Abstract
Repeated exposure to adverse experiences in early life, termed Early Life Stress (ELS), can increase anxiety disorders later in life. Anxiety is directly associated with curiosity, a form of intrinsic drive state associated with increased novelty-seeking behaviour and risk taking for challenging opportunities and could probably modulate learning and memory. In humans, elevated curiosity during adolescence tends to elicit increased exploration, novelty seeking, high risk-taking behaviour and heightened emotionality. Such behaviours are beneficial in maintaining social skills and cognitive functions later in life. We investigated whether ELS-induced anxiety impacts curiosity-like behaviour at adolescence in an animal model. ELS was induced by subjecting Sprague Dawley rat pups to maternal separation and isolation (MS) stress during the stress hyporesponsive period (SHRP) from post-natal days (PND) 4-PND 14. This rat model was tested for anxiety, spontaneous exploratory behaviour and curiosity-like behaviour in a custom-designed arena during adolescence (PND 30–45). ELS-induced changes in the stress were confirmed by corticosterone, while, basal dopamine level was estimated to understand the neurochemical basis of MS stress-induced changes in curiosity. We observed an increase in the levels of anxiety and intrinsic drive state such as curiosity-like behaviour, which was associated with elevated plasma corticosterone and dopamine in MS animals during adolescence suggesting the impact of ELS during SHRP on adolescent behaviour.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When animals are exposed to conflicting environmental conditions, they make decisions based on their motivational states (Neville et al. 2020; Wispinski et al. 2020). One such intrinsic motivational drive is curiosity which is associated with increased exploration, novelty-seeking behaviour and risk-taking abilities in challenging situations. Generally, in the absence of motivational drives, animals avoid potentially threatful situations and approach favourable conditions. However, any adverse events leading to anxiety may influence the exploration (McNaughton and Corr 2004; Mobbs et al. 2020). Based on this concept, several experimental paradigms are designed to measure anxiety-like behaviour, wherein the animals are exposed to conflicting environments of safe versus unsafe zones, for example, centre vs periphery in open field test, light vs dark in light–dark exploration box (Sousa et al. 2006). In the literature, decreased exploration is often associated with heightened anxiety (Heinz et al. 2021) without considering the intrinsic motivation like curiosity which plays a pivotal role in making decisions to approach or avoid exploring the conflicting environments.
In developing rodents, the first two weeks of post-natal day (PND) represent a stress hyporesponsive period (SHRP), wherein the adrenal response to stress or adverse experience is either minimal or non-existent, resulting in stable low levels of circulating glucocorticoids (Sapolsky and Meaney 1986). Any stressful life events such as physical or psychological insults during SHRP may increase the risk for anxiety disorders during adolescence and adulthood, possibly due to the maladaptive hypothalmic-pituitary-adrenal axis (van Bodegom et al. 2017). Studies from our laboratory showed that exposure to early maternal separation and isolation (MS) stress during SHRP, can cause increased anxiety-like behaviour at young adulthood and can significantly impact both affective and cognitive functions (Kambali et al. 2019). These animals, irrespective of the duration and/or timing of MS stress exhibit increased fear memory associated with increased fear generalization during classical fear conditioning test (Sampath et al. 2014). Increased fear with reduced fear extinction was more evidently observed at young adulthood, but not at later stages indicating young adulthood is a critical time to exhibit the impact of MS stress (Mishra et al. 2019). MS rats in comparison to controls exhibit moderately enhanced attentional abilities with increased perseverative behaviour when tested in 5-choice serial reaction time task (5-CSRTT) paradigm and shows enhanced spatial learning abilities with lesser working memory error and reference memory in 4-arm baited radial arm maze task (Kambali et al. 2019).
Curiosity in rats is often described as a perceptual intrinsic drive to explore the novel stimuli which diminishes with continued exposure (Berlyne 1966; Berlyne and Slater 1957). Curiosity also plays an important role in learning, memory and decision making in conflicting situations. Adolescence is the transition stage between childhood and adulthood that is often associated with heightened exploratory, novelty-seeking and risk-taking behaviour which is exaggerated in animals exposed to stress during adolescence (Toledo-Rodriguez and Sandi 2011). A recent study by (Gruber and Fandakova 2021) has elucidated the importance of curiosity in learning during childhood and adolescence. However, experimental evidence for the neural basis of curiosity and its influence on learning and memory are limited. The ceaseless urge for novelty and sensation seeking perhaps leads to elevated levels of curiosity which may further intensify the drive for risk-taking behaviour (Laviola et al. 2003). High levels of curiosity and risk-taking may thus be considered as a feature of personality development during adolescence. However, in some cases due to vigorous search for sensation and elevated curiosity, adolescents might indulge in substance use disorders, which is the flip-side of curiosity and incorrect decision making. Whether such curiosity is altered when it is associated with an adverse experience in early life is not known. Hence it is crucial to establish the relationship between anxiety induced by ELS and its impact on curiosity and learning in adolescence. To address this, we investigated the effect of ELS during SHRP in the MS stress model by assessing anxiety, spontaneous exploratory behaviour and curiosity-like behaviour in adolescence.
Materials and methods
Subjects
Pregnant Sprague Dawley rats at the last week of gestation were procured from the Central animal research facility (CARF), NIMHANS, Bengaluru. Pregnant rats were housed individually in polypropylene cages (22.5 × 35.5 × 15 cm) till delivery in controlled environmental conditions with temperature (25 °C), 12:12 h light–dark cycles. Around 200 lx light intensity was maintained in the cage, during day time. Corn cob was used as a bedding material along with pieces of tissue paper for building the nest for the pups postpartum. The animals had access to ad libitum standard food pellets and water. Care was taken to minimize the discomfort to the animals during maternal separation and the rest of experiments.
Experimental design
Groups
In the Normal control (NC) group, rats were groomed with the dam without any disturbance throughout the prenatal period, except for bedding change. In the Maternal Separation and isolation stress (MS) group, rat pups were exposed to a maternal separation procedure. Rats from the F1 generation were used for the entire study.
Procedure for MS stress
Pregnant females were randomly selected for NC and MS groups. The day of delivery was considered as post-natal day zero (PND0). From PND4 to PND14, rat pups were subjected to MS protocol in a noise-free room, with controlled room temperature (25 °C). During this procedure, rat pups were separated from the mother only after confirming that the milk pouch was full and individual pups were also separated from their littermates and housed individually in the mice cages, with a layer of bedding material, daily for 6 h (10.00–16.00 h). Later, the dam and pups were reunited and undisturbed for the rest of the period till weaning. Weaning was done on PND21 by transferring both male and female pups into separate cages.
Experimental timeline
All behavioural assays were performed during early adolescence, i.e., PND30- PND45. Light dark test was performed on PND30 to assess anxiety-like behaviour. Open field test was performed on PND31 for assessing exploratory behaviour, ambulatory behaviour and anxiety-like behaviour. During PND34-PND36, object retrieval task was performed for curiosity assessment. At PND14 and PND30, pups were decapitated for plasma corticosterone estimation. At PND30, baseline plasma Dopamine was estimated. Rats from multiple litters were used for behavioural and hormonal assays. The timeline for the experimental protocol is shown in Fig. 1A. All experiments were carried out during the daytime between 09.00 h and 18.00 h in a controlled environment.
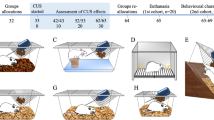
(A) Study design to assess curiosity behaviour in rats exposed to MS stress during stress hyporesponsive period (SHRP). QL1 (Level 1 of curiosity test), QL2 (level 2 curiosity test); PND pos-tnatal days. (B) Experimental procedure consisting of three phases—(i) Habituation (H); (ii) Level 1 curiosity testing (QL1) and Level 2 curiosity testing (QL2); (iii) Cross-sectional view of curiosity chamber. *inside the tray indicates the objects hidden for the object retrieval test
Anxiety-like behaviour
Following MS protocol during SHRP, rats were tested for anxiety-like behaviour in early adolescence age using light–dark test and open field test on two consecutive days, PND30 and PND31, respectively.
Light–dark test
The principle of the paradigm is based on the conflict between avoidance of the aversive brightly illuminated area and approaching the novel dark environment for natural exploration.
Light dark apparatus (Coulbourn Instruments, USA) consisting of two compartments, each with an area of 26 cm × 26 cm, separated by a guillotine door (8 × 8 cm) was used. The light chamber had a ceiling light of 600 lx intensity, while the dark chamber had no direct illumination. At the start of the experiment, the guillotine door was shut and the animal was placed in the light chamber. It was programed to open the door at the 5th second from the start of the protocol and each rat (n = 16 per group) was allowed to explore both the chambers for 5 min. The apparatus was cleaned with 70% Ethanol after each trial to eliminate olfactory cues. The latency to enter the dark compartment, time spent in the dark chamber and number of transitions between the compartments were considered as indices for anxiety-like behaviour, which were assessed offline using cineplex video recording software (Plexon Inc., USA version 1.0, 2005).
Open field test
Open field test was used to assess the anxiety-like behaviour, ambulatory behaviour and exploratory behaviour. Open field arena was made up of a square wooden box (100 × 100 × 40 cm) coated with water-proof black acrylic polyvinyl paint. The floor was divided into 25 squares (20 cm × 20 cm) with 9 squares in the centre and 16 at the periphery. The centre of the arena was illuminated with a light intensity of 30 lx and the periphery with 10 lx. All other light sources in the room were switched off during the experimental session. Each rat (n = 5–7 per group) was placed in the centre of the arena and allowed to explore freely for 5 min in the novel environment. The arena was cleaned with 70% Ethanol after each trial. The activity was video recorded and the distance travelled, velocity, immobility time, latency to enter periphery, frequency of zone transition was analysed using Ethovision® XT software, Noldus (Wagningen, The Netherlands).
Object retrieval task for curiosity assessment
Motivation has two drive states–Extrinsic and Intrinsic motivational drive states. Motivation driven by external reward serves as extrinsic motivation. On the other hand, motivation driven by one’s own curiosity or interest constitutes an intrinsic motivation (Berlyne 1966). Curiosity may be sometimes even associated with a component of risk. To assess the level of curiosity, the present study designed the “Object retrieval task”. In this task, the animal by virtue of its intrinsic motivation i.e., curiosity, performs object retrieval associated with risk.
Apparatus
The apparatus consisted of a 10 mm thick toughened glass chamber 3ft × 3ft (Indigenously designed by Hardik Pandya and group, [Department of Electronic Systems Engineering, Indian Institute of Science (IISc), Bengaluru]. Inside the chamber are two 10 mm thick white polypropylene platforms of 3ft x1ft placed at an adjustable height as shown in Fig. 1B. The two platforms were separated by the empty middle arena into which water was added according to the experimental design. The slopes of the platforms stand obliquely in the middle arena of the chamber, which prevents the escape of the rats below the platforms.
The curiosity chamber consisted of three platforms – (1) start (S) zone, (2) unsafe and risky zone (R) filled with water and (3) curiosity platform (Q) with or without hidden objects. The platform Q contained five novel objects (odourless and tasteless toys, hidden at specific points i.e., at the four corners and one at the centre) hidden in a rectangular plastic tray filled with corncob bedding material up to 2 inch height, which was placed at the centre. The platform S is an exploratory rectangular arena with no objects. To reach the curiosity platform Q, the rat had to cross the risky zone filled with 1.8 inches of water. Finally, to assess the intrinsic drive state as curiosity behaviour and to exclude the extrinsic drive state component, both NC and MS rats were fed with ad libitum food before exposing them into the curiosity task.
Procedure
The entire procedure was carried out in three phases – habituation, Level 1 of curiosity test (QL1) and level 2 curiosity test (QL2) (Fig. 1B).
On day 1, habituation (H) test, each rat was habituated to a novel 3-zone environment, placing the rat in the S zone and allowed to explore S, R and Q zone freely for 10 min. During this stage, R zone was not filled with water and Q zone was not with any hidden objects. At the end of the test, rat was returned to a home cage.
On day 2, to assess the level 1 curiosity test (QL1), each rat was allowed individually to explore both R and Q zone. Here, the rat was individually placed in the S and allowed freely to traverse across the R zone, which was filled with 1.8 inches of water to reach the Q platform (Fig. 1B). The Q zone consisted of a rectangular plastic tray wherein novel objects were hidden. During this period, the rat had to retrieve the objects within 30 min of testing duration.
Finally, on day 3, to assess the learning and adaptability on curiosity test (QL2), object retrieval task was repeated by placing the rat in the S zone. The entire apparatus was cleaned with 70% ethanol between the test procedure.
Analysis of curiosity level
The rat that did not cross the R zone during the QL1 test was considered as a non-performer and was not included in the statistical analysis.
The following parameters were considered to assess the curiosity – Latency to cross water, number of transitions, time spent in Platform S, time spent in platform Q, latency to retrieve the first object, number of objects retrieved, number of rearing, duration of grooming, number of faecal pellets.
Preference for the curiosity platform (in %) was estimated using the formula (Eq. 1):
Curiosity Index (CI) was calculated using the formula (Eq. 2):
Corticosterone (CORT) assay
The corticosterone assay was carried out using ELISA kit method. Rats were sacrificed by decapitation. Trunk blood was collected in heparin-coated tubes, centrifuged (5000 rpm for 10 min at 4 °C) and the plasma supernatant was stored at 20 °C. The corticosterone (CORT) concentration was measured in 1:75 diluted plasma samples using a commercially available ELISA kit (Puregene™, Genetix Biotech Asia Pvt.Ltd, #PG-9430 M) as described by the manufacturer. Each sample was analysed in duplicate and the CORT concentration was determined using the calibration curve based on the external standards. The basal CORT level was measured in two different time points – PND 14 (both sexes) (NC = 8, MS = 9) and PND 30 (male only) (NC = 6, MS = 6) rats.
Dopamine assay
Rats (male) from a different cohort, were sacrificed by decapitation on PND 30 (n = 9 per group) between 3.00 and 4.00PM and trunk blood was collected in the heparin-coated Eppendorf tube. Samples were then centrifuged at 5000 rpm for 10 min at 4 °C to obtain clear plasma. The aliquots of plasma supernatant were stored at 20 °C till the next day for dopamine assay. Neat undiluted samples were used for estimation of dopamine concentration was measured competitive inhibition enzyme immunoassay technique (Cloud Clone Corp, TX, USA, # CEA851Ge) according to the manufacturer's instructions. Later, 50 µl of undiluted plasma or Dopamine standard was added to the antibody-coated wells microplate. Then 50 µl of detection reagent A was added to each well and incubated for 1 h at 37 °C. Then the plate was washed 3 times with wash buffer and 100 µl Detection reagent B was added to each well and incubated for 30 min at 30 °C. Again, the aspiration and wash process repeated 5 times as previously mentioned. Then 90 µl of substrate reagent was added to each well and incubated at 370C for 20 min. When the uniform blue colour appeared then the stop solution was added and yellow colour is formed. The absorbance was read at 450 nm using a microplate within 15 min of adding the stop solution. Each sample was run in duplicate and the concentration of dopamine was determined using the calibration curve based on the concentration of the external standards.
Statistical analysis
All numerical data are presented as mean group values with standard errors of the mean (SEM). Student’s t test was used to compare between the group variables. Paired t test was used to compare within group comparisons at different timepoints. One-way ANOVA with Tukey’s multiple comparison test were applied to compare between groups. All statistical analyses were done using GraphPad Prism software 8.0 version. P value less than 0.05 was considered statistically significant.
Results
MS stress effect on body weight
The MS protocol significantly decreased the body weight of the rats when compared to age-matched NC rats at PND 14 (p < 0.0001), which continued to be low at PND21 (p < 0.0001), but not at PND 35 (p < 0.09) (Fig. 2).
Plasma corticosterone
Baseline levels of plasma corticosterone in MS rats can be a predictive resource for stress impact and thus on anxiety disorders. The plasma corticosterone level did not change immediately after the termination of MS stress at PND 14 but showed a delayed impact with an increased plasma corticosterone at PND 30. As shown in Fig. 3, plasma CORT was significantly higher in PND 30 MS than NC rats (p < 0.02, t1,10 = 2.690), while, an increase in CORT was not found on PND 14 age (p < 0.53, t1,15 = 0.6338). The one-way ANOVA (F3, 25 = 6.847, p < 0.001) followed by Tukey multiple comparison test revealed a significant difference between MS groups of PND14 and PND30 (p < 0.001), but not between NC groups. The data suggest that early MS stress leads to elevated circulating corticosterone at later stages of life, in adolescence age per se, during SHRP.
Plasma dopamine
The impact of MS stress on baseline dopamine was estimated using the ELISA kit method. The statistical analysis using t test revealed that plasma dopamine level was increased significantly at the baseline in MS than NC rat groups (p < 0.009, t1,16 = 2.963) (Fig. 4).
MS-mediated anxiety-like behaviour
Although the impact of MS stress at SHRP in enhancing anxiety has been studied earlier, the findings are inconsistent. Hence, we first investigated whether the MS stress during SHRP has impacted the anxiety-like behaviour in rat offspring at adolescence. In the light–dark test paradigm, MS rats displayed relatively lowered latency to enter the dark chamber (Fig. 5A) (P = 0.02, t1,30 = 2.421), spent a longer time in the dark chamber (Fig. 5B) (P < 0.005, t1,30 = 2.996) and lesser time in the light chamber (P = 0.005, t1,30 = 2.996). The total number of transitions between light and dark chambers was significantly decreased in MS rats when compared to NC rats (Fig. 5C) (P = 0.009, t1,30 = 2.789). Hence, MS stress during the early post-natal period causes heightened anxiety-like behaviour at adolescence if exposed to an anxiety-provoking environment.
Assessment of Anxiety-like behaviour in the light dark test Latency to enter dark chamber (A), Time spent in dark (B), number of transitions between compartments (C) were assessed across maternal separation (MS) and normal control (NC) groups. Data is expressed as Mean ± SEM. **p < 0.02, ***p < 0.005 as compared to NC rats using unpaired t test; (n = 16 per group).
In the Open field test, MS rats showed hyperactivity compared to age-matched NC rats as indicated by the total distance travelled (p < 0.02, t1,10 = 2.695) (Fig. 6A) and velocity of movement (p = 0.02, t1,10 = 2.536) (Fig. 6B), suggesting increased exploratory and ambulatory behaviour. MS animals did not show any statistically significant difference in the time spent in the centre, even though the mean time spent in the centre for MS rats was lesser (10.86 ± 3.318) as compared to controls (14.80 ± 4.977) (Fig. 6C). Collectively, adolescent MS rats exhibit enhanced anxiety-like behaviour, which is comparable to that of adult rats (Kambali et al. 2019).
Effect of MS stress on Anxiety-like behaviour assessed in open field test (A) Total ambulatory distance, (B) velocity of movement, (C) Time spent in the centre were assessed in maternal separation (MS) and normal control (NC) groups. Data is expressed as Mean ± SEM. *p < 0.05 vs. NC, unpaired t test; (N = 5–7 per group).
Curiosity-like behaviour
Object retrieval task revealed consistently increased curiosity-like behaviour in both groups and sexes. Among male rats, 50% of NC (11–22), 25% of MS (6–24) were non-performers, while, 38.46% NC (5–13) and 23.07% MS (3–13) rats were non-performers among female rats in the curiosity task. Hence, statistical analysis was carried out only in performer rats by merging the data from both male and female rats because of statistically insignificant difference in the sex-specific changes in the curiosity behaviour.
MS rats showed a relatively higher level of curiosity-like behaviour by exhibiting reduced latency to reach the curiosity platform by crossing the risky water (R) zone with one-way ANOVA followed by Tukey’s multiple comparison test (F3,90 = 5.553, p < 0.0015, R2 = 0.1562). MS effect analysis using Student’s t test also further revealed that MS rats exhibited relatively higher curiosity by exhibiting reduced latency to reach the curiosity platform than the NC group (t1,45 = 2.73, p < 0.009) (Fig. 7A). The elevated curiosity on day 1 was diminished when re-exposed to the same task after day 2 (QL2) with sustained statistically significant difference (t1,45 = 2.121, p < 0.03) between NC and MS rat groups.
Curiosity-like behaviour during object retrieval task. (A) Latency to cross water (LTW), (B) Latency to retrieve objects (LTR), (C) Number of objects retrieved (NOR) in level 1 (QL1) and level 2 (QL2) of curiosity assessment. Data is expressed as Mean ± SEM. *p < 0.05, **p < 0.02, ***p < 0.0001 as compared to NC in unpaired t test, NC (n = 19) and MS (n = 28)
Further, there was a significant difference in the latency to retrieve the hidden objects as shown with one-way ANOVA (F3,90 = 2.683, p < 0.05, R2 = 0.0821) and also in the total number of objects retrieved in the given time (F3,90 = 2.604, p < 0.05, R2 = 0.07987) across the groups in both the levels of curiosity testing. Student’s t-test analysis revealed that MS rats took lesser time to retrieve the objects compared to the NC rats in the QL1 of object retrieval task (p < 0.05, t1,45 = 1.939), but not at QL2 (p < 0.1784, t1,45 = 1.367) (Fig. 7B). Similarly, the total number of objects retrieved by MS rats were significantly higher than control rat groups in QL1 (p < 0.005, t1,45 = 2.941) but not at QL2 (p < 0.52, t1,45 = 0.5946) (Fig. 7C).
Preference for the curiosity platform (percent time) showed that MS rats exhibited a higher preference for the exploration in the curiosity platform as compared to NC rats, irrespective of gender (One-way ANOVA: F3,90 = 2.3, p < 0.08, R2 = 0.0712). In the QL1 of curiosity testing, Q preference for curiosity platform was significantly higher in MS rats as compared to NC rats as shown with t test (p < 0.02, t1,45 = 2.313) (Fig. 8). However, MS and NC rats did not show statistically significant difference with the preference for curiosity platform at QL2 (Fig. 8) stage (p < 0.2, t1,45 = 1.28).
Finally, the curiosity index was analysed to evaluate the effects of early MS on overall changes in curiosity-like behaviour. While gender-specific changes in curiosity index was not significantly different in both levels (QL1 and QL2) and groups as revealed by one-way ANOVA (F3,90 = 2.445, p < 0.06, R2 = 0.07535). Student’s t test analysis further revealed that MS rats exhibited relatively higher level of curiosity index than NC rats at QL1 (p < 0.01, t1,45 = 2.425) but not at QL2 (p < 0.20, t1,45 = 1.281) (Fig. 9).
Discussion
The present study showed that the MS stress during SHRP has a long-lasting impact on the brain and behaviour during adolescence. The effect of MS stress on anxiety was assessed in light–dark and open field tests, and found to be elevated in both paradigms. We further show that exposure to MS stress was associated with elevated curiosity-like behaviours in adolescence. To the best of our knowledge, this is the first study demonstrating elevated anxiety and curiosity behaviour in adolescent rats, due to MS stress at SHRP. We also observed that the MS stress lead to a marked reduction in body weight until weaning (PND21), which was restored by PND35. In addition, MS stress significantly increased the levels of plasma corticosterone and dopamine in adolescence.
Although adolescence is most often associated with increased energy expenditure, basal metabolic rate and impulsive behaviour (Jung et al. 2011), (Caron and Stephenson 2010), it is less clear whether increased anxiety and energy expenditure may cause body weight loss in adolescence. The reduced body weight in MS rats in the present study is in line with the previous findings (Arp et al. 2016; Bondar et al. 2018), thus, attributable to MS stress at SHRP. While restoration in body weight following weaning indicates, the stress did not have a long-term impact on eating patterns.
In addition, MS stress in rats showed increased basal corticosterone levels. The importance of hypothalmus-pituitary-adrenal (HPA)-axis in the regulation of brain and behaviour has been established (Luo et al. 2014). Previously, we and others have also reported that irrespective of the timing and duration of stress during the critical time window of the development, MS stress caused increased anxiety-like behaviour in adulthood (Costa et al. 2014; Kambali et al. 2019; Roque et al. 2014; Sampath et al. 2014). This indicates that early childhood adversities causes hyper-responsive HPA axis, with increased ACTH and cortisol levels, as predictors of psychological stress both in adolescence and adulthood (Kuhlman et al. 2015; Lupien et al. 2009). In animal models, low maternal care in early life increases plasma ACTH and corticosterone responses to the 20 min period of restraint stress in adults (Liu et al. 1997). On the contrary, MS stress for a brief period increased maternal attention towards pups leading to better attenuation of the stress response (Boccia and Pedersen 2001). These findings together suggest that early maternal care such as licking, grooming, arch back nursing may play an important role in adult behaviour (Buschdorf and Meaney 2015). Hence, we proposed that MS-induced changes in anxiety at adolescence and adulthood are consequences of sustained HPA-axis activation at SHRP due to stress and low maternal care. In line with these evidences, the present study also noted increased plasma corticosterone at PND 30 but not at PND 14, predicted as an index of HPA-axis activation. Elevated serum corticosterone levels were observed even at PND 35 and PND 60 in maternal deprivation rats (Kapor et al. 2020), together indicating that the impact of MS stress was not immediate but surfaced later in life.
Long term effects of MS stress on the dopaminergic system
The most plausible mechanisms mediating curiosity are via increased plasma dopamine would be resultant of increased release of dopamine from the ventral tegmental (VTA) neurons. Animal studies indicate that exposure to psychological or physical stress can influence the release of dopamine (Hausknecht et al. 2013; Holly et al. 2015) which in turn invariably leads to either physiological or pathogenic adaptation (Butts and Phillips 2013; Hausknecht et al. 2013). The increase or decrease in the release of dopamine is based on the time, duration and intensity of psychogenic stressors (McEwen and Wingfield 2003). The bodily changes in the behaviour following stress are most often consequences of adaptive mechanisms by altering the brain functions. Most often it is indicated that hyperactive and impulsive behaviour at adolescence is a result of elevated levels of dopamine (Schultz 1998). Dopamine (DA), produced in the substantial nigra (SN), ventral tegmental area (VTA) and hypothalamus of the brain and plays an important role in motivation, reward and reinforcement learning (Arias-Carrion et al. 2010; Schultz 1998). The rapid increase in dopaminergic activity within the limbic system leads to increased vulnerability to take risky decisions (Freels et al. 2020). Risky decision making in adolescents results from increased sensitivity to rewards in the ventral medial prefrontal cortex and the ventral striatum associated with immature cognitive control abilities (Van Leijenhorst et al. 2010a, b).
Increased plasma dopamine in adolescence is in line with previous reports that MS stress early in life increases excitatory transmission in the VTA dopaminergic neurons and an associated increase in the levels of ACTH and corticosterone (Overton et al. 1996; Spyrka et al. 2020). In the mesolimbic circuitry, both acute and chronic stress have been shown to exhibit either decreased or increased glutamatergic neurotransmission, indicating motivation and reward processing is regulated by the mesolimbic dopaminergic circuitry (Douma and de Kloet 2020). MS stress is shown to increase the volume of substantia nigra (SN)/VTA, increases the number of dopaminergic neurons and the volume of dopaminergic nuclei (Kapor et al. 2020). During adolescence, an increase in dopamine concentration and fibre density in PFC and increased pruning of dopaminergic receptors in the striatum are noted. Consequently, there is increased risk-taking behaviour, drug use, increased exploratory and novelty-seeking behaviour (Spear 2000). ELS further increases the likelihood of substance abuse and risk-taking behaviour which depends mainly on the dopaminergic system (Andersen 2019). MS stress at SHRP dysregulates HPA-axis causing increased plasma corticosterone and activates the dopaminergic system, a condition that lasts for weeks after stressor termination (Horii-Hayashi et al. 2013; Spyrka et al. 2020).
Recently, Wang and Hayden (Wang and Hayden 2021) proposed that curiosity enables and motivates latent learning, by involving two important brain regions of the prefrontal cortex (PFC) – orbitofrontal cortex (OFC) and dorsal anterior cingulate gyrus (dACC). Our study also revealed that repeated exposure to the same task on the second day, reduce the curiosity index in the level 2 object retrieval task. This is in accordance with the response to trivia questions in human subjects wherein the study showed an inverted bell-shaped curve to curiosity-driven task and connected striatal activation with curiosity-driven anticipation (Kang et al. 2009).
Another critical finding unravelled the role of midbrain dopamine neurons in curiosity-induced observation tasks (Bromberg-Martin and Monosov 2020). During the high state of curiosity task, the activity in both the midbrain dopaminergic system and nucleus accumbens was increased and memory was correlated with midbrain SN/VTA and hippocampal activity (Gruber et al. 2014). Although curiosity reflects intrinsic motivation, these results suggest that equivalent mechanisms mediate it as an extrinsically motivating reward. A recent study showed that the neurobiological mechanisms on curiosity encouraging risky behaviour and also motivational lure for curiosity and hunger for food (extrinsic motivation) share common neural correlates, namely nucleus accumbens (ventral striatum) and caudate (dorsal striatum) (Lau et al. 2020).
MS stress and curiosity behaviour
To study the impact of MS stress on the intrinsic drive state, we exposed rats fed with ad libitum food to a non-rewarding task based on risky decisions. Whether such risky decisions are due to hyperactive locomotion or anxiety is not clearly known. MS rats showing elevated anxiety (van Bodegom et al. 2017) and corticosterone have triggered the perseverative behaviour (Kambali et al. 2019) in a reward-oriented task. These findings enforced the present study to search for the consequences of MS stress on intrinsic drive state, such as curiosity behaviour towards the non-rewarding and non-palatable objects using object retrieval test.
Interestingly, elevated curiosity by adolescent MS stress rats viz., increased time in curiosity platform, reduced latency to enter into curiosity platform and increased retrieval of novel objects were observed in the object retrieval task. Curiosity and risk-taking are considered to be normal personality developmental processes during adolescence. As scrutinized in psychological studies, curious people take risks to acquire new experiences (Zuckerman 1996) and are persistently involved in challenging tasks. We hypothesized that curiosity is associated with risk-taking behaviour. Hence, crossing the mild risky water zone with shorter latency and retrieving the more number of non-rewarding objects by crossing the risky water zone by MS than control rats fed with ad libitum food, would be, thus, considered as a consequence of elevated intrinsic drive state. Repeated exposure to the same task led to higher precision in the performance, thereby reducing the marginal difference between the groups in QL2. Nevertheless, the difference between MS and NC male rats was much more apparent in QL1 than in QL2.
Latency to cross water was the first step in deciding performance. Interestingly, 50% of NC males and 38% of NC females did not cross the water throughout the test period and were declared non-performers. In contrast, only 25% of MS males and 23% of MS females remained as non-performers. These observations together indicate that during the object retrieval task, non-performers are more in NC than MS adolescent rats.
Based on the performers data, it was observed that latency to cross water was significantly reduced in MS rats, especially in QL1, when compared to adolescent controls. The increased curiosity-driven performance in object retrieval task could be due to impulsive behaviour (Kambali et al. 2019) with the risky decisions attributed to poor risk assessment abilities of MS rats. The increased retrieval of hidden objects in the QL1 stage by MS rats further indicates enhanced intrinsic motivation is associated with performance. It was intriguing that whether risky decisions was due to vigorous novelty seeking and elevated curiosity. Literature indicates that early life stress has long-term impact in enhancing vulnerability to addictive behaviour in adolescence (Yamaguchi and Kandel 1984). Taken together, the above evidence clearly indicates that increased curiosity and anxiety in adolescents (fed with ad libitum food), thereby indulging in increased risky decisions could be attributed to early life stress. Finally, the changes in basal dopamine and corticosterone levels following MS stress further strengthens the possibility of increased vulnerability for addictive behaviour in adolescence.
Conclusion
We conclude that increased plasma corticosterone and dopamine attribute to elevated anxiety and curiosity during adolescence in rats exposed to MS stress at SHRP.
Abbreviations
- ACC:
-
Anterior cingulate cortex
- ACTH:
-
Adreno corticotropin releasing hormone
- ANOVA:
-
Analysis of variance
- CARF:
-
Central animal research facility
- CI:
-
Curiosity index
- CORT:
-
Corticosterone
- CPCSEA:
-
Committee for the purpose of control and supervision of experiments on animals
- CSIR:
-
Council for Scientific and Industrial Research
- 5-CSRTT:
-
Five choice serial reaction time task
- DA:
-
Dopamine
- ELISA:
-
Enzyme linked Immunosorbent assay
- ELS:
-
Early life stress
- HPA:
-
Hypothalamo pituitary adreanal
- IISc:
-
Indian Institute of science
- MS:
-
Maternal separation
- NC:
-
Normal control
- NIMHANS:
-
National Institute of Mental Health And Neuro sciences
- OFC:
-
Orbito frontal cortex
- PFC:
-
Prefrontal cortex
- PND:
-
Postnatal day
- QL:
-
Curiosity level
- REM:
-
Rapid eye movement
- SEM:
-
Standard error of mean
- SHRP:
-
Stress hypo responsive period
- SN:
-
Substantia nigra
- VTA:
-
Ventral tegmental area
References
Andersen SL (2019) Stress, sensitive periods, and substance abuse. Neurobiol Stress 10:100140
Arias-Carrion O, Stamelou M, Murillo-Rodriguez E, Menendez-Gonzalez M, Poppel E (2010) Dopaminergic reward system: a short integrative review. Int Arch Med 3:24
Arp JM, Ter Horst JP, Loi M, den Blaauwen J, Bangert E et al (2016) Blocking glucocorticoid receptors at adolescent age prevents enhanced freezing between repeated cue-exposures after conditioned fear in adult mice raised under chronic early life stress. Neurobiol Learn Mem 133:30–38
Berlyne DE, Slater J (1957) Perceptual curiosity exploratory behavior, and maze learning. J Comp Physiol Psychol 50:228–232
Berlyne DE (1966) Curiosity and exploration. Science 153:25–33
Boccia ML, Pedersen CA (2001) Brief vs. long maternal separations in infancy: contrasting relationships with adult maternal behavior and lactation levels of aggression and anxiety. Psychoneuroendocrinology 26:657–672
Bondar NP, Lepeshko AA, Reshetnikov VV (2018) Effects of early-life stress on social and anxiety-like behaviors in adult mice: sex-specific effects. Behav Neurol 2018:1538931
Bromberg-Martin ES, Monosov IE (2020) Neural circuitry of information seeking. Curr Opin Behav Sci 35:62–70
Buschdorf JP, Meaney MJ (2015) Epigenetics/programming in the HPA axis. Compr Physiol 6:87–110
Butts KA, Phillips AG (2013) Glucocorticoid receptors in the prefrontal cortex regulate dopamine efflux to stress via descending glutamatergic feedback to the ventral tegmental area. Int J Neuropsychopharmacol 16:1799–1807
Caron AM, Stephenson R (2010) Energy expenditure is affected by rate of accumulation of sleep deficit in rats. Sleep 33:1226–1235
Costa AA, Morato S, Roque AC, Tinos R (2014) A computational model for exploratory activity of rats with different anxiety levels in elevated plus-maze. J Neurosci Methods 236:44–50
Douma EH, de Kloet ER (2020) Stress-induced plasticity and functioning of ventral tegmental dopamine neurons. Neurosci Biobehav Rev 108:48–77
Freels TG, Gabriel DBK, Lester DB, Simon NW (2020) Risky decision-making predicts dopamine release dynamics in nucleus accumbens shell. Neuropsychopharmacology 45:266–275
Gruber MJ, Fandakova Y (2021) Curiosity in childhood and adolescence - what can we learn from the brain. Curr Opin Behav Sci 39:178–184
Gruber MJ, Gelman BD, Ranganath C (2014) States of curiosity modulate hippocampus-dependent learning via the dopaminergic circuit. Neuron 84:486–496
Hausknecht K, Haj-Dahmane S, Shen RY (2013) Prenatal stress exposure increases the excitation of dopamine neurons in the ventral tegmental area and alters their reponses to psychostimulants. Neuropsychopharmacology 38:293–301
Heinz DE, Schottle VA, Nemcova P, Binder FP, Ebert T et al (2021) Exploratory drive, fear, and anxiety are dissociable and independent components in foraging mice. Transl Psychiatry 11:318
Holly EN, DeBold JF, Miczek KA (2015) Increased mesocorticolimbic dopamine during acute and repeated social defeat stress: modulation by corticotropin releasing factor receptors in the ventral tegmental area. Psychopharmacology 232:4469–4479
Horii-Hayashi N, Sasagawa T, Matsunaga W, Matsusue Y, Azuma C, Nishi M (2013) Developmental changes in desensitisation of c-Fos expression induced by repeated maternal separation in pre-weaned mice. J Neuroendocrinol 25:158–167
Jung CM, Melanson EL, Frydendall EJ, Perreault L, Eckel RH, Wright KP (2011) Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J Physiol 589:235–244
Kambali MY, Anshu K, Kutty BM, Muddashetty RS, Laxmi TR (2019) Effect of early maternal separation stress on attention, spatial learning and social interaction behaviour. Exp Brain Res 237:1993–2010
Kang MJ, Hsu M, Krajbich IM, Loewenstein G, McClure SM et al (2009) The wick in the candle of learning: epistemic curiosity activates reward circuitry and enhances memory. Psychol Sci 20:963–973
Kapor S, Aksic M, Puskas L, Jukic M, Poleksic J et al (2020) Long-term effects of maternal deprivation on the volume of dopaminergic nuclei and number of dopaminergic neurons in substantia nigra and ventral tegmental area in rats. Front Neuroanat 14:578900
Kuhlman KR, Geiss EG, Vargas I, Lopez-Duran NL (2015) Differential associations between childhood trauma subtypes and adolescent HPA-axis functioning. Psychoneuroendocrinology 54:103–114
Lau JKL, Ozono H, Kuratomi K, Komiya A, Murayama K (2020) Shared striatal activity in decisions to satisfy curiosity and hunger at the risk of electric shocks. Nat Hum Behav 4:531–543
Laviola G, Macri S, Morley-Fletcher S, Adriani W (2003) Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev 27:19–31
Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D et al (1997) Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science 277:1659–1662
Luo XM, Yuan SN, Guan XT, Xie X, Shao F, Wang WW (2014) Juvenile stress affects anxiety-like behavior and limbic monoamines in adult rats. Physiol Behav 135:7–16
Lupien SJ, McEwen BS, Gunnar MR, Heim C (2009) Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 10:434–445
McEwen BS, Wingfield JC (2003) The concept of allostasis in biology and biomedicine. Horm Behav 43:2–15
McNaughton N, Corr PJ (2004) A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neurosci Biobehav Rev 28:285–305
Mishra PK, Kutty BM, Laxmi TR (2019) The impact of maternal separation and isolation stress during stress hyporesponsive period on fear retention and extinction recall memory from 5-week- to 1-year-old rats. Exp Brain Res 237:181–190
Mobbs D, Headley DB, Ding W, Dayan P (2020) Space, time, and fear: survival computations along defensive circuits. Trends Cogn Sci 24:228–241
Neville V, King J, Gilchrist ID, Dayan P, Paul ES, Mendl M (2020) Reward and punisher experience alter rodent decision-making in a judgement bias task. Sci Rep 10:11839
Overton PG, Tong ZY, Brain PF, Clark D (1996) Preferential occupation of mineralocorticoid receptors by corticosterone enhances glutamate-induced burst firing in rat midbrain dopaminergic neurons. Brain Res 737:146–154
Roque S, Mesquita AR, Palha JA, Sousa N, Correia-Neves M (2014) The behavioral and immunological impact of maternal separation: a matter of timing. Front Behav Neurosci 8:192
Sampath D, Sabitha KR, Hegde P, Jayakrishnan HR, Kutty BM et al (2014) A study on fear memory retrieval and REM sleep in maternal separation and isolation stressed rats. Behav Brain Res 273:144–154
Sapolsky RM, Meaney MJ (1986) Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res 396:64–76
Schultz W (1998) Predictive reward signal of dopamine neurons. J Neurophysiol 80:1–27
Sousa N, Almeida OF, Wotjak CT (2006) A hitchhiker’s guide to behavioral analysis in laboratory rodents. Genes Brain Behav 5(Suppl 2):5–24
Spear LP (2000) The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24:417–463
Spyrka J, Gugula A, Rak A, Tylko G, Hess G, Blasiak A (2020) Early life stress-induced alterations in the activity and morphology of ventral tegmental area neurons in female rats. Neurobiol Stress 13:100250
Toledo-Rodriguez M, Sandi C (2011) Stress during adolescence increases novelty seeking and risk-taking behavior in male and female rats. Front Behav Neurosci 5:17
van Bodegom M, Homberg JR, Henckens M (2017) Modulation of the hypothalamic-pituitary-adrenal axis by early life stress exposure. Front Cell Neurosci 11:87
Van Leijenhorst L, Gunther Moor B, Op de Macks ZA, Rombouts SA, Westenberg PM, Crone EA (2010a) Adolescent risky decision-making: neurocognitive development of reward and control regions. Neuroimage 51:345–355
Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SA, Crone EA (2010b) What motivates the adolescent? brain regions mediating reward sensitivity across adolescence. Cereb Cortex 20:61–69
Wang MZ, Hayden BY (2021) Latent learning, cognitive maps, and curiosity. Curr Opin Behav Sci 38:1–7
Wispinski NJ, Gallivan JP, Chapman CS (2020) Models, movements, and minds: bridging the gap between decision making and action. Ann N Y Acad Sci 1464:30–51
Yamaguchi K, Kandel DB (1984) Patterns of drug use from adolescence to young adulthood: III. predictors of progression. Am J Public Health 74:673–681
Zuckerman M (1996) The psychobiological model for impulsive unsocialized sensation seeking: a comparative approach. Neuropsychobiology 34:125–129
Acknowledgements
Authors also wish to thank Hardik Pandya and Arjun from Indian Institute of Science (IISc, Bengaluru) for constructing the curiosity chamber.
Author information
Authors and Affiliations
Contributions
SSS: Investigation, Data acquisition, data analysis, preparing figures, preparing manuscript; DY, MMSB: Preparing manuscript, revisions and editing, research supervisions; LTR. Funding acquisition, project administration, Study conceptualization and methodology, data analysis, preparing figures, preparing manuscript, revisions and supervisions.
Corresponding author
Ethics declarations
Conflict of interest
The authors (S.S.S., M.M.S.B., Y.D. and T.R.L.) do not have any conflict of interests to declare.
Ethical approval
Prior to experimental protocol, ethical approval was obtained from Institutional Animal ethics committee (AEC/72/ 474/NP) which is under the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India at National Institute of Mental Health and Neurosciences (NIMHANS), Bengaluru.
Additional information
Communicated by Sreedharan Sajikumar.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1
Appendix 1
Equations
Equation 1. Preference for the curiosity platform (in %) was estimated using the formula:
Equation 2. Curiosity Index (CI) was calculated using the formula:
Rights and permissions
About this article
Cite this article
Sharma, S.S., Srinivas Bharath, M.M., Doreswamy, Y. et al. Effects of early life stress during stress hyporesponsive period (SHRP) on anxiety and curiosity in adolescent rats. Exp Brain Res 240, 1127–1138 (2022). https://doi.org/10.1007/s00221-022-06319-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-022-06319-5