showed an increased rate of bone loss among combined PPI and H2RA users in men; however, there were no statistically significant differences in multivariate models for either cohort (Table 3). Similar results were obtained using femoral neck BMD (data not shown).
Fracture Analysis
In the SOF cohort of women, during a mean follow-up time of 7.6 years, 1,410 nonspine fractures occurred, of which 451 were incident hip fractures. In multivariate-adjusted time-dependent Cox regression models, we found an increase in the risk of nonspine fracture among users of PPIs compared to nonusers (RH = 1.34, 95% confidence interval [CI] 1.10–1.64; Table 4). Pooling users of PPIs and/or H2RAs to compare against nonusers yielded similar results for nonspine fractures. Neither PPI nor H2RA use was associated with incident hip fracture risk (Table 4).
In the MrOS cohort of men, during a mean follow-up time of 5.6 years, 489 nonspine fractures occurred, of which 89 were incident hip fractures. In minimally adjusted time-dependent Cox regression models, there was an increased risk of nonspine fracture among men using PPIs compared to nonusers (RH = 1.33, 95% CI 1.01–1.76). This relationship was no longer statistically significant after multivariate adjustment (RH = 1.21, 95% CI 0.91–1.62), although the point estimate for nonspine fracture risk among PPI users was similar to that observed in the SOF.
Secondary analyses were performed with models using SOF and MrOS cohort subsets that excluded users of osteoporosis medications. We found similar results for primary bone loss and fracture outcomes. In addition, secondary analysis which excluded traumatic fractures showed similar results to our primary analysis.
Interactions with Calcium Supplement Use
To test the impact of calcium supplement use on these relationships, we modeled bone loss and fracture outcomes with calcium intake interaction terms. These interaction terms were not significant in either cohort for bone loss outcomes. However, the analysis of calcium supplement use and PPI use did suggest a possible interaction for the nonspine fracture outcome in the MrOS cohort (P = 0.097). Stratified analyses demonstrated that PPI use was associated with an increase in fracture risk among men who were not taking calcium supplements (RH = 1.49, 95% CI 1.04–2.14) but not among men who were taking calcium supplements (RH = 0.88, 95% CI 0.54–1.42). No significant interactions were found for fracture outcomes in the SOF cohort. We found similar results when we stratified by total daily calcium intake.
Discussion
This prospective study examined the relationships between acid-suppressive medication use and skeletal outcomes in two large cohorts, one of men and one of women, which had similar design and included community dwellers older than 65 years. After adjustment for potential confounding factors, use of PPIs in men was associated with mildly lower hip bone density. There was also a suggestion of increased bone loss in men and women who used PPIs, but we did not observe statistically significant differences. Analysis of fracture outcomes showed that use of PPIs conferred a 34% increased risk of nonspine fracture in the cohort of women. Among men who were not taking calcium supplements, there was a 49% greater risk of nonspine fracture compared to those not taking PPIs. Men taking calcium supplements did not have an increased risk of nonspine fracture associated with PPI use.
Based on our nonspine fracture data in the SOF cohort, we estimated that the PPI number-needed-to-harm is such that one extra nonspine fracture would be expected for every 10 women treated for 5 years with a PPI. While this represents a small individual risk, the widespread use of these medications is such that the population-attributable fraction is 3.6%. This means that 3.6% of nonspine fractures in women of this age group could potentially be attributed to use of PPIs. This attributable fraction may become significantly higher given the ever-widening popularity of these medications. Within the time frame of our study, use of acid-suppressive medications within our two cohorts increased by 50%, reflecting the general trend during this time of greater availability and popularity of the PPIs in particular. In addition, individuals that initiate PPI treatment tend to remain on the medication for many years, if not the remainder of their life span [2].
Two case-control studies utilizing national health administrative databases have been reported on this topic. However, neither of the two previous studies examined bone density measurements or interactions with calcium intake. Vestergaard et al. [12] performed a study in Denmark which found an 18% increase in any fracture and a 45% increase in hip fracture for men and women who had used PPIs in the past year. Yang et al. [13] completed a more recent study within the United Kingdom, which also demonstrated an increase in hip fracture risk among users of PPIs. Increasing duration and dose of PPI therapy conferred higher risks of fracture. In addition, they found that the association between PPI use and fracture was more pronounced in men (odds ratio [OR] = 1.78, CI 1.42–2.22 in men; OR = 1.36, CI 1.22–1.53 in women; P = 0.04). By contrast, we did not see any effect of PPI use on hip fracture risk. The Danish study was unable to control for several confounding factors, such as BMI and physical activity. However, removing these covariates from our fracture analysis did not change our estimates of fracture risk (data not shown). Therefore, the reason for this discrepancy is not known, though it may be that our current study is underpowered to see a direct effect on hip fractures and we lack other information such as duration of medication usage to look for time-dependent effects. Nevertheless, the mild increase in overall nonspine fracture risk within the SOF cohort of women is qualitatively similar to these published population-wide case-control studies. This is similar also to the observed nonspine fracture risk among the men not taking calcium supplements in the MrOS cohort.
Three independent studies now suggest that use of PPIs in vivo confers a mildly increased risk of fractures. This is despite in vitro biochemical studies of PPIs that have suggested potential antiresorptive properties by functioning at osteoclast-specific proton pumps [28, 29]. A possible mechanism to explain this increase in fracture risk may be via the decreased absorption of calcium in users of acid-suppressive medications. Achlorhydria is associated with decreased absorption of certain forms of calcium [30]. Several recent studies have demonstrated that hypochlorhydria due to short-term omeprazole use is also associated with impaired intestinal calcium absorption. In one study, participants who were administered a short course of omeprazole had decreased serum calcium and urinary calcium levels compared to placebo [6]. Another recently reported short-term trial found that, compared to placebo, omeprazole use reduced the fractional absorption of calcium from 9.1% to 3.5% [7]. Therefore, it is conceivable that decreased calcium absorption is the mechanism by which PPIs increase fracture risk. If so, individuals with low baseline calcium intake would be at even higher risk for hypocalcemia with PPI use. Interestingly, our data suggest that men with lower intake of calcium who used PPIs had heightened susceptibility to fractures, which is consistent with this hypothesis. Conversely, sufficient levels of calcium supplementation might mitigate the adverse skeletal effects of PPI use.
Whether H2RA use increases fracture risk is still unclear. Grisso et al. [11] first reported an increased risk of fracture for cimetidine users. The Danish study found a dose-dependent decrease in fracture risk with H2RAs [12], while the U.K. study reported a dose-dependent increase in fractures with H2RAs [13]. Our present analysis did not find a statistically significant effect upon fracture in men or women using H2RAs. Theoretically, H2RAs are less effective than PPIs at promoting an acidic gastric environment and, therefore, would have less of a detrimental effect upon calcium absorption. Thus, we would expect a weaker association with fracture rates, which may be hard to discern given the mild effect seen with the more potent PPIs. Further analysis is hampered by diminishing numbers of participants who are prescribed H2RAs in the face of the increasing popularity of PPIs.
Our study should be interpreted with the following limitations in mind. We are reporting the results of two separate cohorts; therefore, direct comparisons between men and women are not possible. However, the design of the MrOS was based on that of the SOF; therefore, they share many similarities. Vertebral fracture data were unavailable, and the number of nonspine fractures was limited. Thus, we may not have had enough power to detect true effects of acid-suppressive medications on fracture risk, especially for hip fractures.
Finally, a main limitation of our study lies in the assessment of acid-suppressive medication use, but all of the difficulties in assessment would have biased the results to the null. First of all, we only assessed use of medications in the 4 weeks prior to the clinic visits; thus, we may have underestimated the number of actual users of acid-suppressive medications. In addition, nonprescription medications were not documented in the MrOS during the baseline visit. We may be underestimating baseline use of H2RAs, but the first over-the-counter PPI was not approved by the U.S. Food and Drug Administration until a year after our baseline visit. We did collect nonprescription medication data on subsequent follow-up MrOS visits, which were incorporated into the analysis. Regrettably, we have limited data on dose and duration of medication usage, but inclusion of short-term users in addition to chronic users would again bias our results to the null. Lastly, increasing numbers of participants were started on acid suppression during the study due to increasing indications and popularity. We attempted to adjust for this by analyzing the fracture outcomes in a time-dependent manner, such that participants could switch medication categories during the follow-up visit and subsequent fractures would be analyzed according to the new category.
In summary, we observed that PPI use is associated with mildly decreased bone density in men. These bone density differences were not seen in women, and a statistically significant effect on rate of bone loss was not observed in either cohort. Nevertheless, we found that use of acid-suppressive medications is associated with a modest increase in nonspine fracture risk in women and perhaps also in men with low calcium intake. Along with previously published studies, these data suggest that PPIs may have unintended negative skeletal effects, although they are likely minor on the individual level. Further studies are warranted to better elucidate the connection between acid-suppressive medications and skeletal outcomes.
References
IMS Health (2005) Leading therapy classes by pharmaceutical sales. http://www.imshealth.com/portal/site/imshealth. Accessed January 2008
Miyamoto M, Haruma K, Kuwabara M, Nagano M, Okamoto T, Tanaka M (2007) Long-term gastroesophageal reflux disease therapy improves reflux symptoms in elderly patients: five-year prospective study in community medicine. J Gastroenterol Hepatol 22:639–644
Laine L, Ahnen D, McClain C, Solcia E, Walsh JH (2000) Potential gastrointestinal effects of long-term acid suppression with proton pump inhibitors. Aliment Pharmacol Ther 14:651–668
Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB (2004) Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA 292:1955–1960
Graziani G, Badalamenti S, Como G, Gallieni M, Finazzi S, Angelini C, Brancaccio D, Ponticelli C (2002) Calcium and phosphate plasma levels in dialysis patients after dietary Ca-P overload. Role of gastric acid secretion. Nephron 91:474–479
Graziani G, Como G, Badalamenti S, Finazzi S, Malesci A, Gallieni M, Brancaccio D, Ponticelli C (1995) Effect of gastric acid secretion on intestinal phosphate and calcium absorption in normal subjects. Nephrol Dial Transplant 10:1376–1380
O’Connell MB, Madden DM, Murray AM, Heaney RP, Kerzner LJ (2005) Effects of proton pump inhibitors on calcium carbonate absorption in women: a randomized crossover trial. Am J Med 118:778–781
Ensrud KE, Duong T, Cauley JA, Heaney RP, Wolf RL, Harris E, Cummings SR (2000) Low fractional calcium absorption increases the risk for hip fracture in women with low calcium intake. Study of Osteoporotic Fractures Research Group. Ann Intern Med 132:345–353
Nordin BE (1997) Calcium and osteoporosis. Nutrition 13:664–686
Cui GL, Syversen U, Zhao CM, Chen D, Waldum HL (2001) Long-term omeprazole treatment suppresses body weight gain and bone mineralization in young male rats. Scand J Gastroenterol 36:1011–1015
Grisso JA, Chiu GY, Maislin G, Steinmann WC, Portale J (1991) Risk factors for hip fractures in men: a preliminary study. J Bone Miner Res 6:865–868
Vestergaard P, Rejnmark L, Mosekilde L (2006) Proton pump inhibitors, histamine h(2) receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int 79:76–83
Yang YX, Lewis JD, Epstein S, Metz DC (2006) Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 296:2947–2953
Cummings SR, Black DM, Nevitt MC, Browner WS, Cauley JA, Genant HK, Mascioli SR, Scott JC, Seeley DG, Steiger P et al (1990) Appendicular bone density and age predict hip fracture in women. The Study of Osteoporotic Fractures Research Group. JAMA 263:665–668
Vogt MT, Rubin DA, Palermo L, Christianson L, Kang JD, Nevitt MC, Cauley JA (2003) Lumbar spine listhesis in older African American women. Spine J 3:255–261
Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, Delay RR (2005) Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials 26:557–568
Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K (2005) Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials 26:569–585
Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P (1994) Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol 10:405–411
Ensrud KE, Palermo L, Black DM, Cauley J, Jergas M, Orwoll ES, Nevitt MC, Fox KM, Cummings SR (1995) Hip and calcaneal bone loss increase with advancing age: longitudinal results from the Study of Osteoporotic Fractures. J Bone Miner Res 10:1778–1787
Steiger P, Cummings SR, Black DM, Spencer NE, Genant HK (1992) Age-related decrements in bone mineral density in women over 65. J Bone Miner Res 7:625–632
Mackey DC, Lui LY, Cawthon PM, Bauer DC, Nevitt MC, Cauley JA, Hillier TA, Lewis CE, Barrett-Connor E, Cummings SR (2007) High-trauma fractures and low bone mineral density in older women and men. JAMA 298:2381–2388
Washburn RA, Smith KW, Jette AM, Janney CA (1993) The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 46:153–162
Block G, Subar AF (1992) Estimates of nutrient intake from a food frequency questionnaire: the 1987 National Health Interview Survey. J Am Diet Assoc 92:969–977
Collett D (1994) Time-dependent variables. Chapman & Hall, London
Allison P (1995) Estimating Cox regression models with PROC PHREG. SAS Institute, Cary, NC
Schafer JL (1999) Multiple imputation: a primer. Stat Methods Med Res 8:3–15
Rubin DB (1997) Estimating causal effects from large data sets using propensity scores. Ann Intern Med 127:757–763
Tuukkanen J, Vaananen HK (1986) Omeprazole, a specific inhibitor of H+-K+-ATPase, inhibits bone resorption in vitro. Calcif Tissue Int 38:123–125
Mizunashi K, Furukawa Y, Katano K, Abe K (1993) Effect of omeprazole, an inhibitor of H+, K+-ATPase, on bone resorption in humans. Calcif Tissue Int 53:21–25
Recker RR (1985) Calcium absorption and achlorhydria. N Engl J Med 313:70–73
Acknowledgements
The SOF is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) and the National Institute on Aging (NIA) under grant numbers AG05407, AR35582, AG05394, AR35584, AR35583, R01 AG005407, R01 AG027576-22, 2 R01 AG005394-22A1, and 2 R01 AG027574-22A1. The MrOS is supported by National Institutes of Health (NIH) funding. The following institutes provide support: NIAMS, NIA, the National Center for Research Resources, and the NIH Roadmap for Medical Research under grant numbers U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, and UL1 RR024140. Midcareer mentoring awards have been given to D. C. B. and N. E. L. (K24-AR-04884).
Conflict of interest statement
Dr. Bauer has received research funding from Merck, Amgen, Proctor and Gamble, and has consultancies for Merck, Zelos, and Tethys. All other authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of the SOF and MrOS Research Groups.
Rights and permissions
About this article
Cite this article
Yu, E.W., Blackwell, T., Ensrud, K.E. et al. Acid-Suppressive Medications and Risk of Bone Loss and Fracture in Older Adults. Calcif Tissue Int 83, 251–259 (2008). https://doi.org/10.1007/s00223-008-9170-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-008-9170-1