Abstract
The brain has the remarkable ability to bind together inputs from different sensory origin into a coherent percept. Behavioral benefits can result from such ability, e.g., a person typically responds faster and more accurately to cross-modal stimuli than to unimodal stimuli. To date, it is, however, largely unknown whether such multisensory benefits, shown for discrete reactive behaviors, generalize to the continuous coordination of movements. The present study addressed multisensory integration from the perspective of bimanual coordination dynamics, where the perceptual activity no longer triggers a single response but continuously guides the motor action. The task consisted in coordinating anti-symmetrically the continuous flexion–extension of the index fingers, while synchronizing with an external pacer. Three different configurations of metronome were tested, for which we examined whether a cross-modal pacing (audio–tactile beats) improved the stability of the coordination in comparison with unimodal pacing condition (auditory or tactile beats). We found a more stable bimanual coordination for cross-modal pacing, but only when the metronome configuration directly matched the anti-symmetric coordination pattern. We conclude that multisensory integration can benefit the continuous coordination of movements; however, this is constrained by whether the perceptual and motor activities match in space and time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In spite of distinct neurophysiological properties subserving the processing of auditory, visual or tactile cues, our brain is remarkably able to bind together inputs from different sensory origin into a coherent percept. These neural operations, characterizing multisensory integration, are involved in the processing of a redundant cross-modal event, i.e., an environmental event captured through different sensory modalities (Stein and Stanford 2008; Driver and Noesselt 2008; Ernst and Bülthoff 2004).
Behavioral and neuronal facilitation has been reported when perceiving and reacting to cross-modal stimuli. Meredith and colleagues first showed increased neuronal responses of specific cells within the cat’s superior colliculus, together with a facilitation of the cat’s orienting behavior (Meredith and Stein 1986; Meredith et al. 1987). Since then, similar observations of improved reactive behaviors in response to cross-modal stimuli have been made for humans, e.g., shorter reaction time to external triggers or better detection of external events (Hughes et al. 1994; Frens et al. 1995; Diederich and Colonius 2004; Bell et al. 2005; Murray et al. 2005). It has been argued that these multisensory benefits, or redundant signal effects (RSE), are constrained by three principles (see Holmes and Spence 2005; Alais et al. 2010 for a discussion): the alignment of the unimodal stimuli in space (spatial rule—Meredith and Stein 1986, 1996; Kadunce et al. 1997), their alignment in time (temporal rule—Meredith et al. 1987; Miller and D’Esposito 2005), and the Inverse Effectiveness Principle predicting that the multisensory benefit is inversely proportional to the intensity of the unimodal stimuli (Stein and Meredith 1993; Wallace et al. 1996; Stanford et al. 2005; Perrault et al. 2005; Stein et al. 2009).
To date, however, it is largely unknown whether multisensory integration can facilitate the continuous coordination of movements, and whether the same three principles apply. Only a few studies have investigated the advantage of multimodal cues when synchronizing a single joint movement with an external pacing (Lagarde and Kelso 2006; Elliott et al. 2010) or when coordinating limb movements (Ronsse et al. 2009; Lagarde et al. 2012; Zelic et al. 2012). The present experiment addressed for the first time the conditions of the stabilization of coordination patterns by multisensory integration. We examined the extent to which a cross-modal metronome can further stabilize the anti-symmetric coordination of the finger movements when compared to its unimodal counterpart.
The patterning and breakdown of continuous rhythmic bimanual coordination has revealed that temporal stability is a hallmark of efficient and healthy behavior (Kelso 1995; Turvey 1990). Two patterns of coordination are naturally stable: a symmetric (i.e., simultaneous flexion–extension of effectors) and an anti-symmetric one (i.e., flexion of one effector synchronized with the extension of the other). However, the less stable anti-symmetric pattern breaks down with increased movement’s rate and is spontaneously replaced by a symmetric coordination pattern (Kelso 1984; Swinnen 2002). Fundamental in our daily actions (Howard et al. 2009), the anti-symmetric coordination between the hands is the one systematically impaired with pathologies of the nervous system (Byblow et al. 2000; Lewis and Byblow 2004; Steenbergen et al. 1996). Also particularly fragile to brain insult, be it by Parkinson syndrome or by experiencing a stroke, this coordination pattern can, however, benefit from the presentation of external cues to rhythm (Bernatzky et al. 2004; Bloem et al. 2004). Indeed, the synchronization of movements with unimodal metronome presented in the environment can stabilize the anti-symmetric coordination and maintain it at higher movement rates. This is likely due to such rhythm providing anchoring to the movement flow (Byblow et al. 1994; Fink et al. 2000; Assisi et al. 2005).
The present study examined whether the synchronization with a cross-modal metronome can reinforce the intensity of such a stabilization effect on anti-symmetric coordination. The efficiency of multisensory integration processes is here addressed when perception and action have to continuously cooperate; this situation contrasts with reactive behaviors for which perception systematically precedes, or triggers, an action. In addition, brain imaging studies have shown that a large-scale reorganization of brain activity underlies the competition between the anti-symmetric and symmetric patterns (Grefkes et al. 2008; Meister et al. 2010; Liuzzi et al. 2011; Hinder 2011). This involves a modification of the inter-hemispheric cross-talk between the sensorimotor cortices: when the mostly inhibitive cross-talk related to the anti-symmetric pattern fails, a less sophisticated cortical network involving facilitative inter-hemispheric exchange takes over, resulting in the behavioral switch to symmetric movements (Meyer-Lindenberg et al. 2002; Aramaki et al. 2006; Barnejee et al. 2012; Diedrichsen et al. 2013). Therefore, whereas faster reactive behaviors indicated the ability of multisensory brain mechanisms to speed up the perceptual processing of cross-modal stimuli, the multisensory stabilization of the anti-symmetric pattern of coordination would reflect the strengthening of the ongoing endogenous sensorimotor processes that support the anti-symmetric coordination.
In the present experiment, the task consisted in coordinating anti-symmetrically the continuous flexion–extension of the right and left index while synchronizing with an external metronome. Three types of metronome were tested in unimodal (auditory or tactile beats) and cross-modal (coincident audio–tactile beats) pacing conditions.
Firstly, the recent identification of strong auditory–tactile interactions in the brain, in particular at the level of the auditory cortex, makes the interaction between hearing and touch a good candidate for investigating multisensory mechanisms. Cortico-cortical connections (Cappe and Barone 2005; Hackett et al. 2007a; Smiley et al. 2007) and cortico-thalamo-cortical connections (Hackett et al. 2007b; Cappe et al. 2009) were identified in the macaque auditory cortex, as well as clusters of auditory–tactile neurons in the caudal belt of auditory association cortex (Schroeder et al. 2001; Fu et al. 2003). There is less evidence of direct interactions in the human brain between the auditory and tactile processing. However, the integration of auditory and tactile stimuli in the posterior regions of the superior temporal cortex (Sperdin et al. 2009; Murray et al. 2005) shows that it is possible that auditory and tactile cues interact via anatomical-linked pathways. Also at the neurophysiological scale, tactile stimuli have been shown to entrain a modulation of the neuronal activity in the auditory association cortex (Kayser et al. 2005) and even in the primary auditory cortex (Kayser and Logothetis 2007). It is this entrainment that likely results in the increased neural responses observed for audio–tactile stimuli (Foxe et al. 2000; Schroeder et al. 2001, 2003; Lakatos et al. 2007; Schroeder and Foxe 2004, 2005; Foxe and Schroeder 2005). Finally, at the behavioral scale, numerous studies have identified benefits from auditory–tactile interaction such as a facilitation in detecting external events (Murray et al. 2005; Zampini et al. 2007) or in tone perception (Schürmann et al. 2004; Gillmeister and Eimer 2007; Guest et al. 2002; Jousmäki and Hari 1998).
Secondly, previous studies demonstrated that the intensity of the stabilization of the anti-symmetric coordination pattern when synchronizing with an external metronome depends on the spatiotemporal configuration of the metronome itself (Fink et al. 2000; Jirsa et al. 2000; Assisi et al. 2005). Therefore, we examined whether and how the multisensory benefit expected from cross-modal pacing was affected by the configuration in space and time of the metronome.
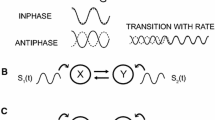
Three configurations of metronome were manipulated for which the intensity of the stabilization effect on the anti-symmetric coordination differs in unimodal pacing conditions. The metronomes and their relations to the anti-symmetric pattern of coordination are presented in Fig. 1 of the “Method” section. The first two (Simple, Double) consisted in a periodic repetition of bilateral beats, simultaneously on the left and on the right side of the participants. Whereas the Simple metronome provided one bilateral beat per movement cycle, the Double metronome consisted of two bilateral beats per movement cycle resulting in a stronger stabilization of the anti-symmetric coordination (Fink et al. 2000; Jirsa et al. 2000). The third metronome (Alternating) provided lateralized beats alternatively on the right and on the left side of the participant, and therefore directly matched the anti-symmetric pattern of movements. Such perceptuo-motor compatibility stabilizes coordination patterns (Zanone and Kelso 1992; Tuller and Kelso 1989) in a way akin to the resonance phenomenon explained by the forced-coupled oscillators theory of coordination (Jirsa et al. 2000; Schöner and Kelso 1988a, b).
The coordination pattern (top) and the configurations of Simple, Double and Alternating metronomes are presented for three cycles of right and left index movement. The black dots represent the occurrence of a metronome beat on the right or left side of the participant. Instructions were given to participants to move their fingers in an anti-symmetrical mode and to synchronize with the metronome beats. Note that the metronome beats could be either audio, tactile, or audio–tactile
We predicted a multisensory enhancement whatever the configuration of the metronome, i.e., a stronger stabilization of the anti-symmetric bimanual coordination with the cross-modal pacing in comparison with the unimodal (audio, tactile) pacing. Furthermore, assuming that the aforementioned principle of inverse effectiveness shown for reactive behaviors would extend to the class of coordinated behaviors, we predicted that for each configuration of metronome, the amplitude of the multisensory benefit would be inversely proportional to the stability of the pattern in unimodal (audio or tactile) pacing conditions.
Methods
Participants
Ten male students (26.1 ± 3.2 years old) from Montpellier 1 University took part in our experiment. One was left handed. None declared abnormal hearing or touch, and all were naive to the purpose of the experiment. All participants gave their written informed consent prior to their inclusion in this study that was approved by the institutional review board and conformed to the 2013 World Medical Association Declaration of Helsinki.
Apparatus
Index finger positions were recorded using electrogoniometers (SG65 from Biometrics Ltd., ±2° accuracy, sampled at 200 Hz).
Audio, tactile, and audio–tactile metronomes were designed as sequences of audio, tactile, and simultaneous audio and tactile stimuli, both consisting in 80-ms square wave pulses with a 300 Hz carrier. The tactile stimuli were provided by electromagnets which responded faster than 2 ms and reproduced the input signal (Lagarde and Kelso 2006). One device was fixed on the pulp of the third phalange of the auricular finger of each hand such that a tactile stimulus could be delivered to each participant on the right and/or on the left side. Participants also wore earphones that were used to provide the audio stimuli on the right and/or on the left side. Note that headphones playing white noise were placed on the top of the earphones in order to isolate participants from any environmental sounds. Right and left audio–tactile stimuli were finally defined by the simultaneous activation of a tactile stimulus and an audio stimulus, together, respectively, on the right or on the left side. A requirement of a strict spatial alignment for multisensory benefits has been shown to not be essential in the case of the audio–tactile interaction (Murray et al. 2005; Zampini et al. 2007; Sperdin et al. 2010 for review). Therefore, in spite of the physical distance between the tactile stimulus (delivered on the auricular finger) and the audio stimulus (directly provided at the ear), we will consider each audio–tactile stimulus as spatially coincident since they consisted in the combination of two unimodal stimuli (audio and tactile) that are applied on the same side (right or left) of the participant.
Procedure
Participants were instructed to perform oscillatory movement, about the metacarpophalangeal joint, with their index fingers moving in an anti-symmetrical pattern at a comfortable amplitude. In an anti-symmetric pattern, the flexion of a finger is performed simultaneously with the extension of the other one, thus while the right finger flexed, the left one extended and vice versa. In addition, the participants had to match their movements with an external metronome whose frequency increased (see the “Experimental conditions” section). They were instructed to stay with the metronome tempo and to not intervene voluntarily if they felt the initial pattern started to change, but to continue with whatever was the most comfortable pattern (“don’t intervene paradigm”, Kelso et al. 1981; Kelso 1995). We also asked the participants to keep their eyes closed during data collection in order to avoid any side effect from visual processing.
We tested each participant in three successive sequences of one trial per experimental condition (see below), and each sequence followed a randomized order, for a total of 27 trials per participant. For each trial, whatever the characteristics of the metronomes, they systematically paced the finger movements from 1 to 2.8 Hz, with the movement rate increasing by steps of 0.2 Hz, each step lasting 10 s.
Experimental conditions
The experiment was organized following a 3 × 3 factorial design.
The experimental factor “Metronome Sensory Modality” had three levels: audio, tactile and audio–tactile. Here, the sensory modality that conveyed the metronome beats was manipulated. That is, the beats of the metronome could be exclusively audio (audio metronomes), exclusively tactile (tactile metronomes) or audio–tactile (audio–tactile metronomes).
The experimental factor “Metronome Configuration” also had three levels (Fig. 1): Simple metronome (bilateral single beats), Double metronome (bilateral double beats), and Alternating metronome (lateralized alternating beats). The Simple metronome consisted in a stream of bilateral beats of the stimuli (audio, tactile, or audio–tactile) provided simultaneously on the right and on the left side of the participants. Participants were asked to complete a full cycle of movement for each metronome beat, by synchronizing the flexion of the right finger on the bilateral beat. The Double metronome also consisted in a stream of bilateral beats, but at twice the frequency of the Simple metronome. Participants were instructed to complete a full cycle of movement for two metronome beats and to synchronize each finger flexion or extension with a bilateral beat (see also Fink et al. 2000). Finally, the Alternating metronome consisted in a stream of lateralized beats presented alternatively on the right and on the left side. Participants had to match the flexion of the right and left finger with the respective right and left metronome beats.
Data processing
The time-series of finger position were low-pass-filtered with a zero-lag 8-Hz second-order Butterworth filter. From these filtered time-series, we extracted the flexion and extension onsets of the right and left fingers that were defined at a threshold of 5 % of peak velocity after the velocity peak of the corresponding flexion or extension movement.
Two variables were computed to assess the synchronization of the participant’s movements with the metronomes.
We first examined the relative errors of synchronization or asynchronies determined by the temporal gap between the right index flexion onsets and the corresponding metronome beats. The standard deviation of the asynchronies time-series produced before the eventual occurrence of the pattern transition was taken as an indicator of the variability of the synchronization of the participant’s movements with the metronomes for the anti-symmetric coordination pattern. However, each participant switched from the intended anti-symmetric pattern of coordination to the symmetric one at a specific frequency plateau. Such a shift affected the length of the asynchronies time-series determined across participants and across experimental conditions, and also, the assessment of the synchronization. As the earliest switch was observed during the fourth frequency plateau (i.e., at 1.6 Hz), we focused the aforementioned analysis on the asynchronies produced during the three first frequency plateaus (from 1 to 1.4 Hz) to ensure a fair comparison across participants and experimental conditions. We also considered as outliers the asynchronies outside of the \(\mu \pm 2\sigma\) interval where \(\mu\) and \(\sigma\), respectively, defined the mean and the standard deviation of the asynchronies time-series.
In accordance with the analyses of previous studies (Fink et al. 2000), for the whole trial, we also examined the correlation (Pearson’s correlation) between the duration of the right finger movement cycles and the duration of the metronome cycles.
Therefore, two variables were considered to assess the synchronization of the participant movements with the metronomes: the standard deviation of the asynchronies during the first three frequency plateaus and the coefficient of correlation between the movement periods and the metronome periods.
Finally, we examined the stability of the anti-symmetric pattern of coordination through two complementary methods (Kelso 1995; Schöner and Kelso 1988a, b).
The first method considers the theoretical and empirical analysis of stationary behavior which estimated the dynamical stability of a coordination pattern established between two oscillators from the circular dispersion (variability) of their relative phase: the larger the dispersion, the less stable the coordination (Kelso 1995; Schöner and Kelso 1988a, b). Therefore, we computed the pointwise relative phase (\(\emptyset\)) time-series such as (1): \(\emptyset = 2\pi *\Delta t/T\) (Kelso et al. 1990), where \(\Delta t\) expresses the latency between a left finger and a right finger flexions, and T the current duration of the right finger movement cycle. The same processing was applied to extension onsets. We then determined the circular dispersion of the relative phase time-series produced before the eventual switch in coordination pattern. For the reasons discussed above, in the analysis of the synchronization of movements with the metronome, we also determined the circular dispersion of the relative phase time-series produced during the three first frequency plateaus. The circular dispersion values ranged within a [0 +1] interval, where 0 indicates the absence of coordination and 1 a perfectly stable coordination.
The second method examined the stability of the anti-symmetric pattern of coordination through an analysis of transient behavior. This method considers the stability of the anti-symmetric coordination pattern as a function of the occurrence of the pattern transition: the later the switch, the more stable the coordination pattern. Such experimental measurement of dynamical stability has been widely applied to processes characterized by two stable states in competition, under the influence of noise (Kelso 1995; Scheffer et al. 2009). For each trial performed, we therefore identified the eventual occurrence of a switch from the initial anti-symmetric coordination pattern (relative phase close to 180°) to the symmetric pattern (relative phase close to 0°). This time onset was then expressed as a ratio of the trial length which will represent the transition time score.
To sum up, the stability of the anti-symmetric pattern of coordination between the right and left finger movements was examined through three variables: the circular dispersion of the pointwise relative phase until the pattern transition, the circular dispersion of the pointwise relative phase for the three first frequency plateaus, and the transition time score.
The statistical analysis consisted in an analysis of variance with repeated measures (ANOVA) with two factors (Metronome Configuration X Metronome Sensory Modality), each one with three levels. We reported all p values after Greenhouse–Geisser correction and used post hoc t tests with Bonferroni adjustment for multiple comparisons. Note that the resulting distributions of correlation coefficients between the movement periods and the metronome periods, of circular dispersion values and of transition time scores, were submitted to statistical analysis after normalization by the arc-sine transformation of the square root such as (2): \(v^{\prime} = {\text{asin}}\left( {\sqrt v } \right)\).
Results
Synchronization with metronomes
The 3 × 3 repeated measures ANOVA on the standard deviation of the asynchronies time-series produced during the three first frequency plateaus showed a significant main effect for Metronome Configuration (F 2, 18 = 6.02, p = 0.019, η 2p = 0.4), a significant main effect for Metronome Sensory Modality (F 2, 18 = 6.34, p = 0.018, η 2p = 0.41), and a significant two-way interaction (F 4, 36 = 3.89, p = 0.034, η 2p = 0.3). Considering the Metronome Configuration main factor effect, post hoc tests indicated lower variability of the asynchronies (i.e., stronger synchronization) when synchronizing with the Double metronomes (mean M = 36 ms, 95 % CI = 5 ms) than with the Simple metronomes (M = 44 ms, 95 % CI = 5 ms, p = 0.009). Considering the Metronome Sensory Modality main factor effect, these post hoc tests revealed lower variability of the asynchronies when synchronizing with the audio metronomes (M = 40 ms, 95 % CI = 7 ms) and the audio–tactile metronomes (M = 39 ms, 95 % CI = 4 ms) than with the tactile metronomes (M = 43 ms, 95 % CI = 5 ms; respectively, p = 0.023 and p = 0.016). Considering the two-way interaction effect, the post hoc tests showed lower variability of the asynchronies when synchronizing with the audio Double metronome (M = 32 ms, 95 % CI = 3 ms) than with the tactile Double metronome (M = 40 ms, 95 % CI = 5 ms; p = 0.023). This post hoc analysis also highlighted the lower variability of the asynchronies when considering the synchronization with the audio Double metronome compared to the audio Alternating metronome (M = 40 ms, 95 % CI = 5 ms; p = 0.029).
The ANOVA on the coefficients of correlation between the period time-series of the right finger movement cycles and those of the metronome (Fink et al. 2000) showed a significant main effect for Metronome Sensory Modality (F 2, 18 = 10.71, p = 0.004, η 2p = 0.54). Post hoc tests indicated a stronger correlation for the audio metronomes (M = 0.979; 95 % CI = 0.004) and audio–tactile metronomes (M = 0.978; 95 % CI = 0.004) when compared to the tactile metronomes (M = 0.972; 95 % CI = 0.01), with, respectively, p = 0.001 and p = 0.005. Note that the mean correlation values are systematically above 0.97, which indicates high level of synchronization whatever the experimental condition. No significant effects were found for Metronome Configuration (F 2, 18 3.35, p > 0.05, η 2p = 0.27) nor for the interaction between Metronome Configuration and Metronome Sensory Modality (F 4, 36 = 0.6, p > 0.05, η 2p = 0.06).
Stability of the anti-symmetric coordination between the finger movements
The 3 × 3 ANOVA on the circular dispersion of the pointwise relative phase time-series computed between the finger movements and produced before the pattern transition showed a significant main effect for Metronome Configuration (F 2, 18 = 16.38, p = 0.002, η 2p = 0.65). The post hoc tests revealed a more stable anti-symmetric coordination when synchronizing with the Double metronomes (M = 0.94, 95 % CI = 0.012) and the Alternating metronomes (M = 0.92, 95 % CI = 0.016) than with the Simple metronomes (M = 0.86, 95 % CI = 0.048; p = 0.005). No significant effects were found for Metronome Sensory Modality (F 2, 18 = 3.22, p > 0.05, η 2p = 0.26) nor for the two-way interaction (F 4, 36 = 2.3, p > 0.05, η 2p = 0.2).
The 3 × 3 ANOVA on the circular dispersion of the pointwise relative phase time-series produced during the three first frequency plateaus yielded a significant main effect for Metronome Configuration (F 2, 18 = 9.08, p = 0.006, η 2p = 0.5). The within-factor post hoc mean comparisons showed that the anti-symmetric coordination was more stable when synchronizing with the Double metronomes (M = 0.92, 95 % CI = 0.049) and the Alternating metronomes (M = 0.93, 95 % CI = 0.015, p = 0.001) than with the Simple metronomes (M = 0.84, 95 % CI = 0.07, p = 0.005). No significant effects were found for Metronome Sensory Modality (F 2, 18 = 2.04, p > 0.05, η 2p = 0.19) nor for the two-way interaction (F 4, 36 = 0.18, p > 0.05, η 2p = 0.02).
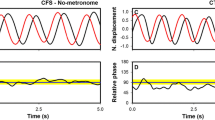
The 3 × 3 repeated measures ANOVA applied on the transition time scores showed a main effect factor for Metronome Configuration (Fig. 2a, F 2, 18 = 8.35, p = 0.0055; η 2p = 0.48) and a significant interaction between Metronome Configuration and Metronome Sensory Modality (Fig. 2b, F 4, 36 = 5.42, p = 0.0072; η 2p = 0.38). The post hoc mean comparisons for the main factor Metronome Configuration revealed that the anti-symmetric coordination pattern was longer maintained when synchronizing with the Alternating metronomes (M = 0.7, 95 % CI = 0.13) than with the Double metronomes (M = 0.6, 95 % CI = 0.14, p = 0.016) and the Simple metronomes (M = 0.57, 95 % CI = 0.13, p 0.004). The post hoc mean comparisons for the two-way interaction indicated a redundant signal effect (RSE—Raab 1962) for the Alternating metronomes. Indeed, the anti-symmetric coordination pattern was maintained longer when synchronizing with the audio–tactile Alternating metronome (M = 0.76, 95 % CI = 0.12) than with the audio Alternating metronome (M = 0.69, 95 % CI = 0.13, p = 0.04) and with the tactile Alternating metronome (M = 0.65, 95 % CI = 0.13, p = 0.001). No significant main effect was found for Metronome Sensory Modality (F 2, 18 = 3.57, p > 0.05; η 2p = 0.28).
Scores of transition time as a function of: a the Metronome Configuration (Simple, Double, Alternating); b the set of experimental conditions (3 Metronome Configuration × 3 Metronome Sensory Modality). The percentage of trial duration at which the pattern transition occurred (coined as score of transition time) is an indicator of the stability of the intended anti-symmetric coordination. Error bars correspond to the 95 % confidence intervals
Two hypotheses can be proposed to clarify the nature of the neural processes mediating redundant signals effects (RSE). On the one hand, RSE may come from the ability of the brain to effectively combine information from different sensory pathways. In this view, the cross-modal benefit from synchronizing with the Alternating audio–tactile metronome results from the emergence of cross-modal interactions in the perceptual processing of the tactile and auditory cues (Coactivation Model hypothesis—Miller 1982). On the other hand, RSE may result from a statistical phenomenon well known as probability summation. In that last case, no cross-modal interaction is involved, and the cross-modal benefit results from the increased probability to stabilize the coordination in view of the simultaneous activation of two independent perceptive processing, respectively, engaged for the audio and tactile rhythmic cues (Race Model hypothesis—Raab 1962; Miller 1982). In the following, we attempted to determine whether the RSE observed in the present experiment (for the Alternating metronome configuration—see above and Fig. 2b) matched the facilitation that can be predicted by the race model hypothesis.
The method proceeds in three steps. First, for each individual, we estimated the cumulative probability of maintaining the intended anti-symmetrical coordination to a certain percentage of time x of the trial length, when paced by the audio (CPa), the tactile (CPt), and the audio–tactile (CPat) Alternating metronomes (i.e., \({\text{CPa}},t,{\text{at}}\left( x \right) = P\left( {X \ge x} \right)\) with \(x \in \left[ {0;100} \right]\) in 0.5 increments). Second, for each individual, we estimated two race model predictions corresponding to two distinct assumptions regarding the dependence of the auditory and tactile unimodal processes when simultaneously activated in a cross-modal condition (Miller 1982). Under the assumption of negative dependence of the two unimodal processes, the race model prediction reads (3): \({\text{CPrm}}\left( x \right) = {\text{CPa}}\left( x \right) + {\text{CPt}}\left( x \right)\). Under the assumption of independence, the race model prediction is (4): \({\text{CPrmi}}\left( x \right) = {\text{CPa}}\left( x \right) + {\text{CPt}}\left( x \right) - \left( {{\text{CPa}}\left( x \right) * {\text{CPt}}\left( x \right)} \right)\). Third, the cumulative probability functions CPrm, CPrmi, and CPat, which were computed for each individual, were group averaged on each quantile. Finally, using pairwise mean comparisons, we assessed difference between CPat and the race model predictions (CPrm and CPrmi) at each quantile. In order to emphasize the sensitivity of the latter analysis, we used paired sample t test without a Bonferroni–Sidak correction (Perneger 1998). The race model hypothesis was considered invalid for each quantile where CPat was found greater than the race model predictions.
As illustrated in Fig. 3, we found that the race model hypothesis under the assumption of negative dependence can fully account for the cross-modal benefit found in the present experiment. However, interestingly, we found that the cross-modal benefit can exceed the race model prediction computed under the assumption of independence (gray area in Fig. 3, p < 0.05 for quantiles 59–60.5 and 73–73.5). This suggests that the phenomenon of statistical redundancy cannot fully account for the multisensory stabilization of the coordination observed with the auditory–tactile Alternating metronome. In contrast, cross-modal interactions within the perceptual processing of the audio and tactile rhythmic cues may have been involved (Coactivation Model hypothesis).
Probability of maintaining the intended anti-symmetric coordination as a function of the percentage of trial length. The fact that, for each curve, the probability of maintaining the intended anti-symmetric coordination decreases with the duration of a trial is an expected result of our paradigm whereby the movement frequency increases all along the trial duration. The group averaged cumulative probabilities are presented for the unimodal Alternating metronomes CPa (audio) and CPt (tactile) and for the audio–tactile Alternating metronome CPat. Predictions of the race model are presented under the two assumptions of dependence of the unimodal processes CPrm (negative dependence) and CPmi (independence). The grayed area indicates when the cross-modal facilitation exceeds the race model predictions
Note that this method is usually employed for rich set of data (e.g., reaction times; Sperdin et al. 2009) and was here adapted for a dataset consisting of relatively few scores (one for each trial so three per experimental conditions for each individual), and this might have limited the statistical power of the aforementioned analysis.
Discussion
The current experiment examined the stability of the anti-symmetric coordination between finger movements when synchronizing with three types of metronome tested in unimodal (auditory or tactile) and cross-modal (audio–tactile) pacing conditions. We predicted that (1) the cross-modal pacing will stabilize the coordination at higher rates than the unimodal pacing conditions for the three types of metronome, and (2) such increased stability will obey a behavioral inverse effectiveness principle, i.e., for a given metronome, the cross-modal gain will be inversely related to the stability of the pattern in unimodal pacing conditions. Results revealed that cross-modal pacing can improve the stability of the coordination. However, in contrast with our initial predictions, we found that such a multisensory benefit (or redundant signal effect: RSE) is not systematic. Multisensory benefit depends on the metronome’s configuration, and in particular, on the compatibility of the perceptual and motor patterns. Our most prominent finding is that effective multisensory integration requires a direct and unique matching between the spatiotemporal configuration of the metronome and the spatiotemporal configuration of the coordination pattern.
This impact of the compatibility of the perceptual and motor patterns on cross-modal behavioral effects was not anticipated. This result suggests interesting consequences for the processes underpinning multisensory integration, which will be discussed in the following.
A multisensory stabilization of the coordination of movement
Previous research has indicated that synchronizing with a unimodal metronome, to some extent, counteracts the loss of temporal stability of the anti-symmetric bimanual coordination when rate is increased. This stabilization effect is likely due to the metronome providing a spatiotemporal anchoring to the movement flow (Fink et al. 2000; Assisi et al. 2005). The present experiment showed that this effect can be strengthened when the pacing is cross-modal (auditory–tactile) rather than unimodal (auditory or tactile). In particular, the anti-symmetric coordination was maintained at higher rates with the Alternating cross-modal metronome in comparison with the Alternating unimodal metronomes.
These findings point to a novel benefit for the combination of sensory cues that complements the well-documented speeding up of a purely reactive behavior: the stabilization of the continuous coordination of movements when synchronizing with an external pacer can be reinforced when the pacer’s beats are cross-modal. In view of the significance of coordination patterns in daily life (Nourrit-Lucas et al. 2013), this result appears highly relevant for our understanding of adapted behavior more generally. Bimanual coordination constitutes an elementary but theoretically grounded window into the study of stability of coordination patterns. This stability acts continuously to maintain efficient movement patterns against neural noise (Schöner and Kelso 1988a, b), the latter being a pervasive component of CNS functioning (Van Beers et al. 2004; Schmidt et al. 1978; Harris and Wolpert 1998; Selen et al. 2005). Finally, a validation of cross-modal stabilization of coordination motivates its use to stabilize new patterns in a learning context or for the recovery of patterns when pathological changes are affecting the CNS.
Multisensory integration is constrained by the perceptuo-motor interaction
We found that the anti-symmetric pattern was maintained until certain critical rates and that this depended on the type of metronome perceived, which was expected. However, we also expected a systematic benefit for synchronizing with a cross-modal pacing, with a gain inversely related to the stability of the pattern in unimodal pacing conditions. Our results revealed a different situation. We found that the behavioral efficacy of multisensory cues varied depending upon changes of metronome configuration: multisensory enhancement existed exclusively for the Alternating metronome.
In an earlier study, we found indications that multisensory integration could be conditioned by the spatiotemporal configuration of metronomes in relation to the coordination pattern (Zelic et al. 2012). This previous study, however, examined different metronome configurations, and few have been shown to fail in triggering multisensory enhancements. In the present experiment, each configuration of metronome tested was found to be efficient in terms of stabilizing the anti-symmetric pattern, albeit in unimodal pacing condition. The Simple metronome has been used in numerous studies to drive bimanual coordination at various frequencies (see Kelso 1995, for a review). The Double metronome has been more seldomly used, but has proved to be efficient to drive bimanual coordination (Fink et al. 2000; Assisi et al. 2005). Also, the Alternating metronome has been shown to drive and stabilize new patterns of coordination in learning experiments (Zanone and Kelso 1992; Tuller and Kelso 1989).
In the following, we discuss the extent to which the configuration of the Alternating metronome differs from those of the bilateral (Simple and Double) metronomes. Then, we consider how such differences might have benefited both the stability of the coordination pattern and the emergence of multisensory enhancement.
Compatibility and symmetry in perception–movement relations
A first step in developing an explanation comes from the analysis of the symmetries in the relation of the coordination pattern and the metronome configuration. The Alternating metronome consisted in sequences of lateralized beats that were provided alternatively on the left and right sides. Participants, respectively, synchronized their right and left finger flexion with these right and left beats; therefore, the action to be synchronized was unequivocally specified as a flexion of the finger located on the side of the beat. Accordingly, the relation between the Alternate metronome and the coordination pattern followed a left–right symmetry. In contrast, the bilateral metronomes provided bilateral beats simultaneously on the left and right sides. Here, the action to be synchronized is necessarily distinct considering the left and right finger, as each bilateral beat triggers the flexion of one finger and the extension of the other. Accordingly, the relation between the bilateral metronomes and the coordination pattern does not follow the aforementioned left–right symmetry.
In bimanual coordination, reduced symmetry often causes reduced stability (Kelso 1995), and this may have limited a behavioral gain in cross-modal bilateral metronomes. Due to its lateralized and alternated structure, the Alternating metronome provided a direct and univocal cueing of the phasing required for the anti-symmetric coordination pattern. In contrast, this phasing was not uniquely specified by the bilateral metronomes which can drive either an anti-symmetric or a symmetric pattern of coordination. This creates the condition for a competition to be solved by the CNS, likely through complementary processes in order to map the syncopated pattern of movements to the bilateral simultaneous pacing.
The analysis of compatibility sketched here is stated in simple terms, but its generalization would need to be made using more formal tools. Importantly such a generalization is required to predict multisensory gains with other coordination patterns involved for example in gait, upright posture, single limb movement, or in speech–gesture coordination (Zelic et al. 2015). This is beyond the scope of the present study, but one can envision analyzing the relation between beat sequences and coordination patterns by comparing their symmetry groups, the same way it was used to establish taxonomies of gait patterns of movement or Gestalt percepts (see Schöner et al. 1990; Pinto and Golubitsky 2006; Turvey et al. 2009; Cassirer 1944).
Lower neuronal load when compatibility is respected
The multisensory efficacy of the compatible Alternating metronome might result from the lower time cost due to the direct projection of the sensory consequence of the stimulation into the hemisphere that initiates the motor response, i.e., the finger flexion. Indeed, the absence of a contralateral event to process when synchronizing with the compatible Alternating metronome likely alleviates the perceptuo-motor mechanisms by avoiding the processing of additional inter-hemispheric exchanges (Umiltà and Nicoletti 1990; Hommel 1996) and the recruitment of supplementary sensorimotor mapping areas (e.g., the left premotor and parietal cortices—Iacoboni et al. 1998). In addition, reducing the inter-hemispheric exchanges involved for these perceptuo-motor mapping operations minimizes the risk of interference with the fragile and critical inhibitive cross-talk that support the anti-symmetric coordination (Grefkes et al. 2008; Meister et al. 2010; Meyer-Lindenberg et al. 2002; Aramaki et al. 2006; Barnejee et al. 2012; Diedrichsen et al. 2013). Therefore, one possibility is that the Alternating metronome involved less sophisticated, likely faster, perceptuo-motor mapping operations, increasing their efficacy at higher rates, when time constraints are maximal. In contrast, the bilateral metronomes likely involved extra perceptuo-motor operations due to the processing of a contralateral information (bilateral beat) which may have inhibited, competed with, or delayed those required for multisensory integration. The left parietal cortex could very conceivably be the place to look for such a competition, as it is assumed to be involved in both perceptuo-motor mapping for incompatible S-R (Iacoboni et al. 1998) and multisensory integration. On that line of thinking, recent studies in computational neuroscience strongly suggest that time delays typically destabilize large-scale networks (Jirsa and Ding 2004). Moreover, the increase in neural noise imposed by supplementary mapping operation may also contribute to a destabilization (Tagliabue and McIntyre 2011).
Onto the benefit of cross-modal neural interaction
Our analysis based on the Miller’s inequality suggests that the stabilization of the coordination observed with the auditory–tactile Alternating metronome cannot be entirely explained by statistical redundancy of independent processes. Rather, the multisensory benefit would be due to cross-modal interactions between the auditory and tactile processing (Fig. 3—Raab 1962; Miller 1982). To date, however, the neurophysiological mechanisms leading to a stabilization of the sensorimotor processes by an external pacer are poorly understood. In the following, we discussed two types of brain processes related to multisensory integration that may have contributed to the multisensory stabilization of the coordination.
Firstly, the cross-modal interaction may simply strengthen perceptuo-motor mechanisms involved in the unimodal pacing conditions without changing its organization. Numerous studies have demonstrated that the output of early, “unisensory” processing is affected by multisensory influences (Lakatos et al. 2008; Diederich et al. 2012; Fiebelkorn et al. 2011; Thorne et al. 2011). In the present case, direct interactions between the low-level cortices (e.g., direct inputs from the somatosensory cortex to the auditory cortex—Smiley et al. 2007; Lakatos et al. 2007) or ascending projections from multisensory subcortical sources (Schroeder and Foxe 2005) might have improved the perceptuo-motor neural processes already at work during the unimodal pacing conditions. Recently, Van Atteveldt et al. (2014) suggested that the combination of canonical, population-level integrative operations such as divisive normalization and phase resetting supports such low-level cortical interactions.
A second possibility is that distinct and more effective perceptuo-motor mechanisms emerged when synchronizing with the cross-modal Alternating metronome. Indeed, previous research has revealed multisensory-specific neural networks exclusively activated for the processing of cross-modal cues (Ghazanfar and Schroeder 2006; Driver and Noesselt 2008; Stein and Stanford 2008; Senkowski et al. 2008). Such multisensory-specific networks have been suggested to speed up the perceptual processing of external cues. In the present experiment, it is possible that a multisensory-specific network has been activated, establishing a novel perceptuo-motor binding more efficient for the stabilization of the coordination. For instance, a more direct coupling could have emerged from sensory to the motor cortices, alleviating the critical temporal cost inherent to the underlying perceptuo-motor mechanisms.
Conclusion
We found that the compatibility of the perceptual and motor patterns is critical for multisensory integration to stabilize bimanual movement coordination. Indeed, multimodal benefits were conditioned by the compatibility of the metronome’s configuration with the spatiotemporal dynamic of the coordination pattern. These original findings suggest that the rules that apply to multisensory integration for reactive behaviors might have to be supplemented in order to generalize to the class of continuous coordinated behaviors. The fact that multisensory integration can facilitate the coordination at the condition of a matching in space and time of patterns of perception and action, provides a fertile new direction for investigating how two fundamental processes, namely motor coordination and multimodal integration, connect in the brain. In addition, enhancing movement coordination via cross-modal auditory–tactile stimuli, which prevents overloading vision, is also promising regarding, for example, the rehabilitation of coordination skills in people post-stroke or in cueing movements for Parkinson patients.
A pressing issue for future research is to unravel the neural mechanisms underlying the stabilization of the coordination when synchronizing with an external pacer and to determine how these perceptuo-motor mechanisms are conditioned by the degree of compatibility of the perceptual and motor patterns. It would constitute a first step toward a better understanding of multisensory integration neural processes from the perspective of coordination dynamics.
References
Alais D, Newell FN, Mamassian P (2010) Multisensory processing in review: from physiology to behaviour. Seeing Perceiving 23:3–38
Aramaki Y, Honda M, Okada T, Sadato N (2006) Neural correlates of the spontaneous phase transition during bimanual coordination. Cereb Cortex 16(9):1338–1348
Assisi CG, Jirsa VK, Kelso JAS (2005) Dynamics of multifrequency coordination using parametric driving: theory and experiment. Biol Cybern 93(1):6–21
Barnejee A, Tognoli E, Kelso JAS, Jirsa V (2012) Spatiotemporal re-organization of large-scale neural assemblies underlies bimanual coordination. Neuroimage 62(3):1582–1592
Bell AH, Meredith MA, Van Opstal AJ, Munoz DP (2005) Crossmodal integration in the primate superior colliculus underlying the preparation and initiation of saccadic eye movements. J Neurophysiol 93(6):3659–3673
Bernatzky G, Bernatzky P, Hesse HP, Staffen W, Ladurner G (2004) Stimulating music increases motor coordination in patients afflicted with Morbus Parkinson. Neurosci Lett 361(1–3):4–8
Bloem BR, Hausdorff JM, Visser JE, Giladi N (2004) Falls and freezing of gait in Parkinson’s disease: a review of two interconnected, episodic phenomena. Mov Disord 19(8):871–884
Byblow WD, Carson RG, Goodman D (1994) Expressions of asymmetries and anchoring in bimanual coordination. Hum Mov Sci 13:3–28
Byblow WD, Summers JJ, Thomas J (2000) Spontaneous and intentional dynamics of bimanual coordination in Parkinson’s disease. Hum Mov Sci 19:223–249
Cappe C, Barone P (2005) Heteromodal connections supporting multisensory integration at low levels of cortical processing in the monkey. Eur J Neurosci 22:2886–2902
Cappe C, Morel A, Barone P, Rouiller EM (2009) The thalamocortical projection systems in primate: an anatomical support for multisensory and sensorimotor interplay. Cereb Cortex 19(9):2025–2037
Cassirer E (1944) The concept of group and the theory of perception. Philos Phenomenol Res 5(1):1–36
Diederich A, Colonius H (2004) Bimodal and trimodal multisensory enhancement: effects of stimulus onset and intensity on reaction time. Percept Psychophys 66(8):1388–1404
Diederich A, Schomburg A, Colonius H (2012) Saccadic reaction times to audiovisual stimuli show effects of oscillatory phase reset. PLoS One 7(10):e44910
Diedrichsen J, Wiestler T, Krakauer JW (2013) Two distinct ipsilateral cortical representations for individuated finger movements. Cereb Cortex 23(6):1362–1377
Driver J, Noesselt T (2008) Multisensory interplay reveals crossmodal influences on sensory-specific’s brain regions, neural responses, and judgments. Neuron 57(1):11–23
Elliott MT, Wing AM, Welchman AE (2010) Multisensory cues improve sensorimotor synchronisation. Eur J Neurosci 31(10):1828–1835
Ernst MO, Bülthoff HH (2004) Merging the senses into a robust percept. Trends Cogn Sci 8:162–169
Fiebelkorn IC, Foxe JJ, Butler JS, Mercier MR, Snyder AC, Molholm S (2011) Ready, set, reset: stimulus-locked periodicity in behavioral performance demonstrates the consequences of cross-sensory phase reset. J Neurosci 31(27):9971–9981
Fink PW, Foo P, Jirsa VK, Kelso JA (2000) Local and global stabilization of coordination by sensory information. Exp Brain Res 134(1):9–20
Foxe JJ, Schroeder CE (2005) The case for feedforward multisensory convergence during early cortical processing. NeuroReport 16:419–423
Foxe JJ, Morocz IA, Murray MM, Higgins BA, Javitt DC, Schroeder CE (2000) Multisensory auditory–somatosensory interactions in early cortical processing revealed by high-density electrical mapping. Cogn Brain Res 10:77–83
Frens MA, Van Opstal AJ, Van der Willigen RF (1995) Spatial and temporal factors determine auditory–visual interactions in human saccadic eye movements. Attent Percept Psycho 57:802–816
Fu KMG, Johnston TA, Shah AS, Arnold L, Smiley J, Hackett TA, Garraghty PE, Schroeder CE (2003) Auditory cortical neurons respond to somatosensory stimulation. J Neurosci 23:7510–7515
Ghazanfar AA, Schroeder CE (2006) Is neocortex essentially multisensory? Trends Cogn Sci 10(6):278–285
Gillmeister H, Eimer M (2007) Tactile enhancement of auditory detection and perceived loudness. Brain Res 1160:58–68
Grefkes C, Eickhoff SB, Nowak DA, Dafotakis M, Fink GR (2008) Dynamic intra-and interhemispheric interactions during unilateral and bilateral hand movements assessed with fMRI and DCM. Neuroimage 41(4):1382–1394
Guest S, Catmur C, Lloyd D, Spence C (2002) Audiotactile interactions in roughness perception. Exp Brain Res 146:161–171
Hackett TA, De La Mothe LA, Ulbert I, Karmos G, Smiley J, Schroeder CE (2007a) Multisensory convergence in auditory cortex, II. Thalamocortical connections of the caudal superior temporal plane. J Comp Neuro 502(6):924–952
Hackett TA, Smiley JF, Ulbert I, Karmos G, Lakatos P, de la Mothe LA, Schroeder CE (2007b) Sources of somatosensory input to the caudal belt areas of auditory cortex. Perception 36(10):1419–1430
Harris CM, Wolpert DM (1998) Signal-dependent noise determines motor planning. Nature 394(6695):780–784
Hinder MR (2011) Interhemispheric connectivity between distinct motor regions as a window into bimanual coordination. J Neurophysiol 107(7):1791–1794
Holmes NP, Spence C (2005) Multisensory integration: space, time and superadditivity. Curr Biol 15(18):R762–R764
Hommel B (1996) SR compatibility effects without response uncertainty. Q J Exp Psychol A 49(3):546–571
Howard IS, Ingram JN, Körding KP, Wolpert DM (2009) Statistics of natural movements are reflected in motor errors. J Neurophysiol 102(3):1902–1910
Hughes HC, Reuter-Lorenz PA, Nozawa G, Fendrich R (1994) Visual-auditory interactions in sensorimotor processing: saccades versus manual responses. J Exp Psychol Hum Percept Perform 20(1):131–153
Iacoboni M, Woods RP, Mazziotta JC (1998) Bimodal (auditory and visual) left frontoparietal circuitry for sensorimotor integration and sensorimotor learning. Brain 121(11):2135–2143
Jirsa VK, Ding M (2004) Will a large complex system with time delays be stable? Phys Rev Lett 93(7):070602
Jirsa VK, Fink P, Foo P, Kelso JAS (2000) Parametric stabilization of biological coordination: a theoretical model. J Biol Phys 26(2):85–112
Jousmäki V, Hari R (1998) Parchment-skin illusion: sound-biased touch. Curr Biol 8(6):R190
Kadunce DC, Vaughan JW, Wallace MT, Benedek G, Stein BE (1997) Mechanisms of within-and cross-modality suppression in the superior colliculus. J Neurophysiol 78:2834–2847
Kayser C, Logothetis NK (2007) Do early sensory cortices integrate cross-modal information? Brain Struct Funct 212(2):121–132
Kayser C, Petkov CI, Augath M, Logothetis NK (2005) Integration of touch and sound in auditory cortex. Neuron 48:373–384
Kelso JA (1984) Phase transitions and critical behavior in human bimanual coordination. Am J Physiol 246:R1000–R1004
Kelso JAS (1995) Dynamic patterns: the self-organization of brain and behavior. The MIT Press, Cambridge
Kelso JAS, Holt KG, Rubin P, Kugler PN (1981) Patterns of human interlimb coordination emerge from the properties of nonlinear limit cycle processes: theory and data. J Mot Behav 13(4):226–261
Kelso JAS, DelColle JD, Schöner G (1990) Action-perception as a pattern formation process. In: Jeannerod M (ed) Attention and performance, vol 13. Erlbaum, Hillsdale, pp 139–169
Lagarde J, Kelso JAS (2006) Binding of movement, sound and touch: multimodal coordination dynamics. Exp Brain Res 173(4):673–688
Lagarde J, Zelic G, Mottet D (2012) Segregated audio–tactile events destabilize the bimanual coordination of distinct rhythms. Exp Brain Res 219(3):409–419
Lakatos P, Chen CM, O’Connell MN, Mills A, Schroeder CE (2007) Neuronal oscillations and multisensory interaction in primary auditory cortex. Neuron 53(2):279–292
Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE (2008) Entrainment of neuronal oscillations as a mechanism of attentional selection. Science 320(5872):110–113
Lewis GN, Byblow WD (2004) Bimanual coordination dynamics in poststroke hemiparetics. J Mot Behav 36(2):174–188
Liuzzi G, Hörnib V, Zimerman M, Gerloff C, Hummel FC (2011) Coordination of uncoupled bimanual movements by strictly timed interhemispheric connectivity. J Neurosci 31(25):9111–9117
Meister IG, Foltys H, Gallea C, Hallett M (2010) How the brain handles temporally uncoupled bimanual movements. Cereb Cortex 20(12):2996–3004
Meredith MA, Stein BE (1986) Spatial factors determine the activity of multisensory neurons in cat superior colliculus. Brain Res 365(2):350–354
Meredith MA, Stein BE (1996) Spatial determinants of multisensory integration in cat superior colliculus neurons. J Neurophysiol 75:1843–1857
Meredith MA, Nemitz JW, Stein BE (1987) Determinants of multisensory integration in superior colliculus neurons. I. Temporal factors. J Neurosci 7(10):3215–3229
Meyer-Lindenberg A, Ziemann U, Hajak G, Cohen L, Berman KF (2002) Transitions between dynamical states of differing stability in the human brain. Proc Natl Acad Sci USA 99(17):10948–10953
Miller J (1982) Divided attention: evidence for coactivation with redundant signals. Cogn Psychol 14(2):247–279
Miller LM, D’Esposito M (2005) Perceptual fusion and stimulus coincidence in the cross-modal integration of speech. J Neurosci 25:5884–5893
Murray MM, Molholm S, Michel CM, Heslenfeld DJ, Ritter W, Javitt DC, Schroeder CE, Foxe JJ (2005) Grabbing your ear: rapid auditory–somatosensory multisensory interactions in low-level sensory cortices are not constrained by stimulus alignment. Cereb Cortex 15(7):963–974
Nourrit-Lucas D, Zelic G, Deschamps T, Hilpron M, Delignières D (2013) Persistent coordination patterns in a complex task after 10 years delay: subtitle: how validate the old saying “once you have learned how to ride a bicycle, you never forget!”. Hum Mov Sci 32(6):1365–1378
Perneger TV (1998) What’s wrong with Bonferroni adjustments. Br Med J 316(7139):1236–1238
Perrault TJ Jr, Vaughan JW, Stein BE, Wallace MT (2005) Superior colliculus neurons use distinct operational modes in the integration of multisensory stimuli. J Neurophysiol 93:2575–2586
Pinto CM, Golubitsky M (2006) Central pattern generators for bipedal locomotion. J Math Biol 53(3):474–489
Raab DH (1962) Statistical facilitation of simple reaction times. Trans NY Acad Sci 24:574–590
Ronsse R, Miall RC, Swinnen SP (2009) Multisensory integration in dynamical behaviors: maximum likelihood estimation across bimanual skill learning. J Neurosci 29(26):8419–8428
Scheffer M, Bascompte J, Brock WA, Brovkin V, Carpenter SR, Dakos V, Sugihara G (2009) Early-warning signals for critical transitions. Nature 461(7260):53–59
Schmidt RA, Zelaznik HN, Frank JS (1978) Sources of inaccuracy in rapid movement. In: Stelmach GE (ed) Information processing in motor control and learning. Academic Press Inc., New York, pp 183–203
Schöner G, Kelso JAS (1988a) A synergetic theory of environmentally-specified and learned patterns of movement coordination. II. Component oscillator dynamics. Biol Cybern 58(2):81–89
Schöner G, Kelso JA (1988b) Dynamic pattern generation in behavioral and neural systems. Science 239(4847):1513–1520
Schöner G, Jiang WY, Kelso JS (1990) A synergetic theory of quadrupedal gaits and gait transitions. J Theor Biol 142(3):359–391
Schroeder CE, Foxe JJ (2004) Multisensory convergence in early cortical processing. In: Calvert GA (ed) The handbook of multisensory processes. MIT Press, Cambridge, pp 295–309
Schroeder CE, Foxe J (2005) Multisensory contributions to low-level, ‘unisensory’processing. Curr Opin Neurobiol 15(4):454–458
Schroeder CE, Lindsley RW, Specht C, Marcovici A, Smiley JF, Javitt DC (2001) Somatosensory input to auditory association cortex in the macaque monkey. J Neurophysiol 85:1322–1327
Schroeder CE, Smiley J, Fu KG, McGinnis T, O’Connell MN, Hackett TA (2003) Anatomical mechanisms and functional implications of multisensory convergence in early cortical processing. Int J Psychophysiol 50(1–2):5–17
Schürmann M, Caetano G, Jousmäki V, Hari R (2004) Hands help hearing: facilitatory audiotactile interaction at low sound-intensity levels. J Acoust Soc Am 115(2):830–832
Selen LP, Beek PJ, van Dieën JH (2005) Can co-activation reduce kinematic variability? A simulation study. Biol Cybern 93(5):373–381
Senkowski D, Schneider TR, Foxe JJ, Engel AK (2008) Crossmodal binding through neural coherence: implications for multisensory processing. Trends Neurosci 31(8):401–409
Smiley JF, Hackett TA, Ulbert I, Karmas G, Lakatos P, Javitt DC, Schroeder CE (2007) Multisensory convergence in auditory cortex, I. Cortical connections of the caudal superior temporal plane in macaque monkeys. J Comp Neurol 502(6):894–923
Sperdin HF, Cappe C, Foxe JJ, Murray MM (2009) Early, low-level auditory–somatosensory multisensory interactions impact reaction time speed. Front Integr Neurosci 3:2
Sperdin HF, Cappe C, Murray MM (2010) Auditory–somatosensory multisensory interactions in humans: dissociating detection and spatial discrimination. Neuropsychologia 48(13):3696–3705
Stanford TR, Quessy S, Stein BE (2005) Evaluating the operations underlying multisensory integration in the cat superior colliculus. J Neurosci 25:6499–6508
Steenbergen B, Hulstijn W, de Vries A, Berger M (1996) Bimanual movement coordination in spastic hemiparesis. Exp Brain Res 110:91–98
Stein BE, Meredith MA (1993) The merging of the senses. The MIT Press, Cambridge
Stein BE, Stanford TR (2008) Multisensory integration: current issues from the perspective of the single neuron. Nat Rev Neurosci 9(4):255–266
Stein BE, Stanford TR, Ramachandran R, Perrault TJ, Rowland BA (2009) Challenges in quantifying multisensory integration: alternative criteria, models, and inverse effectiveness. Exp Brain Res 198:113–126
Swinnen SP (2002) Intermanual coordination: from behavioural principles to neural-network interactions. Nat Rev Neurosci 3(5):348–359
Tagliabue M, McIntyre J (2011) Necessity is the mother of invention: reconstructing missing sensory information in multiple, concurrent reference frames for eye–hand coordination. J Neurosci 31(4):1397–1409
Thorne JD, De Vos M, Viola FC, Debener S (2011) Cross-modal phase reset predicts auditory task performance in humans. J Neurosci 31(10):3853–3861
Tuller B, Kelso JAS (1989) Environmentally-specified patterns of movement coordination in normal and split-brain subjects. Exp Brain Res 75:306–316
Turvey MT (1990) Coordination. Am Psychol 45(8):938–953
Turvey MT, Romaniak-Gross C, Isenhower RW, Arzamarski R, Harrison S, Carello C (2009) Human odometer is gait-symmetry specific. Proc Biol Sci 276(1677):4309–4314
Umiltà C, Nicoletti R (1990) Spatial stimulus-response compatibility. In: Proctor RW, Reeve TG (eds) Stimulus-response compatibility: an integrated perspective. North-Holland, Amsterdam, pp 89–116
Van Atteveldt N, Murray MM, Thut G, Schroeder CE (2014) Multisensory integration: flexible use of general operations. Neuron 81(6):1240–1253
Van Beers RJ, Haggard P, Wolpert DM (2004) The role of execution noise in movement variability. J Neurophysiol 91(2):1050–1063
Wallace MT, Wilkinson LK, Stein BE (1996) Representation and integration of multiple sensory inputs in primate superior colliculus. J Neurophysiol 76:1246–1266
Zampini M, Torresan D, Spence C, Murray MM (2007) Auditory–somatosensory multisensory interactions in front and rear space. Neuropsychologia 45(8):1869–1877
Zanone PG, Kelso JA (1992) Evolution of behavioral attractors with learning: non equilibrium phase transitions. J Exp Psychol Hum Percept Perform 18(2):403–421
Zelic G, Mottet D, Lagarde J (2012) Behavioral impact of unisensory and multisensory audio–tactile events: pros and cons for interlimb coordination in juggling. PLoS One 7(2):e32308
Zelic G, Kim J, Davis C (2015) Articulatory constraints on spontaneous entrainment between speech and manual gesture. Hum Mov Sci 42:232–245
Acknowledgments
This research was supported by SKILLS, an Integrated Project (FP6-IST contract #035005) of the Commission of the European Community. We also gratefully acknowledge the input of the reviewers, with special thanks for the remarkable interaction about the race model predictions that definitely helped us to improve the paper from its original version.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Zelic, G., Mottet, D. & Lagarde, J. Perceptuo-motor compatibility governs multisensory integration in bimanual coordination dynamics. Exp Brain Res 234, 463–474 (2016). https://doi.org/10.1007/s00221-015-4476-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-015-4476-5