Abstract
Motion sickness is more common among women than among men. Previous research has shown that standing body sway differs between women and men. In addition, research has shown that postural sway differs between individuals who experience visually induced motion sickness and those who do not and that those differences exist before exposure to visual motion stimuli. We asked whether sex differences in postural sway would be related to sex differences in the incidence of visually induced motion sickness. We measured unperturbed standing body sway before participants were exposed to visual motion stimuli that induced motion sickness in some participants. During postural testing, participants performed different visual tasks. Results revealed that postural sway was affected by visual tasks, consistent with the literature. In addition, we found a statistically significant three-way interaction between visual tasks, sex, and (subsequent) motion sickness status. These results suggest that sex differences in motion sickness may be related to sex differences in the control of postural balance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Classically, motion sickness has been associated with physical displacement, usually through travel in vehicles. Recent decades have seen a dramatic increase in reports of motion sickness outside the context of travel. Interactive visual technologies can induce motion sickness. In the context of entertainment (e.g., video games), such effects may be inconsequential. However, interactive technologies increasingly are being used for purposes other than entertainment. Motion sickness has been reported among users of tablet computers, such as the iPad (Stoffregen et al. 2014), and there are anecdotal reports of sickness arising from motion graphics in cell phone interfaces (The Guardian 2013). Using “off-the-shelf” devices and applications, the incidence of motion sickness among users of visual interactive technologies can be greater than 50 % (e.g., Dong et al. 2011; Merhi et al. 2007; Stoffregen et al. 2008). In addition, motion sickness is common among users of “immersive” virtual environments of the kind that are widely used in physical rehabilitation (Akiduki et al. 2003; Villard et al. 2008). Motion sickness in these settings can have negative impacts on individuals (e.g., reduced benefit from a rehabilitation intervention) and on society (e.g., the exclusion of susceptible individuals from technology-related careers; Giammarco et al. 2015). The spreading of motion sickness across so many “platforms,” and the general increase in reports of motion sickness suggest that this malady is of general importance at a societal level. Accordingly, there is increased motivation for greater scientific understanding of motion sickness, both in terms of causality or etiology and in terms of our ability to predict and prevent its occurrence.
Sex differences in susceptibility
One of the most commonly observed phenomena of motion sickness is that women are more susceptible than men. This observation has been confirmed in some of the largest field research studies ever conducted. Lawther and Griffin (1988) studied seasickness among more than 20,000 passengers on ocean-going ferries. The severity of seasickness symptoms was greater in women than in men by a ratio of 5.2/3.1. A nearly identical ratio was observed for vomiting, which was more common among women (8.8 %) than among men (5.0 %). Similar results have been obtained in land transportation (Golding 2006; Park and Hu 1999; Turner and Griffin 1999) and in vehicle simulators (e.g., Kennedy et al. 1995). Women are more likely than men to experience motion sickness resulting from wind-induced motion of tall buildings (Lamb et al. 2013).
Sex differences in susceptibility to motion sickness extend to visual motion stimuli in the absence of any inertial displacement. Koslucher et al. (2015) exposed standing participants to visual motion stimuli that oscillated along the line of sight. The stimulus motion was a sum of ten sines in the range 0.1–0.4 Hz, and the maximum amplitude (peak to peak) of room motion was 1.8 cm. Participants were exposed to this motion in up to four trials, each 10 min in duration. Participants were instructed to discontinue participation immediately if they experienced any symptoms of motion sickness, however mild. Koslucher et al. separately evaluated the incidence of motion sickness and the severity of subjective symptoms. Motion sickness incidence was assessed using a yes/no forced choice question, Are you motion sick?, which was administered before and after exposure to visual motion in the moving room. Symptom severity was assessed using the Simulator Sickness Questionnaire or SSQ (Kennedy et al. 1993).
Before exposure, each participant stated that they were not motion sick, and SSQ scores were low (Koslucher et al. 2015). After exposure, the incidence of motion sickness among females was 38 % (26/69), which was significantly greater than incidence among males, which was 9 % (4/45). At post-exposure, symptom severity scores were greater among participants in the sick group. However, post-exposure scores did not differ between men and women in the well group or between men and women in the sick group. That is, there was a sex difference in the incidence of motion sickness, but not in its severity. These results suggest that visual technologies may lead to large sex differences in motion sickness and, consequently, to impacts of motion sickness that are sexist at both the individual and societal levels. The data reported by Koslucher et al. were part of a larger study of sex differences in motion sickness. In the present article, we report data on postural sway and visual performance that were collected from the same participants, as part of that larger study.
Sex differences: causal hypotheses
Why do women and men differ in susceptibility to motion sickness? It has been suggested that reports of subjective symptoms, such as nausea and fatigue, might be affected by social or gender-role issues. For example, women might be more willing than men to acknowledge aversive subjective experiences. However, Golding (2006) rejected this argument. One reason is that the sex difference exists in relation to objective data: As noted above, women vomit more than men (Lawther and Griffin 1988). The sex difference also does not seem to be related to extra habituation to greater ranges of motion environments experienced by risk-taking males (Dobie et al. 2001), nor to gender-biased differential self-selection between males and females when volunteering for laboratory motion sickness experiments (Flanagan et al. 2005). It is sometimes suggested that the sex difference arises from female hormonal cycles (e.g., Howarth and Griffin 2003), but the evidence does not support this view. Golding et al. (2005, p. 972) concluded “only around one-third of the difference between male and female susceptibility could be accounted for by increased or decreased motion susceptibility at certain phases of the menstrual cycle. … any putative menstrual/endocrine effect cannot fully explain sex differences in motion sickness susceptibility.” It has been suggested that sex differences in motion sickness may arise from physiological variables. For example, the level of salivary cortisol is related to motion sickness susceptibility in women. However, this relationship does not exist in men (Meissner et al. 2009). Accordingly, salivary cortisol cannot explain differences between women and men.
The above attempts to explain sex differences in motion sickness are ad hoc, in the sense that they have not been derived from any general theory of motion sickness etiology. That being said, many of the researchers involved are adherents of the sensory conflict theory of motion sickness (e.g., Flanagan et al. 2005; Howarth and Griffin 2003; Golding 2006). The sensory conflict theory claims that everyday interactions with the environment give rise to expectations about relations between inputs from different sensory systems (e.g., Reason 1978). When current patterns of input differ from expected patterns, sensory conflict is produced. When the magnitude of this conflict exceeds some threshold (Oman 1982), motion sickness can result. We are not aware of any attempt to use the sensory conflict theory to explain or predict the existence of sex differences in motion sickness. That is, we are not aware of any claims that the hypothetical internal expectations on which the theory is based should differ between women and men, and we are not aware of any claims that the hypothetical threshold for motion sickness should differ between women and men. The absence of such principled accounts may explain the fact that adherents of the sensory conflict theory have tended to resort to ad hoc explanations for known sex differences.
In the present study, we offer a qualitatively different approach. Our approach is based on the postural instability theory of motion sickness (Riccio and Stoffregen 1991), which argues that motion sickness occurs in the animal–environment system. Many situations, including travel in both physical and virtual vehicles, alter relations between body movement, perceptual stimulation, and the effects of postural control actions. Changes in these dynamics mandate changes in the control actions that are used to stabilize the body. In many situations, these dynamics are not only novel but also variable; an example is the quasi-periodic motion of a ship at sea. Control actions that served to stabilize the body on land generally will be inefficient or even counter productive at sea. Individuals must learn new control actions that are “tuned” to the novel dynamics. As with any form of learning, there will be individual differences in the rate at which new skills are acquired; some individuals will learn more rapidly than others. In addition, there may be individual differences in the way in which new control actions are learned (e.g., Faugloire et al. 2006). Until new control actions are identified (i.e., until the individual learns how to link particular patterns of perceptual stimulation to particular patterns of control outputs), certain aspects of the animal–environment interaction may be uncontrolled or controlled only intermittently. Uncontrolled or intermittently controlled movements tend to be unstable, and instability will persist until new types of control are robust.
The experience of motion sickness often is accompanied (or followed) by visible instability in control of the body: Sufferers may be visibly unsteady in their posture and gait. Instability that is concurrent with or which follows the subjective symptoms of motion sickness is uncontroversial and has no implications for etiology. Riccio and Stoffregen (1991) made a qualitatively different claim. They argued that unstable control of the body should precede any subjective symptoms of motion sickness, and they argued that the subjective symptoms of motion sickness result from instabilities in the control of perceptually guided adjustments to posture. A central prediction of the postural instability theory is that postural activity should differ between persons who experience motion sickness and those who do not and that these differences should exist (and be measurable) before the onset of the subjective symptoms of motion sickness.
Men and women differ in many ways. Which of these differences may underlie sex differences in motion sickness? In the present study, we asked whether sex differences in susceptibility to visually induced motion sickness might be preceded by patterns of postural activity in standing body sway that would differ between women and men.
Postural sway before motion sickness
Motion sickness is associated with degraded control of the body. It is not surprising if people who are suffering from motion sickness have degraded stance and locomotion. More surprising is the repeated finding that such differences in postural control exist before the onset of any of the subjective symptoms of motion sickness and, in fact, before participants are exposed to experimental motion of any kind. This effect has been observed during stance in laboratory devices featuring linear (translational) oscillations (Koslucher et al. 2014; Stoffregen and Smart 1998; Stoffregen et al. 2010), for seated subjects exposed to oscillations rotating around the line of sight (Stoffregen et al. 2000a), and for virtual environments (Villard et al. 2008). It has also been observed in the context of inertial motion, with measures of body sway taken 24 h before the beginning of a sea voyage (Stoffregen et al. 2013).
Effects have been observed in the spatial magnitude of sway (e.g., Smart et al. 2002; Stoffregen and Smart 1998; Stoffregen et al. 2000a; Villard et al. 2008), but also in the temporal dynamics of sway (Stoffregen et al. 2013, 2010; Villard et al. 2008). Accordingly, in the present study, we examined measures of both spatial magnitude and temporal dynamics.
Sex differences in postural sway
Men and women differ in unperturbed standing body sway. Era et al. (2006) measured unperturbed stance in 7979 adults and found that the speed of center of pressure displacements differed between women and men for each of five age groups. Several other studies have also found sex differences in the spatial magnitude of postural sway (Chiari et al. 2002; Kim et al. 2010; Sullivan et al. 2009). There are also sex differences in the temporal dynamics of sway (e.g., Kim et al.). Both spatial and temporal sex differences persist even when data are normalized by subjects’ height (Kim et al. 2010).
Chiari et al. (2002) computed 55 stabilographic parameters from center of pressure data. Men and women differed in several parameters of postural sway for sway in the AP axis. They then compared the stabilometric parameters to several anthropometric parameters that differed significantly between women and men in their sample (height, weight, base of support, foot width, and the angle between the feet). The majority of stabilometric parameters were significantly correlated with one or more of these anthropometric factors. They concluded that most of the effects of sex on standing body sway arose from biomechanical properties rather than neural control.
Given the findings of Chiari et al. (2002), it seems reasonable to ask whether sex differences in postural sway could be related to sex differences in motion sickness. As noted above, Koslucher et al. (2015) exposed standing participants to linear visual oscillation that mimicked the amplitude and frequency characteristics of standing body sway and found that the incidence of motion sickness in women was greater than in men. In addition, for individuals the risk of motion sickness was significantly correlated with parameters of bodily anthropometrics that are sexually dimorphic. Motion sickness incidence was negatively related to overall height when controlling for weight and when controlling for body mass index or BMI. Separately, motion sickness incidence was negatively related to the height of the center of mass, when controlling for BMI. Finally, motion sickness incidence was negatively correlated with foot length (controlling for BMI): Motion sickness was more likely among subjects with shorter feet. Women have lower center of mass and shorter feet even when controlling for sex differences in height (Fessler et al. 2005). Taken together, these findings are consistent with the hypothesis that sex differences in motion sickness incidence may be preceded by distinctive patterns of body sway that are themselves sex-specific.
Postural sway and visual performance
Body sway varies as a function of non-postural tasks that are carried out during stance (e.g., Woollacott and Shumway-Cook 2002). A common finding is that postural sway is reduced during performance of a demanding visual task (searching through a block of text for designated target letters) relative to sway in the absence of visual demand (looking at a blank target); (Stoffregen et al. 2000b; Stoffregen et al. 2011; Yu et al. 2013). Reading places great demands on the visual system: To read each word, the eyes must be pointed with great precision, and to shift gaze from word to work (and from line to line), the eyes must be moved with great precision. The eyes are in the head and the head is atop the torso, such that ocular stabilization will be affected by the position and motion of the torso, including movements that constitute body sway. Arguably, it is for this reason that postural sway varies between different visual tasks (Stoffregen et al. 1999, 2007). Accordingly, a reduced ability to stabilize the body could be related both to visual performance, on the one hand, and to motion sickness susceptibility, on the other.
Anecdotally, the risk of motion sickness can be affected by non-postural tasks: Survey evidence suggests that reading in a car is more provocative than simple looking out the window (Sivak and Schoettle 2015; Turner and Griffin 1999). Some experimental evidence also is relevant. In a study of seasickness, Stoffregen et al. (2013) measured standing body sway while participants looked at the horizon or at a nearby target. The temporal dynamics of sway revealed a statistically significant interaction between visual target distance and the severity of subsequent seasickness. Vehicle motion, such as occurs in automobiles and on ships at sea, poses challenges for overall stabilization of the body, which, in turn, will pose challenges for stabilization of the visual system.
In the present study, we asked whether the modulation of postural sway in response to this variation in visual tasks might be related to postural sway, to subsequent motion sickness, and to sex differences in motion sickness. Our investigation of task effects on postural sway in relation to sex differences in motion sickness was primarily exploratory.
The present study
Koslucher et al. (2015) reported data on the incidence of motion sickness and the severity of symptoms when standing women and men were exposed to oscillating optic flow in a moving room. In that study, the primary aim was to evaluate possible sex differences in the incidence of visually induced motion sickness; that is, the incidence of motion sickness was a dependent variable. The present study comprises data on the kinematics of standing body sway that were collected from the participants in Koslucher et al. before they were exposed to visual motion stimuli. Before participants entered the moving room, we measured standing body sway in during the performance of different visual tasks. We evaluated postural sway and visual performance, and we related these to the incidence of subsequent motion sickness as a function of sex. That is, in the present study, the incidence of motion sickness (as reported by Koslucher et al.) was an independent variable. The use of motion sickness incidence as both a dependent variable and an independent variable is widespread in research on motion sickness (e.g., Bonnet et al. 2006; Chang et al. 2012; Dong et al. 2011; Koslucher et al. 2014; Smart et al. 2002). We predicted that body sway would differ between visual tasks, replicating commonly reported effects. Our central prediction was that before exposure to any stimulus motion body sway would differ between women and men and that these differences would be related to the incidence of motion sickness after exposure to experimental visual motion.
Methods
Participants
The subjects were 114 individuals who participated in exchange for course credit. There were 45 men (mean age 22.81 years, SD 3.43 years; mean weight 82.74 kg, SD 14.45 kg; mean height 1.82 m, SD 0.07 m) and 69 women (mean age 21.78 years, SD 2.23 years; mean weight 65.23 kg, SD 12.37 kb; mean height 1.68 m, SD 0.07 m). The experimental protocol was approved in advance by the University of Minnesota IRB.
Apparatus
Participants stood on a force plate (AccuSway Plus; AMTI, Watertown, MA). We collected the center of pressure (COP) displacement in the anteroposterior (AP) and mediolateral (ML) axes at 50 Hz.
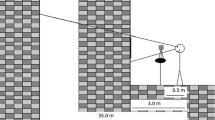
Visual stimulus motion was created using a moving room, which consisted of a cubical frame, 2.44 m on a side, mounted on wheels, and moved along one axis on rails (Fig. 1). The interior surfaces of the walls and ceiling were covered with blue and white marble-patterned adhesive paper. At the center of the front wall was a map of the continental USA (53 cm × 80 cm; 19° × 28°). Illumination was provided by floodlights mounted inside the room and oriented so that shadows were minimized. Movement of the room (oscillation along an axis parallel to the line of sight) was powered by an electric motor under computer control.
Procedure
Following the informed consent procedure participants removed their shoes and responded to a forced choice, yes/no question, Are you motion sick? Participants were instructed (both verbally and on the consent form) to discontinue the experiment immediately if they experienced any motion sickness symptoms, however mild. Anthropometric data were collected, some of which have been reported and analyzed by Koslucher et al. (2015). Next, participants stood with their feet on marked lines so that heels were 17 cm apart and the angle between the feet was 10° (Stoffregen et al. 2010). They were instructed to keep their arms by their sides and to avoid deliberate movement or talking during trials.
Before being exposed to the visual motion stimulus, each participant completed one trial in an inspection task and one in a search task. Visual targets were identical to those used by Stoffregen et al. (2000b). Targets consisted of sheets of white paper 13.5 cm × 17 cm and mounted on rigid cardboard. In the inspection task, the target was a blank sheet of white paper. In the search task, the target was a block of English text, consisting of 14 lines of text printed in a 12-point sans serif font, which was affixed to an otherwise blank card. Before each trial, the participant was asked to count the number of times the letter, r, appeared in the block of text. At the end of the search trial, the participant reported the number of letters counted and their position in the text at the end of the trial. Targets for the inspection and search tasks were 1.0 m in front of the heels, affixed to a stand and adjusted to each participant’s eye height. Each trial was 60 s in duration. Odd-numbered participants began with the search task, while even-numbered participants began with the inspection task.
After the search and inspection trials, targets were removed and participants were exposed to visible motion of the moving room. Room motion was a sum of ten sines in the range 0.1–0.4 Hz, with maximum displacement amplitude of 2.5 cm. Room motion was identical to that used by Bonnet et al. (2006) and Stoffregen et al. (2010), as was the protocol used during moving room trials. Each trial with room motion was 10 min in duration, and subjects were exposed to a maximum of four trials.
Between trials, the moving room was stationary and the subject was required to sit for 1 min. Before each trial, subjects were reminded to discontinue participation immediately if they experienced any symptoms of motion sickness, however mild. Upon discontinuation or after the completion of the experimental protocol (whichever came first), subjects again answered the forced choice, yes/no question, Are you motion sick? Subjects who stated that they were not sick after exposure to room motion were given a printed copy of the forced choice, yes/no question and the SSQ, and asked to fill it out at the time of symptom onset or after 24 h if no symptoms developed. Several studies have reported that the onset of motion sickness can follow exposure by up to 12 h (Miller and Goodson 1960).
Motion sickness incidence was based on a dichotomous classification that was derived solely from answers to the forced choice, yes/no question, Are you motion sick? Subjects who answered this question in the affirmative immediately after exposure to room motion or within 24 h of their participation in the experiment were placed in the sick group. All other subjects were placed in the well group. Following previous research (e.g., Bonnet et al. 2006; Stoffregen and Smart 1998; Stoffregen et al. 2010; Villard et al. 2008), classification into well and sick groups was based solely on responses to the forced choice question.
The data on postural activity reported in this article were collected when the room was stationary. We also collected data on postural sway during room motion; these will be reported elsewhere.
Data analysis
We evaluated performance on the search task in terms of the percent correct, which we defined operationally as the number of letters counted divided by the number of target letters that were in the text. We also evaluated reading speed, which we defined operationally as the number of words read during the 60-s search trial.
We separately evaluated the spatial magnitude and the temporal dynamics of postural activity. We operationalized spatial magnitude in terms of positional variability, that is, the standard deviation of the position of the center of pressure. We evaluated the temporal dynamics of postural activity using detrended fluctuation analysis or DFA. DFA describes the relation between the magnitude of fluctuations in postural motion and the timescale over which those fluctuations are measured (Chen et al. 2002). DFA has been used in studies of the control of stance (Lin et al. 2008; Yu et al. 2013). We conducted inferential tests on α, the scaling exponent of DFA, as derived from the COP data. The scaling exponent is an index of long-range autocorrelation in the data, that is, the extent to which COP positions were self-similar over different timescales. Our DFA did not include integration of the time series (Gao et al. 2006).
Results
Search task performance
We separately evaluated the accuracy of search task performance and the speed of reading during the search task. For each, we conducted a 2 (sex) × 2 (sick) ANOVA. For search task performance (% correct), we found a significant effect of sex, F(1,110) = 9.64, p = .002, partial η 2 = 0.08 (Fig. 2a).
For reading speed (number of words read), we found a significant main effect of sex, F(1,110) = 5.56, p = .02, partial η 2 = 0.048 (Fig. 2b). There were no other significant effects.
Postural sway
We conducted 2 (inspection vs. search) × 2 (body axis, AP vs. ML) × 2 (male vs. female) × 2 (well vs. sick) ANOVAs on the positional variability of the center of pressure and on α of DFA.
Detrended fluctuation analysis revealed two significant effects. The main effect of body axis was significant, F(1,110) = 97.05, p < .001, partial η 2 = .47. The α parameter was greater in the body’s AP axis (mean 1.34, SD .01) than in the body’s ML axis (mean 1.18, SD = .02). In addition, the main effect of visual tasks was significant, F(1,110) = 8.43, p = .004, partial η 2 = .07, which is illustrated in Fig. 3.
For positional variability, we found a significant main effect of body axis, F(1,110) = 82.66, p < .001, partial η 2 = .43. Positional variability in the body’s AP axis (mean 0.388, SD 0.02) was greater than in the body’s ML axis (mean 0.191, SD 0.01). In addition, there was a significant three-way interaction between sex, sickness groups, and visual tasks, F(1,110) = 4.52, p = .036, partial η 2 = .04, which is illustrated in Fig. 3. Post hoc tests revealed a significant difference between men in the well group during performance of the inspection task (95 % CI 0.218–0.275) and women in the sick group during performance of the search task (95 % CI 0.282–0.398), p < .05. At the 99 % level, this comparison was not significant (men/well/inspection 99 % CI 0.209–0.284 vs. women/sick/search 99 % CI 0.264–0.417). There were no other significant effects.
Discussion
We measured unperturbed standing body sway in a large sample of males and females. During postural assessments, participants performed simple visual tasks: looking at a blank target and counting target letters in a block of text. Following postural assessment, participants were exposed to oscillating visual motion that induced motion sickness in 9 % of men and 38 % of women, as reported by Koslucher et al. (2015). Before exposure to oscillating visual motion, reading rate and performance on the search task differed between men and women. The temporal dynamics of postural sway differed during performance of the two visual tasks. Postural sway differed between the body’s AP and ML axes. In addition, unperturbed body sway exhibited a complex interaction between sex, visual tasks, and the subsequent incidence of motion sickness. We discuss these results in turn.
Visual performance
Reading rate was greater among women than among men, but search performance was better among men. Taken together, the results on performance and reading rate suggest that, on average, women may have focused more on speed, while men may have focused more on accuracy. The direction of the difference in search performance (males better than females) is odd and may relate to past literature. Several studies have found that females exceeded males in speed, vocabulary, and comprehension (e.g., Gates 1961). In our search task, performance did not depend upon reading, as such, given that the task was to count letters rather than to comprehend the text.
The interactions between sex and subsequent motion sickness incidence were not significant for search accuracy or for reading rate. Accordingly, we found no evidence that sex differences in search task performance and reading rate were related to sex differences in susceptibility to motion sickness.
Postural sway: sex differences
Contrary to previous studies, we did not find simple (i.e., main effect) differences in postural sway between women and men. Some studies that have reported sex differences in standing body sway have focused exclusively on older adults (e.g., Era et al. 2006; Sullivan et al. 2009). Chiari et al. (2002) and Kim et al. (2010) included participants in the same age range as the present study. However, those studies differed from the present study in at least two ways. First, participants in the present study were taller and heavier and had greater BMI than participants in Chiari et al. and Kim et al. Second, in those studies, participants were invited to select their preferred foot positioning, whereas in the present study we controlled foot positioning. These differences might account for the absence of simple sex differences in postural sway in the present study.
Postural sway: visual tasks and body axes
The temporal dynamics of unperturbed body sway (α of DFA) differed during performance of the search task, relative to sway during performance of the inspection task. This effect replicates similar effects on temporal dynamics in previous studies (e.g., Chen and Stoffregen 2012; Koslucher et al. 2012; Yu et al. 2013). More broadly, the effect replicates the common finding that the kinematics of body sway are influenced by variations in the nature of visual tasks performed during stance (e.g., Stoffregen et al. 1999, 2007; Woollacott and Shumway-Cook 2002).
We also replicated the common finding that during unperturbed stance on land the spatial magnitude of sway is greater in AP than in ML (e.g., Balasubramaniam et al. 2000; Koslucher et al. 2014). We found that the self-similarity of sway was greater in AP than in ML. This finding replicated some studies (e.g., Lin et al. 2008); however, in other studies the reverse pattern has been found (e.g., Koslucher et al. 2014).
Taken together, the effects of visual task and body axis on postural sway confirm that postural sway in our sample was representative of effects that have been widely reported in the literature.
Postural sway: motion sickness, visual tasks, and sex
With respect to the goals of the study, the principal result was the statistically significant interaction between sickness groups (well vs. sick), visual tasks (inspection vs. search), and sex (women vs. men) on the positional variability of unperturbed postural sway before participants were exposed to any motion stimuli. In this section, we discuss several implications of this complex interaction.
First, as predicted, the three-way interaction comprises a difference in pre-exposure, unperturbed postural sway between well and sick groups (Fig. 4). A statistically significant effect involving differences in postural sway between well and sick groups (either a main effect of sickness groups or an interaction comprising sickness groups) has been found in studies using the same stimulus motion in the same moving room (e.g., Bonnet et al. 2006; Koslucher et al. 2014; Stoffregen et al. 2010), using similar stimulus motions in other moving rooms (Smart et al. 2002; Stoffregen and Smart 1998), using similar stimulus motions in a virtual moving room (Villard et al. 2008), and when seated participants were exposed to similar stimulus motions in an fixed-base flight simulator (Stoffregen et al. 2000a, b), as well as when unperturbed standing body sway was measured 24 h before the beginning of a sea voyage (Stoffregen et al. 2013). In each of these studies, spatial and/or temporal aspects of the quantitative kinematics of unperturbed postural sway differed between participants in the well and sick groups, and those differences existed before participants were exposed to stimulus motion. The consistency of these effects across postures (standing vs. seated) and venues (virtual motion in laboratory devices vs. physical motion of a ship at sea) provides robust converging evidence that the effect is general and, accordingly, provides robust support for the postural instability theory of motion sickness (Riccio and Stoffregen 1991).
Second, as part of the three-way interaction, the relation between postural sway and motion sickness was modulated by the visual tasks that participants performed. This is a novel effect. In previous research relating postural activity to motion sickness, researchers have not varied visual tasks during unperturbed stance (e.g., Koslucher et al. 2014; Stoffregen et al. 2010). There is abundant anecdotal evidence that a person’s perceptual and/or cognitive activity can modulate susceptibility to motion sickness. The classic example is reading in an automobile. In automobiles, reading increases the risk of motion sickness (Sivak and Schoettle 2015; Turner and Griffin 1999), which is a major concern for developers of autonomous vehicles (Davies 2015).
Finally, the three-way interaction revealed that the relation between postural sway and motion sickness differed between the sexes. Many studies have identified general differences (i.e., main effects) in postural sway between women and men (e.g., Chiari et al. 2002; Era et al. 2006; Kim et al. 2010; Sullivan et al. 2009). We did not find a simple, (i.e., main effect) difference in postural sway between the sexes. In this sense, we failed to replicate earlier research. In previous comparisons of male and female, postural sway researchers have sometimes varied visual activity, but typically only by evaluating posture with the eyes open versus closed, and have not evaluated possible interactions between sex and visual conditions (Chiari et al.; Era et al.; Sullivan et al.). None of these previous studies has addressed any aspect of motion sickness. Accordingly, our finding of a complex interaction between eyes-open visual tasks, sex, and motion sickness appears to be novel.
The three-way interaction revealed that for men and women there were qualitatively different relationships between sway during different visual tasks and subsequent reports of motion sickness. The post hoc analysis confirmed this relation in terms of a specific contrast; the postural sway of well men during performance of the inspection task differed from the postural sway of sick women during performance of the search task.
Conclusion
We investigated influences on postural sway of sex, visual tasks, and subsequent motion sickness. Taken separately, each of these relations is uncontroversial, in the sense of having been replicated in numerous empirical studies: Men and women differ in postural sway, sway routinely varies as a function of variations in visual tasks, and many studies have demonstrated that motion sickness is preceded by distinctive patterns of postural sway. The results of the present study suggest interrelations between these factors. These interrelations are important because they may provide insight into sex differences in susceptibility to motion sickness and into general relations between postural stability and motion sickness. The existence of relations between motion sickness, sex, and body sway is consistent with our finding, using the same participants, that motion sickness incidence was related to properties of anthropometrics that differ between the sexes (Koslucher et al. 2015).
Previous attempts to explain sex differences in motion sickness have focused on sensory and/or cognitive factors, such as sex differences in spatial processing (e.g., Parsons et al. 2004). In the present study, the inspection and search tasks do not appear to have differed in terms of spatial processing. Perhaps most important, the sensory conflict theory does not predict that postural sway should differ between persons who are susceptible to motion sickness and those who are not. Accordingly, the present results pose a challenge for theories of motion sickness etiology that are based upon the concept of sensory conflict.
Our results are compatible with the postural instability theory of motion sickness etiology (Riccio and Stoffregen 1991). The postural instability theory predicts that postural activity will differ between persons who are susceptible and those who are not and that these differences should exist before the onset of subjective symptoms of motion sickness. The present results are directly compatible with this prediction and extend the theory to the realm of sex differences in motion sickness.
The present findings suggest a novel focus for efforts to assess sex differences in susceptibility to motion sickness. The congruence of our results with those of many other studies relating postural activity to motion sickness (e.g., Bonnet et al. 2006; Stoffregen et al. 2010, 2013; Villard et al. 2008) suggests that postural control may be a defining feature of susceptibility to motion sickness and that sex differences in postural control may be related to sex differences in susceptibility.
References
Akiduki H, Nishiike S, Watanabe H, Matsuoka K, Kubo T, Takeda N (2003) Visual-vestibular conflict induced by virtual reality in humans. Neurosci Lett 340:197–200
Balasubramaniam R, Riley MA, Turvey MT (2000) Specificity of postural sway to the demands of a precision task. Gait Pos 11:12–24
Bonnet CT, Faugloire EM, Riley MA, Bardy BG, Stoffregen TA (2006) Motion sickness preceded by unstable displacements of the center of pressure. Hum Move Sci 25:800–820
Chang C-H, Pan W-W, Tseng L-Y, Stoffregen TA (2012) Postural activity and motion sickness during video game play in children and adults. Exp Brain Res 217:299–309
Chen F-C, Stoffregen TA (2012) Specificity of postural sway to the demands of a precision task at sea. J Exper Psych Appl 18:203–212
Chen Z, Ivanov PC, Hu K, Stanley HE (2002) Effect of nonstationarities on detrended fluctuation analysis. Phys Rev E Stat Nonlin Soft Matter Phys 65(4 (Pt. 1)):041107-1–041107-15
Chiari L, Rocchi L, Cappello A (2002) Stabilometric parameters are affected by anthropometry and foot placement. Clin Biomech 17:666–677
Davies A (2015) A bit of barf can teach us lots about self-driving cars. http://www.wired.com/2015/07/bit-barf-can-teach-us-lots-self-driving-cars/ Accessed July 28 2015
Dobie T, McBride D, Dobie T, May J (2001) The effects of age and sex on susceptibility to motion sickness. Aviat Space Environ Med 72:13–20
Dong X, Yoshida K, Stoffregen TA (2011) Control of a virtual vehicle influences postural activity and motion sickness. J Exp Psychol Appl 17:128–138
Era P, Sainio P, Koskinen S, Haavisto P, Vaara M, Aromaa A (2006) Postural balance in a random sample of 7,979 subjects aged 30 years and over. Gerontol 52:204–213
Faugloire E, Bardy BG, Stoffregen TA (2006) The dynamics of learning new postural patterns. J Motor Behav 38:299–312
Fessler DMT, Haley KJ, Lal RD (2005) Sexual dimorphism in foot length proportionate to stature. Ann Hum Biol 32:44–59
Flanagan MB, May JG, Dobie TG (2005) Sex differences in tolerance to visually-induced motion sickness. Aviat Space Environ Med 76:642–646
Gao J, Hu J, Tung W-W, Cao Y, Sarshar N, Roychowdhury VP (2006) Assessment of long-range correlation in time series: how to avoid pitfalls. Phys Rev E 73:016117
Gates AI (1961) Sex differences in reading ability. Elem Sch J 61:431–434
Giammarco EA, Schneider TJ, Carswell JJ, Knipe WS (2015) Video game preferences and their relation to career interests. Pers Individ Diff 73:98–104
Golding JF (2006) Motion sickness susceptibility. Autonom Neurosci Basic Clin 129:67–76
Golding JF, Kadzere P, Gresty MA (2005) Motion sickness susceptibility fluctuates through the menstrual cycle. Aviat Space Environ Med 76:970–973
Howarth HVC, Griffin MJ (2003) Effect of roll oscillation frequency on motion sickness. Aviat Space Environ Med 74:326–331
Kennedy RS, Lane NE, Berbaum KS, Lilienthal MG (1993) Simulator sickness questionnaire: an enhanced method for quantifying simulator sickness. Int J Aviat Psychol 3(3):203–220
Kennedy RS, Lanham DS, Massey CJ, Drexler JM, Lilienthal MG (1995) Gender differences in simulator sickness incidence: implications for military virtual reality systems. Safe J 25:69–76
Kim JW, Eom GM, Kim CS, Kim DH, Lee JH, Park BK, Hong J (2010) Sex differences in the postural sway characteristics of young and elderly subjects during quiet natural standing. Geriat Geront Int 10:191–198
Koslucher F, Wade MG, Nelson B, Lim K, Chen F-C, Stoffregen TA (2012) Nintendo Wii Balance Board is sensitive to effects of visual tasks on standing sway in healthy elderly adults. Gait Pos 36:605–608
Koslucher FC, Haaland E, Stoffregen TA (2014) Body load and the postural precursors of motion sickness. Gait Pos 39:606–610
Koslucher FC, Haaland E, Malsch A, Webeler J, Stoffregen TA (2015) Sex differences in the incidence of motion sickness induced by linear visual oscillation. Aviat Med Hum Perf 86:787–793
Lamb S, Kwok KCS, Walton D (2013) Occupant comfort in wind-excited tall buildings: motion sickness, compensatory behaviors, and complaint. J Wind Eng Ind Aerodyn 119:1–12
Lawther A, Griffin MJ (1988) A survey of the occurrence of motion sickness amongst passengers at sea. Aviat Space Environ Med 59:399–406
Lin D, Seol H, Nussbaum MA, Madigan ML (2008) Reliability of COP-based postural sway measures and age-related differences. Gait Posture 28:337–342
Meissner K, Enck P, Muth ER, Kellerman S, Klosterhalfen S (2009) Cortisol levels predict motion sickness tolerance in women but not in men. Physiol Behav 97:102–106
Merhi O, Faugloire E, Flanagan M, Stoffregen TA (2007) Motion sickness, console video games, and head mounted displays. Hum Fact 49:920–934
Miller JW, Goodson JE (1960) Motion sickness in a helicopter simulator. Aerospace Med 31:204–212
Oman CM (1982) A heuristic mathematical model for the dynamics of sensory conflict and motion sickness hearing in classical musicians. Acta Otolaryngol 94(Suppl 392):4–44
Park AH-Y, Hu S (1999) Gender differences in motion sickness history and susceptibility to optokinetic rotation-induced motion sickness. Aviat Space Environ Med 70:1077–1080
Parsons TD, Larson P, Kratz K, Thiebaux M, Bluestein B, Buckwalter JG, Rizzo AA (2004) Sex differences in mental rotation and spatial rotation in a virtual environment. Neuropsychologia 42:555–562
Reason JT (1978) Motion sickness adaptation: a neural mismatch model. J R Soc Med 71(11):819–829
Riccio GE, Stoffregen TA (1991) An ecological theory of motion sickness and postural instability. Ecol Psychol 3:195–240
Sivak M, Schoettle B (2015) Motion sickness in self-driving vehicles. UMTRI-2015-12. Ann Arbor: University of Michigan Transportation Research Institute
Smart LJ, Stoffregen TA, Bardy BG (2002) Visually-induced motion sickness predicted by postural instability. Hum Fact 44:451–465
Stoffregen TA, Smart LJ (1998) Postural instability precedes motion sickness. Brain Res Bull 47:437–448
Stoffregen TA, Smart LJ, Bardy BG, Pagulayan RJ (1999) Postural stabilization of looking. J Exper Psych Hum Perc Perf 25:1641–1658
Stoffregen TA, Hettinger LJ, Haas MW, Roe M, Smart LJ (2000a) Postural instability and motion sickness in a fixed-base flight simulator. Hum Fact 42:458–469
Stoffregen TA, Pagulayan RJ, Bardy BG, Hettinger LJ (2000b) Modulating postural control to facilitate visual performance. Hum Move Sci 19:203–220
Stoffregen TA, Hove P, Bardy BG, Riley MA, Bonnet CT (2007) Postural stabilization of perceptual but not cognitive performance. J Mot Beh 39:126–138
Stoffregen TA, Faugloire E, Yoshida K, Flanagan M, Merhi O (2008) Motion sickness and postural sway in console video games. Hum Fact 50:322–331
Stoffregen TA, Yoshida K, Villard S, Scibora L, Bardy BG (2010) Stance width influences postural stability and motion sickness. Ecol Psychol 22:169–191
Stoffregen TA, Villard S, Chen F-C, Yu Y (2011) Standing body sway on land and at sea. Ecol Psychol 23:19–36
Stoffregen TA, Chen F-C, Varlet M, Alcantara C, Bardy BG (2013) Getting your sea legs. PLOS One 8(6):e66949. doi:10.1371/journal.pone.0066949
Stoffregen TA, Chen Y-C, Koslucher FC (2014) Motion control, motion sickness, and the postural dynamics of mobile devices. Exp Brain Res 232:1389–1397. doi:10.1007/s00221-014-3859-3
Sullivan EV, Rose J, Rohlfing T, Pfefferbaum A (2009) Postural sway reduction in aging men and women: relation to brain structure, cognitive status, and stabilizing factors. Neurobiol Aging 30:793–807
The Guardian (2013) http://www.theguardian.com/technology/2013/sep/27/ios-7-motion-sickness-nausea. Accessed July 28 2015
Turner M, Griffin MJ (1999) Motion sickness in public road transport: the relative importance of motion, vision, and individual differences. Br J Psychol 90:519–530
Villard S, Flanagan MB, Albanese G, Stoffregen TA (2008) Postural instability and motion sickness in a virtual moving room. Hum Fact 50:332–345
Woollacott MH, Shumway-Cook A (2002) Attention and the control of posture and gait: a review of an emerging area of research. Gait Pos 16:1–14
Yu Y, Chung H-C, Hemingway L, Stoffregen TA (2013) Standing body sway in women with and without morning sickness in pregnancy. Gait and Posture 37:103–107
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koslucher, F., Haaland, E. & Stoffregen, T.A. Sex differences in visual performance and postural sway precede sex differences in visually induced motion sickness. Exp Brain Res 234, 313–322 (2016). https://doi.org/10.1007/s00221-015-4462-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-015-4462-y