Abstract
It is still unknown whether visuomotor adaptation depends on the time during which a person is exposed to distorted vision, or rather on the number of movements executed under the distortion. To find out, we analysed the pointing errors and movement kinematics of 52 participants adapting with online visual feedback to a 60° visual rotation and 39 participants adapting to a 75° visual rotation without time constraints. We found that movement time was not related with participants’ success during adaptation, whereas peak velocity was inversely associated to adaptive success. However, peak velocity lost its association to adaptation when other parameters were taken into account. Movement kinematics during adaptation had little influence on participants’ performance during de-adaptation. Our data suggest that adaptation does not depend primarily on the duration but rather on the number of movements executed under distorted vision. It further suggests that the measured kinematic parameters are consequences of error corrections rather than determinants of the adaptive success. We further have evidence for the view that adaptive recalibration is independent of movement kinematics during adaptation. This outcome generalizes across different visual rotations and is in accordance with earlier work where online visual feedback of the hand was unavailable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Visuomotor adaptation enables us to act successfully when we encounter unusual situations in everyday life, e.g. when vision is distorted by prescription glasses, when arm length increases due to body growth or tool use and when cursor positions on a vertical PC screen must be mapped onto mouse positions on a horizontal surface. The underlying mechanisms have been thoroughly investigated over the past decades.
Adaptive change is thought to be achieved by an interplay of two distinct phenomena, recalibration of sensory-to-motor pathways and implementation of workaround strategies such as online error corrections. In contrast, the after-effects following removal of the distortion are thought to reflect recalibration processes alone (McNay and Willingham 1998; Redding and Wallace 1996). Recalibration can occur at the visual input stage, at the motor output stage, at the proprioceptive-feedback stage (Cressman and Henriques 2011; Henriques and Cressman 2012) or in central modules which are interlinked with those stages (Bock 2013). Our knowledge of workaround strategies is less well established, but it appears that strategies—unlike recalibration—are linked with cognition, since adaptive change but not after-effects are correlated with cognitive functions such as selective attention (Simon and Bock 2015), divergent thinking (Simon and Bock 2016), reasoning (Werner and Bock 2007), visuospatial functions (Anguera et al. 2010), response speed and decision-making (Bock and Girgenrath 2006). A close link between strategies and cognition could explain why adaptive change is more vulnerable to ageing than after-effects are (Bock 2005; Bock and Girgenrath 2006).
Other work dealt with the time-course of adaptation and established the existence of a fast and a slow adaptive mechanism (Krakauer 2000; Smith et al. 2006) with differential dependence on workspace geometry (Bock and Schmitz 2011). Curiously, however, it is still unknown whether the critical determinant of adaptive time-course is indeed time per se, or rather the number of movements executed. To appreciate the difference between these alternatives, consider two persons who point at visual targets while visual feedback of their arm is distorted, person A pointing double as fast as person B. If time is indeed the critical determinant of adaptation, then performance of A should be worse than that of B after the same number of movements, due to the reduced exposition time. If, however, the number of movements is the critical determinant, the performance of A and B should be similar.
A few studies manipulated the amount of time spent under the distortion by instructing participants to move their arm either with high or low speed. Two studies (Baily 1972; Tseng et al. 2007) found no statistically significant effects of movement speed on adaptation and after-effects. A third study did not analyse those effects statistically, but an inspection of the published figures indicates that slow movers adapted less and showed smaller after-effects (cf. Figs. 4a, 5a of Kitazawa et al. 1997). It is conceivable that participants focused much of their attention on complying with the instructions and thus had fewer spare resources for adapting even with prolonged movement time, resulting in no differences for adaptive success (Baily 1972; Tseng et al. 2007) or even in reduced adaptive success (Kitazawa et al. 1997). We therefore decided to perform a quantitative analysis of the relationship between movement duration (and other kinematic parameters) with adaptation and its after-effects, exploiting the natural between subject variations of movement speed rather than instructing participants to move at certain speeds.
Importantly, fast movements took 210 to 230 ms in all three mentioned studies, which is too short for online visual control. Slow movements took 620 ms in one study (Tseng et al. 2007) and thus allowed online visual control. In the other two studies, slow movements took about 5000 ms (Kitazawa et al. 1997) or 6500 ms (Baily 1972), but arm vision was provided only at movement end. The cited studies therefore provide converging evidence that adaptation without online visual control is comparable to adaptation with 620 ms visual feedback per movement; they leave open whether it is also comparable to adaptation with longer visual feedback availability.
The present study evaluates visuomotor adaptation of unspeeded arm movements with concurrent visual feedback and exploits the natural between-participant variability of those movements to assess the relationship between movement time, visual feedback and adaptation. We performed the analysis for two different visual rotations, in order to yield generalizable conclusions.
Methods
Participants
We analysed data of 89 young (24.01 ± 3.54 years), right handed, healthy participants which signed informed consent before participating. Procedures were all approved by a local institutional ethics committee. All participants were healthy by self-report and had normal or corrected-to-normal vision. Fifty-two participants (mean age: 23.40 ± 3.44 years, 20 females) performed a visuomotor adaptation task with a visual rotation of 60°. Data from these participants have been published before (Simon and Bock 2015, 2016) and are re-analysed here with respect to movement kinematics. The remaining 37 participants (mean age 24.86 ± 3.54 years, 28 females) adapted to a visual rotation of 75°. Experimental hardware and software were the same for all participants.
Experimental procedure
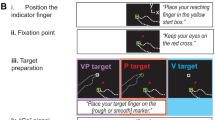
All participants pointed at visual targets on a computer monitor by moving a pen across a digitizing tablet. Sight of the arm was prevented by blinds, but feedback about pen position was provided by a cursor on the screen. Participants were instructed to move the cursor as accurately as possible from a central cross to a target and back (distance one-way 10 cm). The return to the central cross triggered the presentation of the next target at a new position. Targets appeared in a quasi-random sequence at eight different locations on a circle around the central cross (see Fig. 1). Each experimental episode consisted of 24 trials, with three presentations of each target position and was followed by a self-terminated rest break of a few seconds.
Experimental setup for the visuomotor adaptation task. Targets were presented on a vertically orientated screen, and the movements were performed with a digitizing pen on a digitizing tablet placed horizontally in front of the display. Left–right axis remained the same and bottom-top axis became proximal–distal axis. Movement distance on the digitizing tablet was equal to the distances covered on screen. Grey circles represent potential target positions and the black circle a currently displayed target. Only one circle was visible at one time. The black cross represents the starting point for movements
The experimental design included, in fixed order, four episodes of movements with non-rotated visual feedback (baseline episodes), 19 episodes with visual feedback rotated about the central cross by 60° or 75° (adaptation episodes) and four final episodes with non-rotated visual feedback (de-adaptation episodes). The first episode of the baseline conditions was regarded as familiarization and therefore was left out of analysis, resulting in three analysed baseline episodes. Participants were not informed about the feedback manipulation in the adaptation phase, but were told that task difficulty may differ between trials.
Data analysis
An interactive software determined the mean values of following arm movement parameters from each episode:
-
Initial error IE [°]: angular difference between ideal and actual movement direction during the initial 150 ms after movement onset
-
End error EE [°]: angular difference between ideal and actual movement direction at the point of inflection of the movement path
-
Maximum velocity Vmax [cm/s]: highest speed of arm movement
-
Path length PL [cm]: total distance travelled by the finger from central cross to target and back
-
Movement time MT [s]: time from movement onset to end
-
Reaction time RT [s]: time from target appearance to movement onset
Separately for each participant, we calculated the mean value of each parameter across all adaptation episodes; these means will be identified by a subscripted ada (e.g. IEada, MTada, \(V_{{\max_{\text{ada}} }}\)). Parameter IE was explored in further detail by calculating, again separately for each participant, the difference between IE of the first and last adaptation episode (adaptation efficiency, IEeff), the mean across all de-adaptation episodes (IEde-ada) and the score of the first de-adaptation episode (IE st1 de-ada).
For an overview, participants were divided into two kinematic subgroups based on a median split. We determined the median \(V_{{\max_{\text{ada}} }}\) of the 60° rotation group and then subdivided participants from that group into one subgroup whose \(V_{{\max_{\text{ada}} }}\) was smaller than that median, and another subgroup whose \(V_{{\max_{\text{ada}} }}\) was above that median. The same procedure was applied to the 75° rotation group. In an analogous fashion, participants were also split according to their MTada and according to PLada. T tests of independent means were used to compare IEada of subgroups from each rotation group.
The outcome was submitted to one-way repeated-measures ANOVAs with the between-factor subgroup (below-median, above-median i.e. slow, fast or long, short), and the repeated-measures within-factor episode; the dependent variable was IE. Separate ANOVAs were run for the baseline, the adaptation and the de-adaptation phase, separately for each median split and each rotation group, resulting in 3 (phases) × 3 (splits) × 2 (groups) = 18 ANOVAs. Normal distribution of IE was confirmed by a Kolmogorov–Smirnow-Test.
We additionally calculated the Pearson correlation between \(V_{{\max_{\text{ada}} }}\) and MTada and \(V_{{\max_{\text{ada}} }}\) and PLada for each rotation group.
The association of IE with all other parameters was quantified by multiple regression analyses. Four analyses were run for the 60° group and four for the 75° group. The dependent variable for one analysis was IEada, for the second it was IEeff, for the third IEde-ada and for the fourth IE st1 de-ada. Regressors for all analyses were EEada, \(V_{{\max_{\text{ada}} }}\), MTada, RTada and PLada. The outcome was adjusted for multiple testing by the Bonferroni procedure.
Results
Figure 2 depicts the outcome of median splits. The median of \(V_{{\max_{\text{ada}} }}\) was 43.31 cm/s for the 60° rotation group and 36.99 cm/s for the 75° rotation group. When participants were split according to those medians, the resultant subgroups had significantly different peak velocities (60°: t(50) = −10.76, p < .001; 75°: t(35) = −9.47, p < .001). The median of MTada was 3.74 s for the 60° and 3.81 s for the 75° group. Subgroups according to those medians had significantly different movement times (60°: t(50) = 12.63, p < .001; 75°: t(35) = 7.92, p < .001). The median of PLada was 23.62 cm for the 60° group and 26.41 cm for the 75° group. The pertinent subgroups had significantly different path lengths (60°: t(50) = −8.89, p < .001; 75°: t(35) = −6.64, p < .001).
Mean values and standard deviations for the kinematic subgroups of the 60° and the 75° rotation groups. Subgroups were formed by median splits according to peak velocity \(V_{{\max_{\text{ada}} }}\) (A), movement time MTada (B) and path length PLada (C). Subgroups with high kinematic values are presented in grey, those with low kinematic values in white. The medians used for splitting, and 95 % confidence intervals, are displayed in black for each median split
Using the above resulting groups for comparison of the according IEs, no group differences were found during baseline episodes for either split and rotation group (see ANOVAs Table 1, baseline column). However, group differences emerged during the adaptation episodes. As Fig. 3a + b indicates, participants with higher \(V_{{\max_{\text{ada}} }}\) had higher initial errors during adaptation than those with lower \(V_{{\max_{\text{ada}} }}\) (Table 1a, adaptation column). The effect is more pronounced in the 75° group (Table 1d, adaptation column) than in the 60° group and is completely abolished during de-adaptation phase in both rotation groups (Table 1a + d, de-adaptation column). Figure 3c + d illustrates that participants with shorter MTada had comparable IE to those with longer MTada (Table 1b + e, adaptation column) and had comparable after-effects (Table 1b + e, de-adaptation column).
Participants adapting to a 60° rotation (grey) and a 75° degree rotation (black), subdivided with median split into performance groups by a, b \(V_{{\max_{\text{ada}} }}\), c, d MTada and e, f PLada. For each episode, across-participant means and SD are shown. The means of baseline episodes with normal visual feedback are shown as “b”, and those of de-adaptation episodes with normal visual feedback as “d”. Mid-between are the adaptation episodes with rotated visual feedback
Pearson correlation between \(V_{{\max_{\text{ada}} }}\) and the MTada was significant in the 60° rotation group (r (50) = 0.805, p < .001) but not in the 75° rotation group (r (35) = 0.148, p > .05).
Multiple regression results are presented in Table 2 (60° group) and Table 3 (75° group). With IEada as dependent variable, significant predictors were only EEada and PLada in both groups; \(V_{{\max_{\text{ada}} }}\), MTada and RTada were no significant predictors in either model. With IEde-ada as dependent variable, no significant predictor emerged for either rotation group. With IEeff as dependent variable, only EEada was significant and that only in the 60° group. With IE st1 de-ada as dependent variable, only MTada was significant and that only in the 75° group.
Given the outcome for IEada, we decided to split IE once more, this time by the most predictive parameter, PLada. As Fig. 3e + f shows, participants with longer movement paths had higher IE during adaptation than participants with shorter movement paths (Table 1c + f, adaptation column); this effect was more pronounced in the 75° rotation group and was absent during the de-adaptation phase (Table 1c + f, de-adaptation column).
The correlation between \(V_{{\max_{\text{ada}} }}\) and PLada was highly significant in both rotation groups (60°: r (50) = 0.673, p < .001; 75°: r (35) = 0.503, p = .001).
Discussion
This study assessed the influence of kinematic parameters on visuomotor adaptation. We hypothesized that within a given number of trials, natural slow movers will adapt better than natural fast movers if adaptation depends on the duration of visual exposure to the distortion, but they will adapt equally well if adaptation depends on the number of trials.
At a first glance, our data seem to support the former alternative: initial errors during the adaptation phase were indeed smaller in subgroups which moved with a lower peak velocity. However, this benefit of lower V max was not reflected by a corresponding benefit of longer movement times. Moreover, the advantage of lower V max was absent when other movement parameters were taken into account: our multiple regression analyses suggest that the advantage of lower V max was mediated by the two significant regressors, EE and PL. Finally, a benefit of longer V max was also absent when the dependent variable of multiple regressions was the change of initial error (IEeff) rather than its mean value (IEada); in this case, only EE remained as a significant regressor. Taken together, this pattern of findings does not support the view that slow movers are exposed longer to the distortion and therefore produce smaller IEs. It rather supports an interpretation with inversed causality: persons who produce smaller IE achieve a better final accuracy (low EE) with less mid-flight corrections of their movements, hence with a shorter path length—concomitantly—with a lower movement velocity. In contrast, persons producing larger initial errors, correct those errors more perspicuously later during the movement which prolongs the movement path. As a corollary, the persons should have prolonged movement time but this is compensated by increased movement speed. This interpretation is in-line with the concept of isochrony, which maintains that movement velocity in voluntary movements increase with path length in order to keep movement time constant. Isochrony was found to hold for motor skills such as drawing (Viviani and McCollum 1983), writing (Lacquaniti et al. 1983) and, importantly, pointing (Fitts 1954; Stetson and McDill 1923). To sum up MT in naturally speeded movements does not determine adaptation. This casts further doubt on the view that duration of exposure to the distortion is indeed a critical determinant of adaptation. Movement velocity seems to influence adaptation, but should rather be interpreted as consequence of adaptation, reflecting error corrections in movements with free chosen movement speeds.
The proposed interpretation of movement kinematics being a consequence of error corrections makes a specific prediction about performance during the de-adaptation phase. Since initial errors during de-adaptation are thought to reflect the preceding recalibration but not the preceding error corrections (Bock 2005; McNay and Willingham 1998; Redding and Wallace 1996), movement kinematics of the adaptation phase should have little influence on initial errors of the de-adaptation phase. This is indeed what our data seem to indicate. Our regression analyses revealed that PL, EE, Vmax and MT were not correlated with IEde-ada, thus suggesting that error corrections have no appreciable influence on adaptive recalibration. Results of IE st1 deada were inconsistent for both rotation groups, but further implicate no sustainable interaction of movement kinematics during adaptation and after-effects. In fact, even the initial error of the adaptation phase did not influence adaptive recalibration: the correlation between IEada and IEde-ada was non-significant both in the 60° group (r (50) = 0.118; p > .05) and in the 75° group (r (35) = 0.300; p > .05).
As mentioned in the introduction section, earlier studies with forced constant movement velocities also found that movement time does not influence adaptation. The studies differed in the amount of given visual feedback. Adaptation with very short movement time was not poorer than adaptation with somewhat longer movement time with concurrent visual feedback (Tseng et al. 2007). Adaptation with distinctly longer movement time in absence of online visual feedback (Baily 1972; Kitazawa et al. 1997) also showed no difference to adaptation with very short movement time. In the present work, movement time was similar to the long movement times in the latter two studies, but online visual feedback was available. There was again no evidence for an effect of movement time on adaptation. The present data are therefore in agreement with earlier findings, and extend them to arm movements with online visual feedback.
Summing up, we found no evidence for the view that adaptation success depends on exposure duration rather than on the number of trials. Also, different extend of visual online feedback does not influence adaptation. We found preliminary evidence that kinematic parameters during adaptation phase reflect errors and their correction, but are mainly unrelated to recalibration. This evidence is based on two separate data sets, one with a 60° rotation and the other with a 75° rotation and thus seems to be generalizable.
References
Anguera JA, Reuter-Lorenz PA, Willingham DT, Seidler RD (2010) Contributions of spatial working memory to visuomotor learning. J Cogn Neurosci 22(9):1917–1930
Baily JS (1972) Adaptation to prisms: do proprioceptive changes mediate adapted behaviour with ballistic arm movements? Q J Exp Psychol 24(1):8–20. doi:10.1080/14640747208400261
Bock O (2005) Components of sensorimotor adaptation in young and elderly subjects. Exp Brain Res 160(2):259–263. doi:10.1007/s00221-004-2133-5
Bock O (2013) Basic principles of sensorimotor adaptation to different distortions with different effectors and movement types: a review and synthesis of behavioral findings. Front Human Neurosci 7:81. doi:10.3389/fnhum.2013.00081
Bock O, Girgenrath M (2006) Relationship between sensorimotor adaptation and cognitive functions in younger and older subjects. Exp Brain Res 169(3):400–406. doi:10.1007/s00221-005-0153-4
Bock O, Schmitz G (2011) Adaptation to rotated visual feedback depends on the number and spread of target directions. Exp Brain Res 209(3):409–413. doi:10.1007/s00221-011-2564-8
Cressman EK, Henriques DYP (2011) Motor adaptation and proprioceptive recalibration. Prog Brain Res 191:91–99. doi:10.1016/B978-0-444-53752-2.00011-4
Fitts PM (1954) The information capacity of the human motor system in controlling the amplitude of movement. J Exp Psychol 47(6):381
Henriques D, Cressman EK (2012) Visuomotor adaptation and proprioceptive recalibration. J Mot Behav 44(6):435–444. doi:10.1080/00222895.2012.659232
Kitazawa S, Kimura T, Uka T (1997) Prism adaptation of reaching movements: specificity for the velocity of reaching. J Neurosci 17(4):1481–1492
Krakauer JW (2000) Learning of visuomotor transformations for vectorial planning of reaching trajectories
Lacquaniti F, Terzuolo C, Viviani P (1983) The law relating the kinematic and figural aspects of drawing movements. Acta Psychol 54(1–3):115–130. doi:10.1016/0001-6918(83)90027-6
McNay EC, Willingham DB (1998) Deficit in learning of a motor skill requiring strategy, but not of perceptuomotor recalibration, with aging. Learn Mem 4(5):411–420. doi:10.1101/lm.4.5.411
Redding GM, Wallace B (1996) Adaptive spatial alignment and strategic perceptual-motor control. J Exp Psychol Hum Percept Perform 22(2):379
Simon A, Bock O (2015) Does visuomotor adaptation proceed in stages? an examination of the learning model by Chein and Schneider (2012). J Mot Behav. doi:10.1080/00222895.2015.1015677
Simon A, Bock O (2016) Influence of divergent and convergent thinking on visuomotor adaptation in young and older adults. Hum Mov Sci 46:23–29. doi:10.1016/j.humov.2015.11.020
Smith MA, Ghazizadeh A, Shadmehr R (2006) Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biol 4(6):e179. doi:10.1371/journal.pbio.0040179
Stetson RH, McDill JA (1923) Mechanism of the different types of movement. Psychol Monogr 32(3):18
Tseng Y-W, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ (2007) Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol 98(1):54–62
Viviani P, McCollum G (1983) The relation between linear extent and velocity in drawing movements. Neuroscience 10(1):211–218
Werner S, Bock O (2007) Effects of variable practice and declarative knowledge on sensorimotor adaptation to rotated visual feedback. Exp Brain Res 178(4):554–559. doi:10.1007/s00221-007-0925-0
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Simon, A., Bock, O. Influence of movement kinematics on visuomotor adaptation. Exp Brain Res 234, 3083–3090 (2016). https://doi.org/10.1007/s00221-016-4707-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-016-4707-4