Abstract
To evaluate normal and impaired control of anticipatory grip force (GF) modulation, we compared GF production during horizontal arm movements in healthy and post-stroke subjects, and, based on a physiologically feasible dynamic model, determined referent control variables underlying the GF–arm motion coordination in each group. 63% of 13 healthy and 48% of 13 stroke subjects produced low sustained initial force (< 10 N) and increased GF prior to arm movement. Movement-related GF increases were higher during fast compared to self-paced arm extension movements only in the healthy group. Differences in the patterns of anticipatory GF increases before the arm movement onset between groups occurred during fast extension arm movement only. In the stroke group, longer delays between the onset of GF change and elbow motion were related to clinical upper limb deficits. Simulations showed that GFs could emerge from the difference between the actual and the referent hand aperture (Ra) specified by the CNS. Similarly, arm movement could result from changes in the referent elbow position (Re) and could be affected by the co-activation (C) command. A subgroup of stroke subjects, who increased GF before arm movement, could specify different patterns of the referent variables while reproducing the healthy typical pattern of GF–arm coordination. Stroke subjects, who increased GF after arm movement onset, also used different referent strategies than controls. Thus, altered anticipatory GF behavior in stroke subjects may be explained by deficits in referent control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Functional hand use depends on the ability to modulate grip forces (GFs) during manipulative tasks (Blennerhassett et al. 2008). Previous studies of pinch grip forces in stroke subjects focused on grasp-and-lift tasks during vertical point-to-point movements (Blennerhassett et al. 2008; Li et al. 2011; Nowak et al. 2003, 2007; Raghavan et al. 2006). Such tasks, however, may not fully capture performance deficits during other functional activities, which require sustained holding of objects while moving the arm in different directions for tasks such as writing, eating and transferring objects through space.
Anticipatory behavior is usually associated with an increase in GF before (25–165 ms), during or slightly after (< 25 ms) movement onset (Pilon et al. 2007) that is proportional to the load force (Danion and Sarlegna 2007; Flanagan and Tresilian 1994) and is related to the prevention of object slippage when arm movement starts. Individuals with stroke may have impaired anticipatory GF regulation during lifting tasks. GF production was delayed in 49% of individuals with sub-acute stroke compared to healthy subjects. Larger values of grip–lift delay were related to limitations in clinical hand function tests (Blennerhassett et al. 2008). In patients with acute stroke, the time between GF onset and vertical plane arm movement onset was more than two times longer than healthy older adults (Nowak et al. 2003). Larger time intervals between GF onset and acceleration onset for upward movements and smaller time intervals for downward movements suggest that stroke subjects had deficits anticipating and preventing object slippage during arm motion. Possibly to compensate for these deficits, stroke subjects produced exaggerated GFs when lifting and holding an object or during vertical point-to-point movements (Nowak et al. 2003). Timing deficits between GF and arm movement have also been reported in patients with chronic stroke during object transport tasks, but not during cyclical movements (Hermsdörfer et al. 2003). Thus, deficits in anticipatory GF modulation in people with stroke may be task specific and impairments may be related to a lack of temporal coordination of finger, hand and arm movements (Grichting et al. 2000; Sangole and Levin 2009). In addition, GF and load force (LF) scaling may be impaired in individuals with stroke (Allgöwer and Hermsdörfer 2017; Flanagan and Wing 1997; Hermsdörfer et al. 2003) and reduced sensation (Nowak and Hermsdörfer 2003; Nowak et al. 2004). Gaining a better understanding of motor control deficits underlying altered GF modulation during functional movements may provide a better focus for upper limb rehabilitation.
In the present study, we compared GF production during arm movements in healthy and post-stroke subjects based on a physiologically feasible dynamic model of the central control processes underlying motor actions (Pilon et al. 2007). The referent control model was previously used to simulate single-joint movements, isometric force production as well as actions such as jumping, walking and sit-to-stand action (e.g., Feldman 2011; Feldman et al. 2011; Latash 1992; Pilon et al. 2007). Our choice of the model was made by considering controversies arising from two alternative theoretical frameworks addressing the question of how motor actions and GFs are controlled. According to the standard biomechanical framework or its extension, computational framework, the nervous system pre-programs and directly specifies the motor outcome. In contrast, the empirically derived equilibrium point hypothesis, now advanced to the theory of indirect, referent control of action and perception (Feldman 2015, 2019), suggests that motor actions emerge due to changes in specific neurophysiological parameters—referent variables—without computations, pre-programming or internal models.
The choice of the referent control framework is based on the scientifically rigorous criteria that the model (1) is physiologically feasible, (2) is consistent with natural, physical laws and (3) has withstood empirical testing. Based on the correlation between GF and LF during arm movement with a hand-held object, Flanagan and Wing (1997) suggested the existence of an internal model for pre-programming GFs. However, this suggestion does not meet the minimal criteria listed above. Indeed, if GFs are computed, the system can use some EMG–force relationship to determine EMG levels that elicit the requisite muscle forces. This approach does not explain how the system can determine and physically deliver synaptic signals to MNs to force them to generate requisite EMG levels. To do so, the system should convert the computed output (EMG) signals of MNs into input, synaptic signals to MNs. This would be possible to do if MNs had linear properties. However, because of the electrical threshold, i.e., a specific membrane potential that needs to be exceeded to recruit MNs, as well as because of the ability of MNs to autonomously sustain rhythmic discharges (plateau potentials, e.g., Heckman et al. 2005), MNs cannot be considered as linear entities even for small changes in input signals (Feldman 2019). Most importantly, threshold input/output functions of MNs are irreversible, i.e., knowing that a MN discharges, say 12 times per second, one cannot determine which combination of synaptic inputs to the MN was responsible for this outcome. This means that the system cannot determine, nor specify synaptic signals to MNs required for production of pre-programed GFs. Thus, the idea of pre-programming of grip or other muscle forces is physiologically untenable.
Model-based computations of GFs and other computational theories also violate several physical laws (see Feldman 2019). Therefore, we use a dynamical model (Pilon et al. 2007; see Methods) that meets the criteria listed above and has shown that anticipatory modulation of GFs and arm motion in healthy subjects can result from parametric control without internal models or pre-programming.
The present study is the first attempt to use a physiologically realistic model based on the referent control theory (Feldman 2015), to characterize changes in central control variables underlying deficits in GF–arm motion coordination during a functional task following stroke. Our aims were to characterize arm motion and GFs in terms of kinematic and kinetic variables and to provide insights into the causes of deficits in this coordination. We characterized deficits in anticipatory GF modulation in subjects with chronic stroke compared to healthy controls during horizontal arm motion at two different speeds (self-paced and fast) and directions (extension/flexion). Based on the dynamic model of Pilon et al. (2007), we also characterized referent control variables underlying these deficits in terms of reciprocal (Re, Ra) commands targeting arm and hand muscles and co-activation (C) commands targeting arm muscles. Preliminary results have been reported in abstract form (Yamanaka et al. 2010).
Methods
Participants
Thirteen (5 women, 8 men; aged 65.2 ± 9.3 years) individuals who sustained a single left-sided ischemic stroke 3.2 ± 2.3 years previously and 13 age-matched (8 women, 5 men; aged 69.8 ± 8.5 years) healthy subjects participated (Table 1). In all subjects, the dominant right arm was tested. Participants with stroke were included if they had some voluntary flexion/extension of the elbow, fingers and thumb and were able to hold a force transducer while flexing or extending the elbow. They were excluded if they had severe proprioceptive and/or tactile deficits in the elbow, fingers or thumb and difficulties understanding task demands. Subjects in both groups were excluded if they had musculoskeletal or neurological (other than stroke) deficits interfering with task performance. Participants signed informed consent forms approved by the institutional review board of the Center for Interdisciplinary Research in Rehabilitation of Greater Montreal (CRIR).
Clinical examination
Hand and arm sensorimotor function was tested by an experienced clinician. Sensation was evaluated with multiple tests including the Sustained Touch Pressure (STP, reliability: ICC = 0.62–0.92) and Moving Touch Pressure (MTP, ICC = 0.92) tests (Dannenbaum et al. 2002). The STP measures the ability to feel an object held actively or placed passively on the hand for 60 s and scored on a 0–10 Likert scale at 10 s intervals. The level of sensation at the end of 60 s is subtracted from that at the beginning so that a score of 0 indicates that no fading occurred. The MTP measures the ability to estimate tactile intensity of brushes of different textures (percent of correct answers). Tactile thresholds on thumb and index finger pads were tested with Semmes–Weinstein monofilaments (Anderson and Croft 1999; weighted kappa = 0.92). Motor impairment was assessed with the Chedoke McMaster Arm and Hand Impairment Inventory for which a score of 7 reflects full recovery (Gowland et al. 1993). Grip strength was measured with a Jamar dynamometer (Lindstrom-Hazel et al. 2009; ICC = 0.99). Upper limb dexterity was assessed with the Box and Blocks Test (BBT, Platz et al. 2005; ICC > 0.95) as the number of blocks moved from one side of a box to the other in 60 s. Fine motor function was assessed with the Purdue Pegboard Test (PPT) as the number of pegs placed on a wooden pegboard in 60 s (Desrosiers et al. 1995; ICC > 0.66). For grip strength, BBT and PPT, the average of the two attempts was reported.
Experimental procedures and data recording
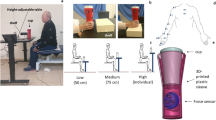
Subjects sat on a chair with a solid back support. The hips and knees were flexed to 90° and the feet were supported on a foot rest. The axis of rotation of the right elbow was aligned with that of a horizontal manipulandum on which the forearm in the neutral position was secured with Velcro straps. The hand extended beyond the end of the manipulandum so that wrist movements were unrestrained. A cylindrical object (force transducer, 7 cm diameter, 3.9 cm high, 63.5 g) was placed near the distal end of the manipulandum. The moment of inertia of the manipulandum with the arm was about 0.1 kg m2. Because of the low mass, the moment of inertia of the transducer was negligible (less than 0.1%), compared to the inertia of the manipulandum with the arm.
At a “ready” cue, subjects lifted the object by 2–3 cm and held it between the index finger and thumb. 3–5 s after a steady grip force (GF) was reached, a second cue (‘go’) instructed subjects to flex or extend the elbow while holding the object, 60° from an initial position of 160° (flexion) or 70° (extension), respectively (full elbow extension was 180°), and then to hold the arm in the final position for 2–3 s. A 15 s or longer rest period was allowed between trials to avoid fatigue. Flexion (F) or extension (E) movements were made at self-paced (S) or fast (F) speeds resulting in four conditions done in a randomized order: fast extension (FE), fast flexion (FF), self-paced extension (SE) and self-paced flexion (SF). Each condition included 10 trials (40 trials in total).
An accelerometer attached to the manipulandum 26.2 cm from the axis of rotation recorded tangential and centrifugal components of elbow movement acceleration. GF was recorded with the force transducer. Axoscope 10 software (Molecular Devices, Downingtown, PA) recorded kinetic and kinematic data at a sampling rate of 2000 Hz.
Data analysis
Empirical data
The task was divided into three phases (i.e., lift, hold, transport) based on GF timing and elbow flexion/extension acceleration (Fig. 1a). The lift phase was defined as the time from initial finger contact with the force transducer when GF rate increased and remained above 3SDs of baseline GF for at least 50 ms. The hold phase was defined as the time between the end of the lift phase, when GF fell below 3SDs of the mean GF rate during the last 0.5 s of the plateau, and elbow movement onset. The end of the hold phase was found by moving backward from the second peak in GF associated with elbow movement (transport phase) to the point at which GF fell below 3SDs of the peak GF rate, when present. When there was no peak GF in the transport phase, the end of the hold phase was defined from the peak elbow acceleration. The transport phase was defined as the time between the beginning and end of the elbow movement, when the arm acceleration reached and remained near zero. The start of the transport phase (i.e., arm movement onset) was defined as the first time point when arm acceleration exceeded 3SDs of the mean acceleration during the first second of the trial (when there was no movement).
Examples of Pattern A (low sustained force (< 10 N), grip force (GF) precedes arm movement) for fast flexion (a) and Pattern E (low sustained force (< 10 N) with no change in GF during arm movement) for self-paced flexion (b) in typical healthy subjects. The task was divided into three phases. The lift phase was defined as the time from initial contact of the fingers with the force transducer until the GF reached a plateau. The hold phase was the time between the end of the lift phase and elbow movement onset. The transport phase was defined as the time between the beginning and end of the elbow movement, when the arm acceleration reached and remained near zero level
Main outcome measures were sustained GF (N) during the hold phase, force–acceleration delay (Delay, ms) and change in GF (∆GF, N) during the transport phase. Sustained GF was calculated as the mean GF during the hold phase. The level of sustained GF was considered low if values were ≤ 10 N, corresponding to the level of GF in preliminary data from healthy subjects. Indeed, values > 10 N were rarely observed in our sample and their Z scores were higher than two standard deviations from their means per condition. i.e., Z ≥ 2. The Delay (ms) was the difference in time between the arm movement onset and the end of the hold phase. Positive values (> 0 ms) indicated that GF started increasing before arm movement onset, marking the start of the transport phase. The ∆GF (N) was defined as the difference between the value of sustained GF and the peak GF during the transport phase. Additional outcome measures were the peak arm velocity (°/s) and coefficient of variability (CV, %) of the Delay. Velocity was integrated from the accelerometer data using the trapezoidal rule. Data were filtered using a triangular moving average filter, with a window size of 15 ms.
We also computed GF/LF ratios. One part of LF is the gravitational force (weight of the transducer) acting vertically. This constant part of LF was compensated when the object (transducer m = 63.5 g, weight mg = 0.62 N) was lifted and moved. Of the two inertial components of the load (radial and tangential to the hand trajectory), only the radial component required GF to prevent the transducer from sliding off the fingers. Thus, the variable part of LF is ma, where ‘a’ is the radial acceleration measured by the accelerometer. GF/LF ratios were calculated at each time sample, averaged for each trial over the hold and transport phases, and then averaged across trials for each subject/condition.
The following movement patterns were identified for each condition based on a combination of Delay (positive: > 0 ms, negative: < 0 ms, no change in GF during arm movement) and sustained GF (≤ 10 N, > 10 N), since these are the main variables related to arm–hand coordination during functional activity. Pattern A—low sustained GF during the hold phase and a positive Delay; Pattern B—high sustained GF and a positive Delay; Pattern C—low sustained GF and a negative Delay; Pattern D—high sustained GF and negative Delay. Patterns E and F—low or high sustained GF, respectively, with no change in GF during arm movement. Pattern G—none of the above.
Simulations
The simulation was based on the following model assumptions. Parametric control of GFs is directly related to the notion of parametric control of MNs (for details see Feldman 1986, 2016, 2019). A muscle is activated if the difference between the current muscle length (x) and the threshold muscle length (λ*) is positive:
The asterisk signifies that the threshold is velocity dependent: it decreases with the increasing velocity (v) of muscle lengthening; to a first approximation, λ* = λ − µv, where µ is a time-dimensional parameter presumably controlled by dynamic γ-MNs (Feldman 1986).
A specific form of threshold position control is applied to multiple skeletal muscles and joints. Such control is presumably accomplished by changes in the referent (R) configuration of the entire body or its segments (Feldman and Levin 1995; Feldman 2015), such that the activity of each skeletal muscle depends on the deflection of the current body configuration (Q) from the referent configuration. Indeed, in the presence of external forces, the body reaches position Q at which muscle forces begin to balance external forces. By applying these notions to the arm movement with an anticipatory increase in the GF (Pilon et al. 2007), the R of this system is composed of two components, one defining the referent shape (aperture) of the hand (Ra; Fig. 2a, b), and another defining the referent position of the elbow joint (Re; Fig. 2c). In the present analysis, the hand shape was characterized by the actual (Qa) and the referent aperture (Ra) between the thumb and the index fingers. Thus, the referent position of the arm–hand system is defined as,
Referent control of grip force and arm movement. (a) Hand muscles generate activity and force proportional to the difference between the actual (Qa) hand aperture constrained by the object size held between the index and the thumb, and the centrally specified referent (Ra) hand aperture that defines a virtual distance between the index and the thumb. GF emerges because the object prevents the fingers from reaching the threshold, referent position. b When the object is forcefully taken away from the fingers (horizontal arrow), the fingers move to the referent position (“unloading reflex”). c Elbow motion results from shifts in the referent elbow position (Re). d Referent control of elbow motion (Re) and hand aperture (Ra) while moving the arm with the object. Note the delay d between the Ra and Re, when the onset of change of Ra precedes that of Re. Reproduced with modification from Pilon et al. (2007) with permission
Changes in each referent component are accomplished by reciprocal influences on MNs of agonist and antagonist muscles of the respective arm segments. Reciprocal influences can be combined with descending facilitation of agonist and antagonist MNs, resulting in changes in their activation threshold positions. These changes can be visualized as setting angular ranges, called C-zones or C commands, in which muscles can be coactivated. It is assumed that to move the arm with an object between the fingers, the system monotonically changes both components at chosen rates. Changes in Re give rise to arm movement and changes in Ra result in GF generation. Since referent control of arm movements has been described previously (e.g., Pilon and Feldman 2006), we focused on referent control of GFs (see also Pilon et al. 2007). Physically, GF emerges from the interaction of the hand with the object. Specifically, to hold an object, the system predetermines a threshold (referent) aperture of the hand (Ra) from which thumb and finger flexor muscles begin to be activated. At this hand position, the fingers virtually penetrate the object (Fig. 2a). In other words, the hand aperture at the referent hand position is smaller than the actual hand aperture (Qa) constrained by the object size. The presence of the object prevents hand muscles from reaching their activation thresholds. Muscles are subsequently activated depending on the difference between the actual and the referent hand aperture, resulting in the emergence of GF. If the emerging GF is unsatisfactory, the system increases or decreases the referent hand aperture Ra until the desired GF is achieved:
where k is a coefficient that depends on the elasticity the finger pads via which fingers contact the object (Pilon et al. 2007). Note that in the absence of slippage, friction force is equal to the applied force and is not defined via a friction coefficient.
The referent aperture is reached when the object is forcefully pulled away from the fingers (Fig. 2b, horizontal arrow), a phenomenon similar to the unloading reflex. To hold an object, the system diminishes Ra to a value that is sufficient to prevent the object from sliding off the fingers. The ratio of the applied grip force to the minimal GF at which sliding is prevented (i.e., the maximal value of Ra) is called the safety margin (Cole and Johansson 1993).
The predominant patterns in healthy (Pattern A) and stroke subjects (Patterns A and C) were simulated for FE movement for individual trials based on the model. Only simulated trials with r2 ≥ 0.7 between predicted and actual data were included in the statistical analysis. In addition to the equation of elbow motion, the model includes constants characterizing muscle force production in response to reflex and/or central muscle activation as well as control, referent variables underlying changes in muscle activation (defined in Pilon et al. 2007; Table 2). Individual elbow movements were simulated by choosing appropriate rates (°/s) of Re and Ce commands, durations of Ce command and delays before the Re commands, i.e., initiation time of the task-related change in R. The duration of Re was then calculated as the ratio between 60° (the range of elbow movement) and the rate of Re. Individual GFs were simulated by choosing an appropriate rate and duration of the Ra command, the delay before Ra and the initial angle between the thumb and index fingers (°). Ramp-shaped patterns of, Re and Ce commands were used to reproduce experimental movement patterns. The values of referent variables described above were found using the constrained non-linear optimization (fmincon function) in Matlab (R2015a), by minimizing the root mean square error (RMSE) between simulated and empirical trajectories and GFs.
Statistical analysis
Empirical data
The number of occurrences of each movement pattern for each movement direction and speed were summed across individuals and the proportion of individuals who used each pattern was computed for each condition. Chi square or Fisher’s exact tests were used to compare the proportion of subjects (healthy vs. stroke) using different patterns (healthy typical vs. non-healthy typical) in each condition and who modified the pattern when changing movement speed and direction.
The effects of speed and direction in healthy and stroke groups on the three main outcomes (mean sustained GF, Delay and ∆GF) were investigated. Due to non-normal data distributions or missing values, non-parametric tests were used (Mann–Whitney test and Wilcoxon test with FDR corrections for multiple comparisons).
Peak velocities were normally distributed and compared using RM-ANOVAs with speed and direction as within-subject factors and group as the between-subject factor to confirm that movement speeds differed as instructed between conditions. CVs of Delay and GF/LF ratios for the hold and transport phases were compared between groups using Mann–Whitney test with FDR correction for multiple comparisons.
The three main outcome measures were correlated with clinical scores using Spearman rho statistics to describe the relationship between movement variables and stroke severity. FDR corrections were used. All tests were done using SPSS (version 24.0) with initial significance levels of p < 0.05. Homogeneity of variance assumptions were assessed with Levene’s tests.
Simulations
Resemblances between simulated and experimental curves for elbow acceleration and GF (“goodness of fit”) for FE movement were evaluated by the coefficient of correlation (r2) and the RMSE. Control variables were compared between trials of stroke and healthy subjects using Pattern A with Mann–Whitney test. In addition, trials of stroke subjects using Pattern C were compared to those of healthy subjects using Pattern A with Mann–Whitney test. For all comparisons, significance levels were p < 0.05.
Results
Movement characteristics
Mean values of peak velocity (°/s), sustained GF (N), Delay (ms), ∆GF (N) and GF/LF ratios by group and condition are shown in Table 3. Peak velocity and sustained GF values are based on all subjects. However, since Delay and ∆GF could not be calculated for subjects who had patterns characterized by no movement-related increase in GF, sample sizes for these variables were smaller for fast movements: healthy: n = 13, stroke: n = 10; and for SE: healthy: n = 9, stroke: n = 6 and SF; healthy: n = 5, stroke: n = 5.
Movement speeds were matched between groups for the self-paced condition and were significantly faster for the fast conditions (self-paced: 143 ± 56°/s; fast: 345 ± 93°/s; F1,19 = 124, p < 0.001; healthy: t(12) = 12.77, p < 0.001; stroke: t(10) = 5.14, p < 0.001). Extension movements were slower than flexion movements in both groups (extension: 222 ± 62°/s; flexion: 267 ± 75°/s; F1,19 = 20, p < 0.001).
For healthy subjects only, sustained GF tended to be lower in self-paced compared to fast extension (self-paced: 0.341 ± 0.351 N, fast: 0.410 ± 0.422 N; p = 0.023, pFDR = 0.092), and ∆GF was significantly lower in self-paced compared to fast movements for extension movements (self-paced: 0.101 ± 0.116 N, fast: 0.569 ± 0.289 N; p = 0.008, pFDR = 0.032). ∆GF tended to be higher for stroke (0.322 ± 0.382 N) subjects for SE (p = 0.026, pFDR = 0.104) compared to controls.
The CV of Delay did not differ between subgroups of stroke and healthy subjects with detectable GF onsets (Patterns A, B, C and D; FE and FF: healthy: n = 13, stroke: n = 10; SE: healthy: n = 9, stroke: n = 6; SF: healthy: n = 5, stroke: n = 5).
Mean GF/LF ratios for the hold phase varied from 5.39 to 7.55 for healthy subjects and 6.27–8.28 for subjects with stroke. Ratios for the transport phase were 4.19–9.05 and 9.16–11.97 for healthy and stroke subjects, respectively. No significant differences (using Mann–Whitney test) were found between groups.
Grip force and movement patterns in healthy subjects
During FE and FF, the majority (92% and 85% respectively, Fig. 3a) of subjects used Pattern A (low pre-movement sustained GF with positive Delay; Fig. 2a). Sustained GF was < 10 N for both FE and FF and began to increase (∆GF 0.484 ± 0.291 N for FE and 0.665 ± 0.360 N for FF) prior to or simultaneously with arm motion (Delay 33 ± 19 ms for FE and 39 ± 23 ms for FF). Pattern B was observed in only one healthy subject for FF. This subject produced a high pre-movement GF (~ 15 N). Only one subject used Pattern D for FF characterized by a high sustained GF (14 N) and a long delay (200 ms) for changes in GF (Fig. 3a).
Types and prevalence of patterns of grip force (GF) modulation for subjects making horizontal arm movements at two different speeds (fast/self-paced) and directions (extension/flexion) in healthy (a) and stroke (b) subjects. Patterns were defined as follows: Pattern A—low sustained GF during the hold phase and a positive Delay; Pattern B—high sustained GF and a positive Delay; Pattern C—low sustained GF and a negative Delay; Pattern D- high sustained GF and negative Delay. Patterns E and F—low or high sustained GF, respectively, with no change in GF during arm movement. Pattern G—none of the above
Movement patterns during self-paced movements were more diverse than those during fast movements. Pattern A was observed in 46% of subjects for SE (sustained GF: 0.237 ± 0.237 N, ∆GF: 0.130 ± 0.135 N, Delay: 100 ± 96 ms). In contrast, for SF, Pattern E (Fig. 2b), predominated in 54% of subjects (sustained GF 0.408 ± 0.289 N with no movement-related delay; Fig. 3a).
Effect of changing speed and direction on use of movement patterns in healthy subjects
Most subjects used different movement patterns for SE (54%; patterns: C, E and F) and SF (70%; patterns: C, E and F) compared to fast movement (FE patterns: A and D; FF patterns: A, B, D). Only 8% of subjects used a different movement pattern (B) for FF compared to FE (A), whereas most subjects (62%) used different movement patterns when changing the direction of self-paced movement (SF patterns: C, E, F; SE patterns: A, C, F).
Grip force and movement patterns in post-stroke subjects
Use of Pattern A during FE and FF was characteristic of 46% and 75% of stroke subjects, respectively. In these subjects, sustained GF was < 10 N for both FE and FF and began to increase (∆GF 0.702 ± 0.290 N for FE and 0.878 ± 0.858 N for FF) prior to or simultaneously with arm motion (Delay 151 ± 171 ms for FE and 71 ± 85 ms for FF). The other patterns, B (FE: 9%; FF: 8%), C (FE: 36%) and E (FE: 9%; FF: 17%) occurred in fewer subjects (Figs. 3b, 4).
Examples of Pattern C (low sustained force (< 10 N), arm movement precedes grip force (GF) onset) for fast extension (a) and Pattern B (high sustained force (>10 N), GF precedes arm movement) for fast flexion (b) in stroke subjects. The task was divided into three phases as described for Fig. 2: lift, hold, transport
Self-paced movement patterns were more variable than those during fast movements. Pattern A was observed in 47% of subjects in SE (sustained GF: 0.329 ± 0.161 N, ∆GF: 0.373 ± 0.403 N, Delay: 205 ± 144 ms). In contrast, during SF, Pattern E was predominant in 46% of subjects (sustained GF 0.266 ± 0.074 N with no movement-related delay). Patterns B, C, F and G were used occasionally during self-paced movements (9% each of patterns; Fig. 3b).
Effect of changing speed and direction on use of movement patterns in stroke subjects
Similar to healthy subjects, most subjects with stroke used different movement patterns for SE (56%; patterns: C, E, F, G) and SF (60%; patterns: C, E G) when changing from fast to self-paced speed (FE patterns: A, B, C; FF pattern: A) and half used different movement patterns when changing the direction of self-paced movement (50.0%; SF patterns: B, C, E; SE patterns: C, E, F, G). Almost half of the subjects used different movement patterns for FF (A, E) compared to FE (46%; patterns: A, C).
Comparison of movement patterns between healthy and stroke subjects
Stroke subjects used Pattern A significantly less often than healthy subjects only for FE (Fisher’s exact test; p = 0.023). Patterns were similarly modified in healthy and stroke subjects when changing movement speed from fast to self-paced for extension and flexion movements. Stroke subjects tended to modify their patterns more than healthy subjects when changing movement direction during fast movement (Fisher’s exact test; p = 0.061 (but modified them similarly to healthy subjects when changing movement direction in self-paced movements.
Relationship between hemiparetic hand function and variables of anticipatory GF control
In the stroke group, only two measures of hand function were related to GF variables. Better scores on the Purdue Pegboard test (r = − 0.77, p = 0.010, pFDR = 0.030) were related to shorter delays during FE, and higher grip strengths (r = − 0.87, p = 0.019, pFDR = 0.057) tended to correlate with shorter delays during SE.
Simulations
Comparisons between simulated and experimental data in healthy and stroke subjects in FE movement
The predominant patterns (A and C) in both groups were simulated for FE movement. Pattern A was used by 12 healthy subjects and 5 stroke subjects, while Pattern C was used by 4 stroke subjects. For Pattern A, GF and elbow acceleration of all 12 healthy subjects (S2-S13) and of 3 of 5 stroke subjects (H5, H9, H11) were simulated. Ramp-shaped referent inputs R and C effectively reproduced the motor output matching the patterns in healthy subjects (GF: healthy; r2 = 0.67–0.99, p < 0.001; stroke: r2 = 0.90–0.98, p < 0.001; elbow movements: healthy; r2 = 0.65–0.94, p < 0.001; stroke: r2 = 0.67–0.91, p < 0.001). Figure 5a, b show simulations of Pattern A trials in typical healthy and stroke subjects, respectively.
For Pattern C (Fig. 6), which characterized FE only in stroke subjects, GF and elbow acceleration of 2 of 4 stroke subjects (S4: 71.4% of trials, S12: 100% of trials) were simulated. R and C referent inputs were effective in eliciting the motor output that matched experimental GF (r2 = 0.94–0.98, p < 0.001) and elbow movement (r2 = 0.72–0.93, p < 0.001) patterns.
Differences in control variables between healthy and stroke subjects in FE movement
For GF simulations in Pattern A, the rate of Ra for stroke subjects (85.8 ± 34.8°/s) was higher compared to healthy subjects (40.2 ± 26.3°/s, p < 0.001), while the duration of Ra (stroke 0.16 ± 0.04 s; healthy: 0.27 ± 0.16 s, p = 0.002) was significantly shorter. There were no differences for initial angle and delay before Ra. For elbow movement simulation, the rate and duration of Re and Ce were similar between groups. The delay before Re was shorter for the stroke subjects (0.67 ± 0.04 s) compared to healthy subjects (0.69 ± 0.04 s; p = 0.038, Table 4).
The second most prevalent pattern (Pattern C) for FE in stroke subjects, which did not occur in healthy subjects for FE, was compared to Pattern A in healthy subjects. For GF simulations, the rate of Ra (92.7 ± 20.6°/s), duration of Ra (0.17 ± 0.04 s) and initial angle (− 7.44 ± 2.74) of Pattern C differed from Pattern A (p < 0.001, p = 0.031, p = 0.015, respectively). The delay before Re was similar between groups. For elbow movement simulation, rate of Re (494.2 ± 167.6°/s), duration of Re (0.14 ± 0.08 s) and delay before Re (0.63 ± 0.03 s) of Pattern C differed significantly from that of Pattern A (p < 0.001 for all). No significant differences were found for rate and duration of Ce (Table 4).
Discussion
The control of a grip and transport task performed at two different speeds in the horizontal plane in subjects with chronic stroke was investigated. GF and movement patterns were modeled based on R and C commands that specify the referent configuration of the hand and arm (Feldman 2015; Pilon et al. 2007).
Empirical data
Anticipatory control of GF–arm motion coordination in healthy and stroke subjects
Predominantly, both healthy and stroke subjects produced low sustained GF during the hold phase and increased GF just before the transport phase (Pattern A). Other patterns were also used in both groups but their prevalence differed, especially in the fast extension task. Pattern A has also been previously described as characteristic of lifting an object (Flanagan and Tresilian 1994; Raghavan et al. 2006) and of GF–arm coordination during motion in a horizontal plane in healthy subjects (Danion et al. 2007; Danion and Sarlegna 2007; Pilon et al. 2007).
In 37% of healthy and 52% of stroke subjects across all four conditions, only a late or no arm movement-related increase in GF was observed. The absence of an anticipatory GF increase might be age related, since it has been more often observed in healthy young adults (Danion et al. 2007; Pilon et al. 2007). For example, during rhythmical movement of a hand-held object in the horizontal plane, GF preceded load force by 7 ms in young healthy adults (25 years), but lagged behind load force by 25 ms in older subjects (66 years; Danion et al. 2007). Such a lag may be related to slowness in initiation and execution of movements (Inglin and Woollacott 1988; Stelmach and Nahom 1992) and a decline in visuoperceptual abilities with increasing age (Eslinger and Benton 1983). However, during vertical load displacements, GF modulation was synchronized with load force fluctuations in both young and elderly subjects (Gilles and Wing 2003).
In the subgroup of subjects with detectable GF onsets, the interval between arm movement onset and GF increase (Delay) did not differ between healthy and stroke subjects, suggesting that the majority of stroke subjects (69%) had no deficits in temporal coupling, although prolonged delays between the hemiparetic and less-affected hand (McDonnell et al. 2006) and between the hemiparetic hand and hands of healthy subjects have been reported for some tasks (e.g., grip–lift, Nowak et al. 2003; drawer- pulling; Wiesendanger and Serrien 2001) but not in others (Blennerhassett et al. 2008; Hermsdörfer et al. 2003).
Sustained GF and ΔGF did not differ between stroke and healthy subjects. Previous findings are equivocal about the ability of subjects with stroke to sustain and modulate GF. Subjects with subacute and chronic stroke produced markedly increased GFs when lifting, holding and performing reaching and vertical pointing movements (Blennerhassett et al. 2008; Nowak et al. 2003, 2007; Wenzelburger et al. 2005). On the other hand, not all participants with stroke had excessive GF prior to commencing a lifting task (Blennerhassett et al. 2006) and other manipulative tasks such as stationary holding, transport and vertical cyclic movements of an instrumented object (Hermsdörfer et al. 2003). The inconsistencies may be related to differences in stroke lesion location (Feys et al. 2000; Shelton and Reding 2001). Indeed, at least one study reported that the more posterior the lesion was located within the posterior limb of the internal capsule, the more pronounced were the alterations in timing and precision of GF modulation during reaching-to-grasp movement in the parasagittal plane (Wenzelburger et al. 2005). However, lesion effects were not considered in our study. The inconsistencies may also be related to different sensory deficits of participants across studies. GF increases were found in healthy subjects after local anesthesia of the fingers (Nowak et al. 2001). The fact that participants in our sample did not have severe sensory deficits may have reduced the differences in sustained GF and ΔGF between stroke and healthy subjects.
Effects of speed and direction on anticipatory control in healthy and stroke subjects
The majority of subjects in both groups used different movement patterns for self-paced movements made at different speeds and in different directions. However, stroke subjects tended to modify their patterns more than healthy subjects when changing movement direction during fast movements. Also, unlike healthy participants, stroke subjects failed to increase GF in fast compared to self-paced extension.
Previous studies have reported that movement patterns can change for elbow or wrist movements made slower or faster than self-paced speeds (Adam and Paas 1996; Doeringer and Hogan 1998; Nagasaki 1989; Park et al. 2017; Werremeyer and Cole 1997). For example, profiles of displacement, velocity, acceleration and jerk for elbow flexion movements were more asymmetrical for movements made more slowly or faster than comfortable speed (Nagasaki 1989). For wrist movements made in the horizontal plane while holding a low-mass object, the timing of GF increase was also related to movement speed. GF began to increase slightly before the start of wrist motion for fast movements but simultaneously with wrist motion onset for medium speed movements. The size of the increase in grip–load ratio was significantly greater for the fast wrist flexion movements.
It can be suggested that, similar to controls, stroke subjects were able to exploit, to some degree, the remaining redundancy of the damaged sensorimotor system to stabilize the task goal (i.e., maintain GF) by altering their hand configuration for gripping (Latash and Anson 1996; Raghavan et al. 2010). However, adaptability was limited as shown by difficulties to sufficiently increase GF between self-paced and fast elbow extension movements. Decreased task-specific adaptability is consistent with previous findings in which subjects with stroke had deficits in rapidly adapting shoulder–elbow interjoint coordination when unexpected perturbations occurred during various reaching tasks compared to healthy subjects (Levin et al. 2016; Shaikh et al. 2014; Tomita et al. 2017). Such deficits led to the inability to stabilize the task goal of endpoint trajectory preservation when different body segments were involved in the movement (Shaikh et al. 2014).
Simulated data: referent control of grip force and arm movement
The predominant movement patterns in healthy (Pattern A) and stroke subjects (Patterns A and C) were simulated for FE movement, in which movement patterns differed significantly between groups. For Pattern A, the model of the referent arm–hand configuration successfully simulated arm movement and GF in 80% and 65% of healthy and stroke trials, and for Pattern C in 71% and 100% of trials in two stroke subjects.
Model variables of the timing of elbow acceleration and aperture components of the referent configuration successfully simulated increases in GF starting before (Pattern A) or after (Pattern C) arm movement onset. This has previously been shown for younger healthy adults in Pilon et al. (2007). In our study, the ranges of values for the rate of Re and Ra (for elbow and GF simulations) as well as for rate and duration of Ce (for elbow simulation only) were wider for both healthy and stroke groups compared to the previous study. This may be related to the older age of our participants, since precision grip force control capacity declines with advancing age (Kinoshita and Francis 1996). Older adults produce larger and more variable grip forces compared to younger subjects (Cole 1991; Danion et al. 2007) which is also consistent with our finding of wider ranges of the control variables in this group.
For Pattern A, the magnitude and duration of the rate of the R command were higher and shorter, respectively, in stroke compared to healthy trials for GF simulation, i.e., the changes in the referent position of GF were steeper and faster, possibly less regulated, in the trials of stroke subjects. The rate and duration of the R and C command for the elbow simulation did not differ between healthy and stroke trials. In addition, the delay before R was shorter for the participants with stroke compared to the healthy participants for the elbow simulation. These differences were found for a subgroup of stroke subjects with good hand and arm recovery who manifested the same pattern typically used in healthy subjects. This suggests that in this subgroup, R and C commands can be modified by neural control levels such that deficits in movement patterns and related variables of anticipatory control are not observed. This emphasizes the flexibility of the neural control system in setting a wide range of values of control variables that are manifested in the same movement pattern.
In another subgroup of stroke trials, categorized as Pattern C, the change in control variables produced a different pattern, not typically used by healthy subjects during FE. The rate of the R command was higher and its duration was shorter in stroke compared to healthy trials of Pattern A for both simulations of arm acceleration and GF. Also here, for the arm acceleration simulation, the rate and duration of the C command did not differ between healthy and stroke trials, and the delay before R was shorter in the participants with stroke compared to the healthy participants. These differences may indicate maladaptive neuroplasticity processes occurring in this subgroup of stroke subjects resulting in non-healthy typical patterns. The finding that different values of control variables following stroke describe movement patterns and related variables suggests that impaired anticipatory GF control in subjects with stroke may be explained by deficits in referent control. The results of the simulation show, using referent control variables, that movement patterns emerge without direct programming of muscle activation, forces and kinematics (Feldman 2015). Thus, the values of the kinematic and force variables observed in this study likely emerged from the difference between the actual arm–hand configuration and its virtual, referent configuration, modified by neural control levels.
Limitations
The model successfully reproduced the anticipatory increases in GF observed after the arm movement onset. Less successful were reproductions in cases in which the GF started increasing before the arm movement onset. Nevertheless, the simulation did produce a longer delay in Pattern C compared to A, as predicted. It is likely that the optimization method used in the simulation did not fully capture the time courses of all possible variations of this action. In other words, this is not a problem of the model as such but rather of the optimization method.
The control variable R of both GF and elbow movement simulations and variable C of elbow movement simulation were mathematically described by ramp-shaped time functions. It is possible that a model characterized by only one ramp shape cannot simulate trials of older adults and stroke subjects who use more feedback than feedforward patterns (Raghavan et al. 2006). The multiple peaks observed in the GF rate in these cases are likely due to multiple specifications of the referent position, which cannot be captured well by a single ramp. In addition, the ramp shape may not be able to simulate the anticipatory increases in GF before the arm movement onset (Pattern A) due to the gradual GF increase that characterized some of the experimental trials. Finally, the relatively large number of fixed variables may not have well described the movements, and as the optimization procedure is sensitive to the initial estimate and several variables were used, the best-fit simulations may have been local minima rather than the actual optimal solution.
Clinical implications
Deficits in temporal coupling between the arm and hand were related to clinical functional ability of the upper limb in the stroke group. This is similar to previous studies in which temporal coupling between the rate of change of GF and load force during the lift correlated highly with the Action Research Arm Test (McDonnell et al. 2006), and deficits in pre-lift delay were related to worse performance on the Jebsen–Taylor Hand Function Test, Motor Assessment Scale and a custom-designed survey about daily hand use (Blennerhassett et al. 2008). Such correlations point to the functional significance of task-specific temporal coupling, and suggest that this aspect of movement production may be important to assess and train during rehabilitation of stroke survivors with elbow movement and grip deficits. Our finding that healthy typical and non-healthy typical patterns were produced by changing R and C commands in a subgroup of stroke subjects suggests that the underlying cause of the motor dysfunction is related to deficits in parametric control. Improvements in motor function due to recovery or training may result from recovery of the ability to specify and regulate descending threshold control variables.
Conclusions
The significantly fewer healthy typical patterns observed in stroke subjects during FE arm movements, impaired ability to increase movement-related GF during fast compared to self-paced arm extension, together with the observed association between longer delays and upper limb motor impairment, describe several deficits of anticipatory GF control in subjects with stroke. These deficits may be related to limitations in parametric control, defined as inability to specify appropriate R and C commands.
References
Adam JJ, Paas FGWC (1996) Dwell time in reciprocal aiming tasks. Hum Mov Sci 15:1–24. https://doi.org/10.1016/0167-9457(95)00041-0
Allgöwer K, Hermsdörfer J (2017) Fine motor skills predict performance in the Jebsen Taylor Hand Function Test after stroke. Clin Neurophysiol 128:1858–1871. https://doi.org/10.1016/j.clinph.2017.07.408
Anderson AM, Croft RP (1999) Reliability of Semmes Weinstein monofilament and ballpoint sensory testing, and voluntary muscle testing in Bangladesh. Lepr Rev 70:305–313
Blennerhassett JM, Carey LM, Matyas TA (2006) Grip force regulation during pinch grip lifts under somatosensory guidance: comparison between people with stroke and healthy controls. Arch Phys Med Rehabil 87:418–429. https://doi.org/10.1016/j.apmr.2005.11.018
Blennerhassett JM, Carey LM, Matyas TA (2008) Clinical measures of handgrip limitation relate to impaired pinch grip force control after stroke. J Hand Ther 21:245–253. https://doi.org/10.1197/j.jht.2007.10.021
Cole KJ (1991) Grasp force control in older adults. J Mot Behav 23:251–258. https://doi.org/10.1080/00222895.1991.9942036
Cole KJ, Johansson RS (1993) Friction at the digit-object interface scales the sensorimotor transformation for grip responses to pulling loads. Exp Brain Res 95(3):523–532. https://doi.org/10.1007/BF00227146)
Danion F, Sarlegna FR (2007) Can the human brain predict the consequences of arm movement corrections when transporting an object? Hints from grip force adjustments. J Neurosci 27:12839–12843. https://doi.org/10.1523/JNEUROSCI.3110-07.2007
Danion F, Descoins M, Bootsma RJ (2007) Aging affects the predictive control of grip force during object manipulation. Exp Brain Res 180:123–137. https://doi.org/10.1007/s00221-006-0846-3
Dannenbaum RM, Michaelsen SM, Desrosiers J, Levin MF (2002) Development and validation of two new sensory tests of the hand for patients with stroke. Clin Rehabil 16:630–639. https://doi.org/10.1191/0269215502cr532oa
Desrosiers J, Hébert R, Bravo G, Dutil E (1995) The Purdue Pegboard Test: normative data for people aged 60 and over. Disabil Rehabil 17:217–224. https://doi.org/10.3109/09638289509166638
Doeringer JA, Hogan N (1998) Intermittency in preplanned elbow movements persists in the absence of visual feedback. J Neurophysiol 80:1787–1799. https://doi.org/10.1152/jn.1998.80.4.1787
Eslinger PJ, Benton AL (1983) Visuoperceptual performances in aging and dementia: clinical and theoretical implications. J Clin Neuropsychol 5:213–220
Feldman AG (1986) Once more on the equilibrium-point hypothesis (lambda model) for motor control. J Mot Behav 18(1):17–54
Feldman AG (2011) Space and time in the context of equilibrium-point theory. Wiley Interdiscip Rev Cogn Sci 2:287–304. https://doi.org/10.1002/wcs.108
Feldman AG (2015) Referent control of action and perception: Challenging conventional theories in behavioural neuroscience. Springer, New York
Feldman AG (2016) Active sensing without efference copy: referent control of perception. J Neurophysiol 116(3):960–976. https://doi.org/10.1152/jn.00016.2016
Feldman AG (2019) Indirect, referent control of motor actions underlies directional tuning of neurons. J Neurophysiol (review, el.publication ahead of print)
Feldman AG, Levin MF (1995) The origin and use of positional frames of reference in motor control. Behav Brain Sci 18:723–744. https://doi.org/10.1017/S0140525X0004070X
Feldman AG, Krasovsky T, Baniña MC, Lamontagne A, Levin MF (2011) Changes in the referent body location and configuration may underlie human gait, as confirmed by findings of multi-muscle activity minimizations and phase resetting. Exp Brain Res 210:91–115. https://doi.org/10.1007/s00221-011-2608-0
Feys H, Hetebrij J, Wilms G, Dom R, De Weerdt W (2000) Predicting arm recovery following stroke: value of site of lesion. Acta Neurol Scand 102:371–377
Flanagan JR, Tresilian JR (1994) Grip-load force coupling: a general control strategy for transporting objects. J Exp Psychol Hum Percept Perform 20:944–957. https://doi.org/10.1037/0096-1523.20.5.944
Flanagan JR, Wing AM (1997) The role of internal models in motion planning and control: evidence from grip force adjustments during movements of hand-held loads. J Neurosci 17(4):1519–1528
Gilles MA, Wing AM (2003) Age-related changes in grip force and dynamics of hand movement. J Mot Behav 35:79–85. https://doi.org/10.1080/00222890309602123
Gowland C, Stratford P, Ward M, Moreland J, Torresin W, Van Hullenaar S, Sanford J, Barreca S, Bernadette V, Plews N (1993) Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke 24:58–63
Grichting B, Hediger V, Kaluzny P, Wiesendanger M (2000) Impaired proactive and reactive grip force control in chronic hemiparetic patients. Clin Neurophysiol 111:1661–1671. https://doi.org/10.1016/S1388-2457(00)00355-2
Heckman CJ, Gorassini MA, Bennett DJ (2005) Persistent inward currents in motoneuron dendrites: Implications for motor output. Muscle Nerve 31:135–156
Hermsdörfer J, Hagl E, Nowak DA, Marquardt C (2003) Grip force control during object manipulation in cerebral stroke. Clin Neurophysiol 114:915–929. https://doi.org/10.1016/S1388-2457(03)00042-7
Inglin B, Woollacott M (1988) Age-related changes in anticipatory postural adjustments associated with arm movements. J Gerontol 43:M105–M113. https://doi.org/10.1093/geronj/43.4.M105
Kinoshita H, Francis PR (1996) A comparison of prehension force control in young and elderly individuals. Eur J Appl Physiol Occup Physiol 74:450–460. https://doi.org/10.1007/BF02337726
Latash ML (1992) Virtual trajectories, joint stiffness, and changes in the limb natural frequency during single-joint oscillatory movements. Neurosci 49:209–220. https://doi.org/10.1016/0306-4522(92)90089-K
Latash ML, Anson JG (1996) What are “normal movements” in atypical populations? Behav Brain Sci 19:55–68. https://doi.org/10.1017/S0140525X00041467
Levin MF, Liebermann DG, Parmet Y, Berman S (2016) Compensatory versus noncompensatory shoulder movements used for reaching in stroke. Neurorehabil Neural Repair 30:635–646. https://doi.org/10.1177/1545968315613863
Li Y, Randerath J, Goldenberg G, Hermsdörfer J (2011) Size-weight illusion and anticipatory grip force scaling following unilateral cortical brain lesion. Neuropsychologia 49:914–923. https://doi.org/10.1016/j.neuropsychologia.2011.02.018
Lindstrom-Hazel D, Kratt A, Bix L (2009) Interrater reliability of students using hand and pinch dynamometers. Am J Occup Ther 63:193–197
McDonnell MN, Hillier SL, Ridding MC, Miles TS (2006) Impairments in precision grip correlate with functional measures in adult hemiplegia. Clin Neurophysiol 117:1474–1480. https://doi.org/10.1016/j.clinph.2006.02.027
Nagasaki H (1989) Asymmetric velocity and acceleration profiles of human arm movements. Exp Brain Res 74:319–326. https://doi.org/10.1007/BF00248865
Nowak DA, Hermsdörfer J (2003) Selective deficits of grip force control during object manipulation in patients with reduced sensibility of the grasping digits. Neurosci Res 47:65–72. https://doi.org/10.1016/S0168-0102(03)00182-2
Nowak DA, Hermsdörfer J, Glasauer S, Philipp J, Meyer L, Mai N (2001) The effects of digital anaesthesia on predictive grip force adjustments during vertical movements of a grasped object. Eur J Neurosci 14:756–762. https://doi.org/10.1046/j.0953-816X.2001.01697.x
Nowak DA, Hermsdörfer J, Topka H (2003) Deficits of predictive grip force control during object manipulation in acute stroke. J Neurol 250:850–860. https://doi.org/10.1007/s00415-003-1095-z
Nowak DA, Glasauer S, Hermsdörfer J (2004) How predictive is grip force control in the complete absence of somatosensory feedback? Brain 127:182–192. https://doi.org/10.1093/brain/awh016
Nowak DA, Grefkes C, Dafotakis M, Küst J, Karbe H, Fink GR (2007) Dexterity is impaired at both hands following unilateral subcortical middle cerebral artery stroke. Eur J Neurosci 25:3173–3184. https://doi.org/10.1111/j.1460-9568.2007.05551.x
Park S-W, Marino H, Charles SK, Sternad D, Hogan N (2017) Moving slowly is hard for humans: limitations of dynamic primitives. J Neurophysiol 118:69–83. https://doi.org/10.1152/jn.00643.2016
Pilon JF, Feldman AG (2006) Threshold control of motor actions prevents destabilizing effects of proprioceptive delays. Exp Brain Res 174(2):229–239
Pilon JF, De Serres SJ, Feldman AG (2007) Threshold position control of arm movement with anticipatory increase in grip force. Exp Brain Res 181:49–67. https://doi.org/10.1007/s00221-007-0901-8
Platz T, Pinkowski C, van Wijck F, Kim IH, Di Bella P, Johnson G (2005) Reliability and validity of arm function assessment with standardized guidelines for the Fugl-Meyer Test, Action Research Arm Test and Box and Block Test: A multicentre study. Clin Rehabil 19:404–411. https://doi.org/10.1191/0269215505cr832oa
Raghavan P, Krakauer JW, Gordon AM (2006) Impaired anticipatory control of fingertip forces in patients with a pure motor or sensorimotor lacunar syndrome. Brain 129:1415–1425. https://doi.org/10.1093/brain/awl070
Raghavan P, Santello M, Gordon AM, Krakauer JW (2010) Compensatory motor control after stroke: An alternative joint strategy for object-dependent shaping of hand posture. J Neurophysiol 103:3034–3043. https://doi.org/10.1152/jn.00936.2009
Sangole AP, Levin MF (2009) Palmar arch modulation in patients with hemiparesis after a stroke. Exp Brain Res 199:59–70. https://doi.org/10.1007/s00221-009-1972-5
Shaikh T, Goussev V, Feldman AG, Levin MF (2014) Arm-trunk coordination for beyond-the-reach movements in adults with stroke. Neurorehabil Neural Repair 28:355–366. https://doi.org/10.1177/1545968313510973
Shelton FN, Reding MJ (2001) Effect of lesion location on upper limb motor recovery after stroke. Stroke 32:107–112
Stelmach GE, Nahom A (1992) Cognitive-motor abilities of the elderly driver. Hum Factors 34:53–65. https://doi.org/10.1177/001872089203400107
Tomita Y, Feldman AG, Levin MF (2017) Referent control and motor equivalence of reaching from standing. J Neurophysiol 117:303–315. https://doi.org/10.1152/jn.00292.2016
Wenzelburger R, Kopper F, Frenzel A, Stolze H, Klebe S, Brossmann A, Kuhtz-Buschbeck J, Gölge M, Illert M, Deuschl G (2005) Hand coordination following capsular stroke. Brain 128:64–74. https://doi.org/10.1093/brain/awh317
Werremeyer MM, Cole KJ (1997) Wrist action affects precision grip force. J Neurophysiol 78:271–280. https://doi.org/10.1152/jn.1997.78.1.271
Wiesendanger M, Serrien DJ (2001) Neurological problems affecting hand dexterity. Brain Res Rev 36:161–168. https://doi.org/10.1016/S0165-0173(01)00091-1
Yamanaka J, Feldman AG, Levin MF (2010) Deficits in coordination between grip force and arm movement in stroke. Abstract presented at the American Congress of Rehabilitation Medicine, Montreal, October, 2010. Arch Phys Med Rehabil 91(10):e30
Acknowledgements
The research was partially funded by a grant from CHRP-CIHR-NSERC (Canada) to AGF and MFL, and support from the Eldee Foundation and the Bloomfield family of Montreal, Canada, granted through Tel Aviv University (SFT). Thanks to Ruth Dannenbaum and Valeri Goussev for help with data collection and analysis and to the participants who volunteered for the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Frenkel-Toledo, S., Yamanaka, J., Friedman, J. et al. Referent control of anticipatory grip force during reaching in stroke: an experimental and modeling study. Exp Brain Res 237, 1655–1672 (2019). https://doi.org/10.1007/s00221-019-05498-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-019-05498-y