Abstract
We exposed standing men and women to motion relative to the illuminated environment in a moving room. During room motion, we measured the kinematics of standing body sway. Participants were instructed to discontinue immediately if they experienced any symptoms of motion sickness, however mild. For this reason, our analysis of body sway included only movement before the onset of motion sickness. We analyzed the spatial magnitude of postural sway in terms of the positional variability and mean velocity of the center of pressure. We analyzed the multifractality of postural sway in terms of the width of the multifractal spectrum and the degree of multiplicativity of center of pressure positions. Results revealed that postural sway differed between participants who later reported motion sickness and those who did not, replicating previous effects. In a novel effect, postural responses to motion of the illuminated environment differed between women and men. In addition, we identified statistically significant interactions that involved both Sex and motion sickness status. Effects were observed separately in the spatial magnitude and multifractality of sway. The results were consistent with the postural instability theory of motion sickness (Riccio and Stoffregen in Ecol Psychol 3:195–240, 1991) and suggest that Sex differences in motion sickness may be related to Sex differences in the control and stabilization of bodily activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Susceptibility to motion sickness differs between the sexes. When asked to rate their generalized susceptibility to motion sickness, women give higher ratings than men (e.g., Flanagan et al. 2005; Paillard et al. 2013; Park and Hu 1999). Sex differences also exist in studies that have evaluated actual motion sickness in specific situations, including seasickness (Lawther and Griffin 1988), and motion sickness in terrestrial vehicles (Forstberg et al. 1999; Kennedy et al. 1995). Motion sickness is greater among women even in wind-blown tall buildings (Lamb et al. 2013). Typically, field research has indicated a Sex difference in the range of 5/3; that is, for every five women who report motion sickness in a particular setting, only three men report motion sickness. A nearly identical ratio obtains with the more objective measure of vomiting, which is more common among women (8.8 %) than among men (5.0 %) (Lawther and Griffin 1988).
The most widely accepted theory of the etiology of motion sickness centers on the concept of intersensory conflict. In general terms, it is argued that ordinary interactions with the environment give rise to internalized expectations about patterns of multisensory stimulation. In novel motion environments, patterns of multisensory stimulation also are novel, such that incoming multisensory patterns could differ from hypothetical internal models. These hypothetical differences are interpreted as intersensory conflict and are alleged to cause motion sickness (e.g., Reason 1978), provided that the magnitude of the differences exceeds a hypothetical internal threshold (Oman 1982). Given these ideas, advocates of the sensory conflict theory might claim that women have greater sensory conflict than men, or that the hypothetical threshold for motion sickness is lower in women than in men. To the best of our knowledge, no proponent of the sensory conflict theory has made either prediction. Rather, proponents of the sensory conflict theory have offered explanations that are ad hoc, such as the idea that increased female susceptibility might help to protect the fetus from toxins (e.g., Golding 2006).
Postural sway: sex differences
Men and women differ in unperturbed standing body sway. Era et al. (2006) measured unperturbed stance in 7979 adults and found that the speed of center of pressure displacements differed between women and men for each of five age groups. Several other studies have also found Sex differences in the spatial magnitude of postural sway (Chiari et al. 2002; Kim et al. 2010; Sullivan et al. 2009). There are also Sex differences in the temporal dynamics of sway (e.g., Kim et al. 2010).
Chiari et al. (2002) computed 55 stabilographic parameters from center of pressure data and found that men and women differed in several parameters of postural sway for sway in the AP Axis. They then compared the stabilometric parameters to several anthropometric parameters that differed significantly between women and men in their sample (height, weight, base of support, foot width, and the angle between the feet). The majority of stabilometric parameters were significantly correlated with one or more of these anthropometric factors. Chiari et al. concluded that most of the effects of Sex on standing body sway arose from biomechanical properties rather than from “neural control.”
Historically, the existence of Sex differences in the kinematics of postural sway has not been seen as having relevance to the existence of Sex differences in motion sickness. In the next section, we describe recent research that motivates consideration of the hypothesis that Sex differences in postural activity may be related to Sex differences in motion sickness.
Postural sway and motion sickness
It is not surprising if people who are suffering from motion sickness have degraded stance and locomotion, and degraded postural control that follows motion sickness has no implications for motion sickness etiology. More surprising is the finding that such differences in postural control exist before the onset of any of the subjective symptoms of motion sickness. This effect has been observed during stance in laboratory devices featuring linear (translational) oscillations (Koslucher et al. 2014; Stoffregen and Smart 1998; Stoffregen et al. 2010), for seated subjects exposed to oscillations rotating around the line of sight (Stoffregen et al. 2000), for virtual environments (Villard et al. 2008), during the playing of video games while seated (e.g., Dong et al. 2011; Stoffregen et al. 2014), and in relation to seasickness (Stoffregen et al. 2013). Effects have been observed in the spatial magnitude of sway (e.g., Smart et al. 2002; Stoffregen and Smart 1998; Stoffregen et al. 2000; Villard et al. 2008), but also in the temporal dynamics of sway (Stoffregen et al. 2010, 2013; Villard et al. 2008). These effects confirm a prediction of the postural instability theory of motion sickness (Riccio and Stoffregen 1991).
The existence of Sex differences in motion sickness susceptibility (e.g., Lawther and Griffin 1988), together with the existence of Sex differences in postural sway (e.g., Chiari et al. 2002), and the finding that postural sway differs between persons who experience motion sickness and those who do not (e.g., Stoffregen et al. 2010) suggest that Sex differences in postural sway might precede Sex differences in motion sickness. This prediction is not ad hoc; rather, it follows directly from the postural instability theory of motion sickness (Riccio and Stoffregen 1991). Such an effect might occur in at least two ways.
First, individual differences in postural sway that were related to both Sex and motion sickness might exist in patterns of unperturbed body sway, before individuals were exposed to potentially nauseogenic motion stimuli. Such an effect would be an extension to the domain of Sex differences of results from several previous studies (e.g., Koslucher et al. 2014; Stoffregen et al. 2010; 2013; Stoffregen and Smart 1998). Koslucher et al. (2016) evaluated relations between Sex and motion sickness in the context of postural sway measured before participants were exposed to potentially nauseogenic visual motion stimuli. Unperturbed standing body sway was measured while participants performed visual tasks that made differing demands on the precision of gaze (reading vs. looking at a blank target). The results revealed a complex interaction between Sex, visual tasks, and participants’ reports of motion sickness when they were subsequently exposed to linear visual oscillation in a moving room. This interaction was consistent with the hypothesis that Sex differences in unperturbed standing body sway might be related to Sex differences in visually induced motion sickness.
Separately, individual differences in postural sway that were related to both Sex and motion sickness might exist during exposure to potentially nauseogenic motion stimuli, but before the onset of motion sickness. This would be an extension to the domain of Sex differences of previous studies (e.g., Koslucher et al. 2014; Stoffregen et al. 2010; Stoffregen and Smart 1998; Villard et al. 2008). In the present study, our focus was on relations between Sex and susceptibility in postural sway measured during exposure to potentially nauseogenic linear visual oscillation in a moving room.
The present study was part of a larger project that included the studies of Koslucher et al. (2015, 2016). Our overall goal was to evaluate relations between motion sickness and Sex in the context of motion relative to the illuminated environment. In this larger project, a single sample of participants was exposed to linear visual oscillation in a moving room. Koslucher et al. (2015) reported data on Sex differences in the incidence and severity of motion sickness, together with relations between motion sickness, Sex, and anthropometric measures. In that study, motion sickness incidence was a dependent variable. Koslucher et al. (2016) reported data on the kinematics of standing body sway before participants were exposed to potentially nauseogenic visual motion. In that study, motion sickness incidence was an independent variable in analyses of the kinematics of standing body sway. The present study comprises the third segment of the overall study: We report data on postural sway during exposure to potentially nauseogenic visual motion. As in Koslucher et al. (2016), in this article the incidence of motion sickness was an independent variable. Because data on motion sickness incidence and severity were reported by Koslucher et al. (2015), in the present study we report those data only to support our use of them as an independent variable in our analyses of postural activity.
Postural responses to motion of the illuminated environment
Many researchers study unperturbed postural sway. Another paradigm examines postural responses to imposed motion. Imposed motion can be inertial, as in moving platform posturography (e.g., Nashner and McCollum 1985). Imposed motion can also be optical, in what is known as the moving room paradigm (Dijkstra et al. 1994). People often sway when exposed to global optic flow, whether it is produced by physical displacement of the illuminated environment, as in a moving room (e.g., Lee and Lishman 1975; Stoffregen 1985) or by virtual displacement of a simulated environment (e.g., Dijkstra et al. 1994; Stoffregen et al. 2004). We are not aware of any previous research that has evaluated the possible existence of Sex differences in postural responses to motion of the illuminated environment. Given that unperturbed sway varies as a function of Sex (Era et al. 2006; Kim et al. 2010; Sullivan et al. 2009), we might expect to find Sex differences in postural responses to perturbation of the illuminated environment. In the present study, we predicted that there would be Sex differences in postural responses to motion of the illuminated environment.
Multifractality as a measure of postural stability
Traditionally, scientists have evaluated postural activity in terms of the spatial magnitude of movement. Typically, “more sway” has been interpreted to indicate “less stability” (e.g., Goldie et al. 1989; Lin et al. 2008). Recent decades have brought a surge of interest in the temporal structure of animate movement, that is, the ways in which patterns of movement vary in time. The temporal structure of movement differs qualitatively from its spatial structure, such that one type of measure cannot be substituted for the other. Put another way, spatial and temporal measures provide qualitatively different information about movement. Many scientists now examine the temporal structure of postural activity. One aspect of temporal structure is its fractality, the tendency of temporal patterns to be nested in time, that is, the tendency for similar patterns to occur across different time scales. For example, a given movement pattern might occur rapidly (e.g., over a few milliseconds), and very slowly (e.g., over a few minutes), while simultaneously occurring over some intermediate time scale (e.g., over a few seconds). It might be assumed that the fractality of a system is fixed. However, research has revealed that the temporal nesting of movement patterns can change over time within a system. Changes in fractality fall under the rubric of multifractality (e.g., Ihlen and Vereijken 2013; Kelty-Stephen et al. 2013). Multifractality has been identified in standing body sway (e.g., Ihlen et al. 2013; Thurner et al. 2000).
Multifractality may have special relevance to relations between postural activity and motion sickness. Ihlen and Vereijken (2013) argued that individual differences in multifractality of movement can be used to distinguish health from disease. Shimizu et al. (2002) evaluated unperturbed standing body sway in healthy adults and in patients suffering from cirrhosis of the liver, which can affect postural control. They found that these groups differed in the degree of multifractality of postural sway, consistent with Ihlen and Vereijken’s suggestion that multifractality might serve as an indicator of health disorders. If motion sickness is understood as a clinical condition (albeit temporary), then we might expect postural precursors of motion sickness to include atypical patterns of multifractality in postural sway. Riccio and Stoffregen (1991) explicitly predicted that postural instability relating to motion sickness would be observed in temporal metrics. Multifractal analysis did not exist in 1991; however, their prediction can be evaluated in this context. Stoffregen (2011) argued that motion sickness is a type of movement disorder, that is, that the subjective symptoms of motion sickness may arise from a (transient) disorder in the control of posture. These arguments motivate a prediction that the multifractality of postural sway will differ between individuals who become motion sick and those who do not.
The present study
In the present study, standing participants were exposed to linear oscillation of the illuminated environment, and we recorded data on body sway during this exposure. The linear oscillation stimulus induced motion sickness in some participants but not in others, as reported by Koslucher et al. (2015). In the present study, our principal aim was to evaluate postural activity during exposure to linear oscillation of the illuminated environment, and to compare these data between the sexes and between participants who reported motion sickness and those who did not. Using a standardized methodology, we instructed participants to discontinue participation immediately if they experienced any symptoms of motion sickness, however mild. For this reason, the postural activity that we analyzed was measured before the onset of subjective symptoms and, therefore, the data are meaningfully related to the hypothesis that postural sway should differ between persons who experience motion sickness and those who do not, and that such differences should exist before the onset of subjective symptoms of motion sickness (Riccio and Stoffregen 1991). This study was quasi-experimental in the sense that Sex and motion sickness were not determined via random assignment. The dependent variables were data on body sway.
Methods
Participants
The subjects were 114 individuals who participated in exchange for course credit. There were 45 men (mean age 22.81 years, SD 3.43 years) and 69 women (mean age 21.78 years, SD 2.23 years). The experimental protocol was approved in advance by the University of Minnesota IRB. Additional data on anthropometrics of the sample were reported by Koslucher et al. (2015).
Apparatus
Participants stood on a force plate (AccuSway Plus; AMTI, Watertown, MA). We recorded the position of the center of pressure in the anteroposterior (AP) and mediolateral (ML) axes at 50 Hz.
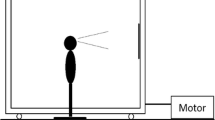
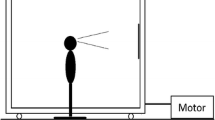
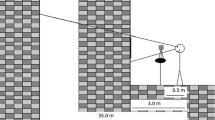
Visual stimulus motion was created using a moving room, which consisted of a cubical frame, 2.44 m on a side, mounted on wheels and moved along one Axis on rails (Fig. 1). The interior surfaces of the walls and ceiling were covered with blue and white marble-patterned adhesive paper. At the center of the front wall was a map of the continental USA (53 cm × 80 cm; 19° × 28°). Illumination was provided by floodlights mounted inside the room and oriented so that shadows were minimized. Movement of the room (oscillation along an Axis parallel to the line of sight) was powered by an electric motor under computer control.
Procedure
Following the informed consent procedure participants removed their shoes and responded to a forced-choice, yes/no question, Are you motion sick? They also completed the Simulator Sickness Questionnaire, or SSQ, which yields intensity ratings for 16 different symptoms (Kennedy et al. 1993). Participants were instructed (both verbally and on the consent form) that they might experience motion sickness and that if they experienced any symptoms of motion sickness, however mild, then they should discontinue the experiment immediately. Participants stood with their feet on marked lines so that heels were 17 cm apart and the angle between the feet was 10° (Stoffregen et al. 2010). They were instructed to keep their arms by their sides and to avoid talking or deliberate movement during trials.
Before being exposed to the visual motion stimulus, we measured unperturbed stance during performance of simple visual tasks. These data have been reported elsewhere (Koslucher et al. 2016). After these trials, participants were exposed to visible motion of the moving room. There was not a single fixation point; participants were asked to keep their gaze on the map on the front wall.
Room motion was a sum of ten sines in the range 0.1–0.4 Hz, with maximum displacement amplitude of 2.5 cm (Fig. 2). Room motion was identical to that used by Bonnet et al. (2006) and Stoffregen et al. (2010), as was the protocol used during moving room trials. Each trial with room motion was 10 min in duration, and subjects were exposed to a maximum of four trials. Data on postural activity were recorded continuously for each trial.
Before each trial, subjects were reminded to discontinue participation immediately if they experienced any symptoms of motion sickness, however mild. Between trials, the moving room was stationary and the subject was required to sit for 1 min. Upon discontinuation or after the completion of the experimental protocol (whichever came first), subjects again answered the forced-choice, yes/no question, Are you motion sick? Subjects who stated that they were not sick after exposure to room motion were given a printed copy of the SSQ and the forced-choice, yes/no question and asked to fill it out at the time of symptom onset or after 24 h if no symptoms developed. Several studies have reported that the onset of motion sickness can follow exposure by up to 12 h (Miller and Goodson 1960).
Motion sickness incidence was based on a dichotomous classification that was derived solely from answers to the forced-choice, yes/no question, Are you motion sick? Participants who answered this question in the affirmative immediately after exposure to room motion or within 24 h of their participation in the experiment were assigned to the Sick Group. All other participants were assigned to the Well Group. Data on the severity of symptoms, as obtained using the SSQ, were used solely to compare symptom levels in the Well and Sick groups (for details, see Koslucher et al. 2015).
The data on postural activity reported in this article were collected when the room was in motion. We also collected data on postural sway when the room was stationary; these data have been reported elsewhere (Koslucher et al. 2016).
Data analysis
We evaluated the evolution of sway over the course of exposure to the sum-of-sines stimulus. To evaluate the evolution of sway over time, we selected three 2-min Time Windows from the data, using the method of Bonnet et al. (Bonnet et al. 2006; Koslucher et al. 2014; Stoffregen et al. 2010). Due to discontinuation, participants in the Sick and Well groups did not have the same duration of exposure to the sum-of-sines stimulus. We judged it to be important to ensure that the windows for the Sick and Well groups represented similar exposure durations. To ensure this, we tied the selection of windows for the Well Group to the mean exposure duration of the Sick Group. For the Sick Group, we choose the first, the middle, and the final 2 min for each participant, with the restriction that no window included a boundary between two trials (i.e., each window included only continuous data from within a single trial). For the Well Group, windows were selected based on the mean exposure of participants in the Sick Group. This selection ensured that the sway data for both groups corresponded to the same mean duration of exposure to the sum-of-sines stimulus.
We evaluated four aspects of postural activity. We evaluated the spatial magnitude of postural activity using two different measures. We operationalized spatial magnitude in terms of the positional variability of the center of pressure (i.e., the standard deviation of the position of the center of pressure). Separately, we operationalized spatial magnitude in terms of the mean velocity of the center of pressure.
To examine the temporal dynamics of postural activity, we evaluated the multifractality of postural activity, which we operationalized in two ways. Multifractality is not a dichotomous feature of a time series. It exists on a continuum: A given time series can exhibit more or less multifractality. To quantify the degree of multifractality in a time series, we examined the width of the multifractal spectrum, using multifractal detrended fluctuation analysis (Ihlen et al. 2013; cf. Kantelhardt et al. 2002). The wider the multifractal spectrum, the more multifractal the time series (Kelty-Stephen et al. 2013). To evaluate spectrum width, we used the range of the singularity exponent, h(q), which was calculated with the open source code MFDFA1 (Ihlen 2012), set to a minimum scale of q = −10, increasing incrementally by 1 to q = 10, and a polynomial trend fit set to 3. We selected a minimum scaling range of 16 data points with 19 evenly spaced increasing segment sizes to a maximum of the length of the time series. This range was the same for each time series. We computed spectrum width for each time series and conducted inferential statistics on these values. While spectrum width is a useful measure of the overall degree of multifractality, it is not sensitive to certain details of multifractal systems. One such detail is the degree to which the interaction between fractal dimensions is multiplicative, rather than additive. Multiplicativity can be evaluated using an iterated amplitude-adjusted Fourier transform (IAAFT) surrogate analysis (Ihlen et al. 2013). We used the IAAFT to differentiate multifractal processes that exhibited multiplicative interactions from those that did not.
We evaluated positional variability, velocity, and the width of the multifractal spectrum using separate 3 (Time Windows) × 2 (Axis) × 2 (Sex) × 2 (Sickness Groups) ANOVAs, with repeated measures on the Time Windows factor. Time Windows and Axis were within-participants factors, while Sex and Sickness Groups were between-participants factors. Use of the range of h(q) to estimate spectrum width is susceptible to outliers (Ihlen 2012; cf. Kelty-Stephen et al. 2013). For this reason, before conducting ANOVA on spectrum width we removed data from participants for which spectrum width was greater than three standard deviations from the overall mean (across trials and participants). For each ANOVA, we estimated effect sizes using the partial η2 statistic.
We generated 30 surrogate time series using the IAAFT. We then compared the spectrum width of the original time series to the distribution of surrogate spectrum widths (Palatinus et al. 2013). We evaluated the percentage of time series that differed significantly from the surrogates. Using t tests, we compared these percentages between axes (AP vs. ML), between Sexes (male vs. female, separately for AP and ML), and between Sickness Groups (well vs. sick, separately for AP and ML).
Results
As reported by Koslucher et al. (2015), the incidence of motion sickness was significantly greater among women (38 %; 26/69) than among men (9 %; 4/45). Also as reported by Koslucher et al., at post-exposure symptom severity was greater in the Sick Group than in the Well Group.
For positional variability, we found a significant effect of Axis, F(1,109) = 75.00, p < 0.001, partial η 2 = 0.41. Positional variability was greater in the AP Axis (mean = 0.61, SD = 0.40) than in the ML Axis (mean = 0.31, SD = 0.26). The main effect of Time Windows also was significant, F(2,167.56) = 6.589, p = 0.004, partial η 2 = 0.06 (Fig. 3a).
For the mean velocity of the center of pressure, the main effect of Axis was significant, F(1,178) = 233.05, p < .001, partial η 2 = 0.72. The mean velocity of the center of pressure was greater in the AP Axis (mean = 1.24, SD = 0.54) than in the ML Axis (mean = 0.647, SD = 0.30). In addition, we found a significant main effect of Time Windows, F(2,178) = 3.805, p = 0.024, partial η 2 = 0.04, which is illustrated in Fig. 3b. The interaction between Axis and Sex was significant, F(1,178) = 6.76, p = .01, partial η 2 = 0.07 (Fig. 4a), as was the interaction between Axis and Sickness Groups, F(1,178) = 8.27, p = .005, partial η2 = 0.09 (Fig. 4b). There was a significant three-way interaction between Time Windows, Sex, and Sickness Groups F(2,178) = 3.88, p = .022, partial η 2 = 0.04, which is illustrated in Fig. 5. Finally, there was a significant four-way interaction between Time Windows, Axis, Sex, and Sickness Groups, F(2,178) = 3.39, p = .036, partial η 2 = 0.04 (Fig. 6).
For the spectrum width of MF-DFA, the main effect of Time Windows was significant, F(1.96,213.76) = 5.81, p = .003, partial η 2 = 0.052 (Fig. 3c). The main effect of Axis was significant, F(1,109) = 12.81, p < .001, partial η 2 = 0.11. Spectrum width was greater in the AP Axis (mean = 0.65, SE = 0.01) than in the ML Axis (mean = 0.62, SE = 0.01). The main effect of Sickness Groups was also significant, F(1,109) = 8.79, p = 0.004, partial η 2 = 0.08. Spectrum width was greater for the Sick Group (mean = 0.67, SD = 0.02) than for the Well Group (mean = 0.61, SD = 0.01). The Time Windows × Axis interaction was significant, F(2,218) = 5.86, p = .003, partial η 2 = 0.05 (Fig. 7). Finally, there was a significant Time Windows × Sickness Groups interaction, F(1.96,213.76) = 4.23, p = 0.02, partial η 2 = 0.04 (Fig. 8).
For the IAAFT surrogate analysis, the percentage of trials that differed from surrogates was greater for postural activity in the AP Axis (97.94 %; 332 of 339) than in the ML Axis (86.73 %; 294 of 339), t = −5.504, p < 0.001, indicating that on the same trials the degree of multifractality tended to be greater for movements in the AP Axis than for movements in the ML Axis. We found no differences between women and men, or between Well and Sick, each p > .05.
Discussion
Standing participants were exposed to linear oscillation of the illuminated environment in a moving room. Participants were instructed to discontinue the protocol immediately if they experienced any symptoms of motion sickness, however mild. Accordingly, we evaluated the kinematics of body sway during room motion but before the experience of subjective symptoms of motion sickness. Replicating previously reported effects, we found that postural sway differed between body axes (Koslucher et al. 2014), that it changed over time (Bonnet et al. 2006; Koslucher et al.; Stoffregen et al. 2010), and that it differed between participants who later reported motion sickness and those who did not (Bonnet et al.; Koslucher et al.; Stoffregen et al.). In a novel effect, we found that patterns of sway that preceded motion sickness differed between women and men.
Axis effects
Postural activity differed between the body’s AP and ML axes. In our analyses of the spatial magnitude of sway, we found main effects of Axis for the positional variability and velocity of the center of pressure. These differences are consistent with previous studies in which standing participants were exposed to oscillatory motion of the illuminated environment in the body’s AP Axis (e.g., Koslucher et al. 2014). In our analyses of the fractality of sway, we found main effects of Axis for the width of the multifractal spectrum, which was greater for postural activity in the AP Axis. Separately, the degree of multiplicativity was greater for postural activity in the AP Axis than for postural activity in the ML Axis.
The most likely explanation for these Axis effects relates to the motion stimulus that we used: The room oscillated along the line of sight, which in our study was aligned with the body’s AP Axis. It makes sense that postural responses to stimulus motion would be greatest in the Axis of stimulation. The relation between the line of sight and the body’s AP Axis could be reversed, for example, by rotating the head (i.e., instructing participants to turn their heads to look at the side wall), or by rotating the body (i.e., turning the body to the side while the head continued to face the front wall; Stoffregen 1985).
Temporal evolution of postural activity
Postural activity changed over time during exposure to room motion. The main effect of Time Windows was statistically significant for the positional variability (Fig. 3a) and velocity (Fig. 3b) of the center of pressure. These effects are consistent with previous studies of postural responses to motion of the illuminated environment (e.g., Smart et al. 2002). In addition, the main effect of Time Windows was statistically significant for the width of the multifractal spectrum (Fig. 3c). This effect is novel and reveals that multifractality is plastic with respect to continuous changes in visual motion stimuli (cf. Palatinus et al. 2013). These main effects demonstrate the powerful influence of linear visual oscillations on postural activity, regardless of body Axis, Sex, or subsequent motion sickness.
For the width of the multifractal spectrum, we also found a significant interaction between Axes and Time Windows (Fig. 7). Interestingly, for postural activity in the AP Axis spectrum width did not change over time. Rather, change over time was observed solely in the ML Axis. Thus, room motion, which oscillated along the body’s AP Axis, was associated with gradual change in multifractality in the body’s ML Axis. Over time, the spectrum width in the ML Axis gradually became identical with the spectrum width in the AP Axis. It may be that visual motion established a stable pattern of multifractality in the body’s AP Axis and that this pattern served as an attractor for sway in the body’s ML Axis.
Effects relating to Sex
Ours may be the first study to investigate possible Sex differences in body sway using the perturbation paradigm. For this reason, generalized Sex differences are of interest. For the mean velocity of the center of pressure, women and men differed by sway Axis, independent of the incidence of motion sickness (Fig. 4a). This is a novel effect, and it suggests that Sex differences in postural activity are not limited to the control of unperturbed stance, but exist also in postural responses to motion of the illuminated environment. Relative to men, women may be less likely to couple postural sway to motion of the illuminated environment. It may be that this Sex difference is related to Sex differences in field dependence/independence, such as those commonly reported in the rod and frame test (e.g., Voyer et al. 1995) or other aspects of spatial processing (e.g., Giammarco et al. 2015; Kimura 1997). However, the effect may also be related to Sex differences in anthropometrics that correlate with Sex differences in unperturbed postural sway (e.g., Chiari et al. 2002; Kim et al. 2010; Sullivan et al. 2009). In future research, it will be important to develop methods that make it possible to differentiate effects on postural activity of physical differences between the sexes (e.g., anthropometrics) from effects of cognitive or psychological differences between the sexes.
Postural precursors of motion sickness: magnitude
Consistent with previous research (Faugloire et al. 2007; Smart et al. 2002; Stoffregen and Smart 1998), during exposure to room motion the velocity of center of pressure displacements differed between participants who later reported motion sickness and those who did not. However, the main effect of motion Sickness Groups was not significant: Rather, Sickness Groups interacted with other factors.
A statistically significant interaction between Sickness Groups and Axis (Fig. 4b) revealed that in the AP Axis mean velocity was greater among participants who later became sick. Of greater relevance to the focus of the present study, postural sway preceding motion sickness differed between women and men. During exposure to room motion, the velocity of the center of pressure varied as a function of Sex and subsequent motion sickness. That is, postural responses to the moving room differed between participants who later became sick and those who did not, but these differences varied as a function of Sex. The interaction between Sickness Groups and Sex was embedded in a statistically significant three-way interaction that included Time Windows (Fig. 5). That interaction, in turn, was embedded within a statistically significant four-way interaction involving Time Windows, Sex, Sickness Groups, and Axis (Fig. 6). Using the same participants, Koslucher et al. (2015) found that women and men were differentially susceptible to motion sickness arising from linear visual oscillation (with greater susceptibility among women). Koslucher et al. (2016) evaluated postural sway prior to room motion and found that the same men and women swayed differently in ways that were related to subsequent motion sickness. Taken together, the three studies demonstrate that Sex differences exist in susceptibility to motion sickness induced by linear visual oscillation and that they are related to Sex differences in postural activity both before and during exposure to the visual motion stimulus.
Postural precursors of motion sickness: multifractality
In a main effect, the width of the multifractal spectrum was greater for participants who reported motion sickness than for those who did not. The main effect was embedded within a significant interaction between Sickness Groups and Time Windows. Figure 8 suggests that for the Well Group spectrum width was stable over time, whereas spectrum width gradually increased over time for the Sick Group. These effects are novel and constitute the first demonstration that changes in the fractality of postural activity precede the subjective symptoms of motion sickness.
The postural instability theory predicts that the postural sway of well and sick individuals will differ before the onset of subjective symptoms. Riccio and Stoffregen (1991) were explicit in stating that differences need not be confined to measures of the spatial magnitude of sway. Previous research using detrended fluctuation analysis has shown differences in the temporal dynamics of sway between participants in the well and Sick groups (e.g., Stoffregen et al. 2010, 2013; Villard et al. 2008). The results of the present study extend this finding to the multifractality of sway.
The analysis of multifractality in human movement is relatively novel, and researchers have stated it is not entirely clear how variations in multifractality should be interpreted (Palatinus et al. 2013). The postural instability theory (Riccio and Stoffregen 1991) can help us to interpret multifractality in postural sway. Postural instability theory dictates that patterns of sway among participants who will become sick are less stable; hence, the larger value of spectrum width in the Sick Group corresponds to lower stability. This result can help us to interpret patterns of multifractality in other settings. For example, Munafo et al. (2016) found that spectrum width was greater among older adults than among younger adults. It is widely assumed that postural sway is less stable among older adults than among younger adults. Accordingly, results of the present study are consistent with the findings of Munafo et al.
The IAAFT revealed no statistically significant differences relating to motion sickness. That is, we found no evidence that subsequent motion sickness was related to the degree of multiplicativity of postural sway.
Linear versus angular motion
Sex differences in motion sickness appear to be related to the axes in which motion stimuli are presented. When seated participants have been exposed to rotational motion stimuli, Sex differences have been small or absent, for both gravitoinertial rotation (Klosterhalfen et al. 2005) and optical rotation (e.g., Klosterhalfen et al. 2006). Sex differences are stronger when imposed motion includes linear components. As reported by Koslucher et al. (2015), the participants in the present study exhibited a Sex difference of more than 4:1 when exposed standing participants to linear visual oscillation. Similarly, Lawther and Griffin (1986) found that seasickness was most strongly related to linear components of ship motion.
The results of the present study, together with those of Koslucher et al. (2015, 2016), are compatible with the idea that Sex differences in motion sickness are related to the control of posture. During stance, postural activity comprises rotations primarily around the ankles and hips: Rotation around the body’s vertical Axis is exiguous (the same is true for seated posture). That is, physical (and, presumably, psychological) differences between the sexes do not appear to lead to Sex differences in rotation around the body’s vertical Axis. Differences between well and sick participants have been observed in seated postural sway (e.g., Chen et al. 2012; Dong et al. 2011; Merhi et al. 2007; Stoffregen et al. 2000, 2008, 2014), but in these studies Sex was not a factor in data analysis. This is an important area for future research on seated posture.
Implications for theory
The sensory conflict theory asserts that motion sickness arises from conflict between current patterns of intersensory stimulation and those expected on the basis of past experience, as modulated by an internal threshold (e.g., Oman 1982). Within this theoretical framework, the existence of robust Sex differences in motion sickness, across venues (e.g., visually induced motion sickness vs. seasickness), across response measures (e.g., self-reports of generalized susceptibility vs. motion sickness induced in laboratory settings), and across subjective and objective measures (e.g., symptom ratings vs. vomiting) would appear to imply the existence of reliable differences between the sexes in the generation of hypothetical intersensory conflict, in the threshold for the triggering of symptoms, or both. We are not aware of any published arguments to this effect; that is, the sensory conflict theory does not appear to offer a basis to understand or predict that susceptibility to motion sickness should be affected by Sex. As noted in Introduction, in the literature on motion sickness explanations for the Sex difference typically have been ad hoc (Golding 2006).
The postural instability theory of motion sickness (Riccio and Stoffregen 1991) predicts that postural activity should differ between individuals who experience motion sickness and those who do not and that such differences should exist (and be measurable) before the onset of any subjective symptoms of motion sickness. The theory implies that individual differences in postural activity may be related to individual differences in susceptibility to motion sickness. Women and men reliably differ in the quantitative kinematics of standing body sway. Chiari et al. (2002) argued that Sex differences in standing body sway arise principally from sexual dimorphism, and not from Sex differences in information processing or neural strategies. Accordingly, relations between Sex differences in body sway and Sex differences in motion sickness are meaningfully related to the postural instability theory of motion sickness, rather than being ad hoc.
Conclusion
The present study reports the final dataset from a larger investigation of Sex differences in visually induced motion sickness. Koslucher et al. (2015) exposed women and men to oscillation of the illuminated environment in a moving room. They found that the incidence of motion sickness was greater among women (39 %) than among men (9 %). In addition, Sex differences in motion sickness incidence were significantly correlated with sexual dimorphism, including Sex differences in overall height, in the height of the body’s center of mass, and in foot length. Using the same participants, Koslucher et al. (2016) examined unperturbed postural sway before exposure to room motion. They found that Sex differences in the incidence of visually induced motion sickness were preceded by differences in the kinematics of unperturbed body sway, in a complex interaction comprising Sex, Sickness Groups, and visual tasks performed during unperturbed stance. That is, motion sickness was related to Sex differences in body sway that existed before participants were exposed to any experimental motion stimuli. This effect was limited to the positional variability of the center of pressure, that is, to a measure of the spatial magnitude of postural activity.
In the present study, we evaluated the kinematics of standing body sway as the same participants were exposed to linear oscillation of the illuminated environment in a moving room. Participants were instructed to discontinue participation immediately if they experienced subjective symptoms of motion sickness, however mild. Accordingly, we analyzed postural activity before the onset of subjective symptoms of motion sickness. The mean velocity of the center of pressure differed between participants who later became motion sick and those who did not, replicating previous findings (Smart et al. 2002; Stoffregen and Smart 1998). Separately, men and women differed in their postural responses to the visual motion stimulus, a novel finding in research relating postural control to optic flow (cf. Lee and Lishman 1975). In addition, statistically significant interactions revealed that patterns of mean velocity that preceded motion sickness differed by Sex.
Our analysis of movement dynamics revealed that the multifractality of postural activity differed between participants who later became motion sick and those who did not and that this difference evolved over time during exposure to visual motion stimuli. These were novel findings, for the first time revealing that individual differences in the multifractality of movement may be related to the subjective experience of motion sickness.
The results of the present study, together with those of Koslucher et al. (2015, 2016), are consistent with the following hypotheses: (1) Sex differences exist in susceptibility to visually induced motion sickness, (2) Sex differences in susceptibility are related to Sex differences in anthropometric measures that are, themselves, related to the kinematics of body sway, and (3) Sex differences in susceptibility to motion sickness have their basis in Sex differences in the control of body posture, both before and during exposure to potentially nauseogenic visual motion stimuli. These results suggest that it may be possible to explain Sex differences in susceptibility to motion sickness within the framework of the postural instability theory of motion sickness (Riccio and Stoffregen 1991).
References
Bonnet CT, Faugloire EM, Riley MA, Bardy BG, Stoffregen TA (2006) Motion sickness preceded by unstable displacements of the center of pressure. Hum Move Sci 25:800–820
Chen Y-C, Dong X, Chen F-C, Stoffregen TA (2012) Control of a virtual avatar influences postural activity and motion sickness. Ecol Psychol 24:279–299
Chiari L, Rocchi L, Cappello A (2002) Stabilometric parameters are affected by anthropometry and foot placement. Clin Biomech 17:666–677
Dijkstra TMH, Schöner G, Gielen CCAM (1994) Temporal stability of the action-perception cycle for postural control in a moving visual environment. Exper Brain Res 97:477–486
Dong X, Yoshida K, Stoffregen TA (2011) Control of a virtual vehicle influences postural activity and motion sickness. J Exper Psychol Appl 17:128–138
Era P, Sainio P, Koskinen S, Haavisto P, Vaara M, Aromaa A (2006) Postural balance in a random sample of 7,979 subjects aged 30 years and over. Gerontol 52:204–213
Faugloire E, Bonnet CT, Riley MA, Bardy BG, Stoffregen TA (2007) Motion sickness, body movement, and claustrophobia during passive restraint. Exper Brain Res 177:520–532
Flanagan MB, May JG, Dobie TG (2005) Sex differences in tolerance to visually-induced motion sickness. Aviat Space Environ Med 76:642–646
Forstberg J, Andersson E, Ledin T (1999) Influence of different conditions for tilt compensation on symptoms of motion sickness in tilting trains. Brain Res Bull 47:525–535
Giammarco EA, Schneider TJ, Carswell JJ, Knipe WS (2015) Video game preferences and their relation to career interests. Pers Individ Diff 73:98–104
Goldie PA, Bach TM, Evans OM (1989) Force platform measures for evaluating postural control: reliability and validity. Arch Phys Med Rehab 70:510–517
Golding JF (2006) Motion sickness susceptibility. Autonom Neurosci Basic Clin 129:67–76
Ihlen EA (2012) Introduction to multifractal detrended fluctuation analysis in Matlab. Front Physiol 3:141. doi:10.3389/fhys.201200141
Ihlen EA, Vereijken B (2013) Multifractal formalisms of human behavior. Hum Move Sci 32:633–651. doi:10.1016/j.humov.2013.01.008
Ihlen EA, Skjaeret N, Vereijken B (2013) The influence of center-of-mass movements on the variation in the structure of human postural sway. J Biomech 46:484–490
Kantelhardt JW, Zschiegner SA, Doscielny-Bunde E, Havlin S, Bunde A, Stanley HE (2002) Multifractal detrended fluctuation analysis of nonstationary time series. Phys A 316:87–114
Kelty-Stephen DG, Palatinus K, Saltzman E, Dixon JA (2013) A tutorial on multifractality, cascades, and interactivity for empirical times series in ecological science. Ecol Psychol 25:1–62
Kennedy RS, Lane NE, Berbaum KS, Lilienthal MG (1993) Simulator sickness questionnaire: an enhanced method for quantifying simulator sickness. Int J Aviat Psychol 3:203–220
Kennedy RS, Lanham DS, Massey CJ, Drexler JM, Lilienthal MG (1995) Gender differences in simulator sickness incidence: implications for military virtual reality systems. SAFE J 25:69–76
Kim JW, Eom GM, Kim CS, Kim DH, Lee JH, Park BK, Hong J (2010) Sex differences in the postural sway characteristics of young and elderly subjects during quiet natural standing. Geriat Geront Int 10:191–198
Kimura D (1997) Sex, sexual orientation and Sex hormones influence human cognitive functions. Biomed Rev 7:33–39
Klosterhalfen S, Kellerman S, Pan F, Stockhorst U, Hall G, Enck P (2005) Effects of ethnicity and gender on motion sickness susceptibility. Aviat Space Environ Med 76:1051–1057
Klosterhalfen S, Pan F, Kellerman S, Enck P (2006) Gender and race as determinants of nausea induced by circular vection. Gender Med 3:236–242
Koslucher FC, Haaland E, Stoffregen TA (2014) Body load and the postural precursors of motion sickness. Gait Pos 39:606–610
Koslucher FC, Haaland E, Malsch A, Webeler J, Stoffregen TA (2015) Sex differences in the incidence of motion sickness induced by linear visual oscillation. Aviat Med Hum Perform 86:787–793
Koslucher FC, Haaland E, Stoffregen TA (2016) Sex differences in visual performance and postural sway precede Sex differences in visually induced motion sickness. Exper Brain Res 234:313–322
Lamb S, Kwok KCS, Walton D (2013) Occupant comfort in wind-excited tall buildings: motion sickness, compensatory behaviors, and complaint. J Wind Eng Indus Aerodyn 119:1–12
Lawther A, Griffin MJ (1986) The motion of a ship at sea and the consequent motion sickness amongst passengers. Ergon 29:535–552
Lawther A, Griffin MJ (1988) A survey of the occurrence of motion sickness amongst passengers at sea. Aviat Space Environ Med 59:399–406
Lee DN, Lishman JR (1975) Visual proprioceptive control of stance. J Hum Mov Stud 1:87–95
Lin D, Seol H, Nussbaum MA, Madigan ML (2008) Reliability of COP-based postural sway measures and age-related differences. Gait Pos 28:337–342
Merhi O, Faugloire E, Flanagan M, Stoffregen TA (2007) Motion sickness, console video games, and head mounted displays. Hum Fact 49:920–934
Miller JW, Goodson JE (1960) Motion sickness in a helicopter simulator. Aerosp Med 31:204–212
Munafo J, Wade MG, Stoffregen TA (2016). The distance of visual targets affects the spatial magnitude and multifractal scaling of standing body sway in younger and older adults. Exp Brain Res (under review)
Nashner LM, McCollum G (1985) The organization of human postural movements: a formal basis and experimental synthesis. Behav Brain Sci 8:135–150
Oman CM (1982) A heuristic mathematical model for the dynamics of sensory conflict and motion sickness. Acta Otolaryngol 44(Suppl 392):4–44
Paillard AC, Quarck G, Paolino F, Denise P, Paolino M, Golding JF, Ghulyan-Bedikian V (2013) Motion sickness susceptibility in healthy subjects and vestibular patients: effects of gender, age, and trait-anxiety. J Vestib Res 23:203–210
Palatinus Z, Dixon JA, Kelty-Stephen DG (2013) Fractal fluctuations in quiet standing predict the use of mechanical information for haptic perception. Ann Biomed Eng 41:1626–1634
Park AHY, Hu S (1999) Gender differences in motion sickness history and susceptibility to optokinetic rotation-induced motion sickness. Aviat Space Environ Med 70:1077–1080
Reason JT (1978) Motion sickness adaptation: a neural mismatch model. J R Soc Med 71:819–829
Riccio GE, Stoffregen TA (1991) An ecological theory of motion sickness and postural instability. Ecol Psychol 3:195–240
Shimizu Y, Thurner S, Ehrenberger K (2002) Multifractal spectra as a measure of complexity in human posture. Fractals 10L:103–116. doi:10.1142/S0218348X02001130
Smart LJ, Stoffregen TA, Bardy BG (2002) Visually-induced motion sickness predicted by postural instability. Hum Fact 44:451–465
Stoffregen TA (1985) Flow structure versus retinal location in the optical control of stance. J Exper Psychol Hum Perc Perform 11:554–565
Stoffregen TA (2011) Le mal des transports comme trouble du mouvement [Motion sickness considered as a movement disorder]. Sci Motricité 74:19–30
Stoffregen TA, Smart LJ (1998) Postural instability precedes motion sickness. Brain Res Bull 47:437–448
Stoffregen TA, Hettinger LJ, Haas MW, Roe M, Smart LJ (2000) Postural instability and motion sickness in a fixed-base flight simulator. Hum Fact 42:458–469
Stoffregen TA, Bardy BG, Merhi O, Oullier O (2004) Postural responses to two technologies for generating optical flow. Presence 13:601–615
Stoffregen TA, Faugloire E, Yoshida K, Flanagan M, Merhi O (2008) Motion sickness and postural sway in console video games. Hum Fact 50:322–331
Stoffregen TA, Yoshida K, Villard S, Scibora L, Bardy BG (2010) Stance width influences postural stability and motion sickness. Ecol Psychol 22:169–191
Stoffregen TA, Chen F-C, Varlet M, Alcantara C, Bardy BG (2013) Getting your sea legs. PLoS ONE 8(6):e66949. doi:10.1371/jour-nal.pone.0066949
Stoffregen TA, Chen Y-C, Koslucher FC (2014) Motion control, motion sickness, and the postural dynamics of mobile devices. Exper Brain Res 232:1389–1397. doi:10.1007/s00221-014-3859-3
Sullivan EV, Rose J, Rohlfing T, Pfefferbaum A (2009) Postural sway reduction in aging men and women: relation to brain structure, cognitive status, and stabilizing factors. Neurobiol Aging 30:793–807
Thurner S, Mittermaier C, Hanel R, Ehrenberger K (2000) Scaling-violation phenomena and fractality in the human posture control system. Phys Rev E 62:4018–4024
Villard S, Flanagan MB, Albanese G, Stoffregen TA (2008) Postural instability and motion sickness in a virtual moving room. Hum Fact 50:332–345
Voyer D, Voyer S, Bryden MP (1995) Magnitude of Sex differences in spatial abilities: a meta-analysis and consideration of critical variables. Psychol Bull 117:250–270
Acknowledgments
We thank Eric Haaland, Amy Malsch, and Jennifer Webeler, who assisted with data collection and analysis. We thank also Damian Kelty-Stephen, for assistance with MF-DFA. This project was supported by the University of Minnesota’s Undergraduate Research Opportunities Program. Justin Munafo’s participation was supported by a fellowship from the University of Minnesota Diversity of Views and Experience program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koslucher, F., Munafo, J. & Stoffregen, T.A. Postural sway in men and women during nauseogenic motion of the illuminated environment. Exp Brain Res 234, 2709–2720 (2016). https://doi.org/10.1007/s00221-016-4675-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-016-4675-8