Abstract
Light touch at the fingertip has been shown to influence postural control during standing and walking. Interlimb cutaneous reflexes have been proposed to provide a neural link between the upper and lower limbs to assist in interlimb coordination during activities such as walking. In this study, we tested the hypothesis that cutaneous sensory pathways linking the arm and leg will be facilitated if subjects use light touch to assist with postural control during treadmill walking. To test this, interlimb cutaneous reflexes from the median nerve, serving the skin contact region, and radial nerve, serving an irrelevant sensory territory, were tested in the legs of subjects walking on treadmill in an unstable environment. Interlimb cutaneous reflexes were tested while subjects (a) touched or (b) did not touch a stable contact with their fingertip, and while the eyes were either (c) open or (d) closed. Reflexes arising from both nerves were facilitated when vision was removed that was then ameliorated when touch was provided. These changes in reflex amplitude during the eyes closed conditions were mirrored by changes in background muscle activity. We suggest that this facilitation of interlimb reflexes from both nerves arises from a generalized increase in excitability related to the postural anxiety of walking on a treadmill with the eyes closed, which is then restored by the provision of light touch. However, the influence of touch when the eyes were open differed depending upon the nerve stimulated. Radial nerve reflexes in the legs were suppressed when touch was provided, mirroring a suppression in the background muscle activity. In contrast, median nerve reflexes in the leg were larger when touch was provided with the eyes open, despite a suppression of background muscle activity. This nerve-specific effect of touch on the amplitude of the interlimb cutaneous reflexes suggests that touch sensory information from the median nerve was facilitated when that input was functionally relevant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Perturbations delivered during walking evoke corrective responses in the legs that are accompanied by activation of muscles in the arms (Marigold and Misiaszek 2009). Arm responses can assist in balance control by dissipating the center of mass momentum generated from the perturbation (Roos et al. 2008), or by protecting from injury by reaching for nearby handles (Maki and McIlroy 2006) or absorbing the impact of a fall (Feldman and Robinovitch 2007). In addition to the direct role, the arms play during balance regulation, and they also play an indirect role. For example, when the movement of the arms is restricted, responses in leg muscles to perturbations delivered at the waist during walking are stronger than when arms are free to move (Misiaszek and Krauss 2005). In contrast, holding on to stable handles suppresses the responses in the legs (Misiaszek et al. 2000; Misiaszek and Krauss 2004). Corrective responses in the legs are strongly modulated by the task engaged by the arms.
Light touch has been shown to have a strong effect on balance regulation. During standing, natural body sway is increased in the absence of vision, but largely reduced by active (Holden et al. 1994; Jeka and Lackner 1994; Wing et al. 2011) and passive (Rogers et al. 2001) light touch of a stable surface. The effect of touch has been dissociated from mechanical support by maintaining the load applied to the touch surface below 1 N, a non-mechanically supportive level (Holden et al. 1994). Moreover, the effect of touch was abolished by applying tourniquet ischemia to the upper limb, thereby inducing a partial block of the sensory afferents (Kouzaki and Masani 2008). The implication is that cutaneous feedback from the hands in the absence of mechanical support is important during balance control.
The effect of light touch on balance regulation is also present during walking. The provision of light touch of a stable surface during treadmill walking results in a reduction in body sway whether subjects walk with the eyes open or closed (Dickstein and Laufer 2004). In addition, corrective reactions to balance perturbations delivered during walking are adapted with the provision of touch. Responses in the legs to perturbations delivered with backward pulls applied at the waist have an increased magnitude with the provision of touch independent from the availability of vision (Forero and Misiaszek 2013). Moreover, Forero and Misiaszek (2013) also showed that blindfolded subjects required fewer steps to restore balance following a perturbation if they were lightly touching a stable reference. These results indicate that the provision of light touch provides sensory cues capable of adapting balance regulation for the lack of visual inputs.
Dietz (2002) suggested that upper and lower limb movements are coordinated during human gait as a residual function of quadrupedal locomotion. There is evidence of a neuronal linkage between upper and lower limbs. In particular, cutaneous reflex responses are evoked in various muscles in the body after stimulation of nerves serving the hands and feet of subjects maintaining tonic contractions (Zehr et al. 2001), as well as of subjects performing locomotor activities such as walking (Zehr and Haridas 2003) and cycling (Sakamoto et al. 2006). In addition, interlimb cutaneous reflex responses evoked when subjects perform a locomotor activity show a phase modulation (Haridas and Zehr 2003). These results suggest that interlimb cutaneous reflexes are relevant in assisting movement coordination between the arms and legs during motor tasks (for review, see Zehr and Duysens 2004).
Recently, we demonstrated that intralimb cutaneous reflexes evoked in the arms during treadmill walking are regulated depending upon whether the stimulated nerve innervated the skin relevant to a light touch reference (Forero and Misiaszek 2014a). It was argued that the change in the amplitude of the evoked reflex reflected an increased functional relevance of the sensory information related to the stabilizing touch reference. An unresolved question is whether similar adaptations to interlimb cutaneous reflexes from the arm to the leg would also be observed. Given that interlimb cutaneous reflexes are argued to assist with movement coordination between the arms and legs during locomotor tasks (Zehr and Duysens 2004), we predict that interlimb cutaneous reflexes originating from the nerve innervating the skin of the fingertip will be adapted when light touch is used to enhance balance. Therefore, the purpose of the current study was to use interlimb cutaneous reflexes elicited in muscles of the legs from stimulation of nerves serving the hand as a neural probe to test the effect of light touch on sensorimotor pathways that could link the actions of the arms and legs. We hypothesized that interlimb cutaneous reflexes resulting from stimulation of the median nerve (serving the palmar surface of the index finger) and those of the radial nerve (serving the dorsal surface of the index finger) would be modulated differently when light touch of the fingertip was used to enhance stability during walking.
Methods
Subjects
A total of 12 subjects (seven males and five females) were included in the experiment. Ages, heights and weights ranged from 21 to 36 years (mean ± SD, 27.5 ± 5.16 years), 154 to 190 cm (169.6 ± 9.87 cm) and 52 to 87 kg (66.6 ± 11.30 kg), respectively. Subjects provided written consent of their participation in a protocol approved by the University of Alberta Research Ethics Board.
Protocol
During the experiment, subjects were asked to walk on a motorized treadmill. For this study, subjects were required to walk on the treadmill with their eyes closed in some conditions. To ensure the walking speed for a subject was consistent across conditions, the subjects were asked to select a comfortable walking speed with their eyes closed (0.8–1.1 m/s) which was then used for all conditions. In order to increase the level of postural threat experienced throughout the experiment, the subjects also received pulls to the waist delivered randomly (approximately 10–20 s between each disturbance) throughout each of the walking conditions (Haridas et al. 2006). Backward pulls of the waist were applied through a steel cable fastened to the front and back of a harness worn by the subjects about the pelvis (Fig. 1). A force of about 20 % body weight was used to pull the subjects. This produces a disturbance that elevates postural threat, but does not normally cause subjects to trip or fall (Misiaszek 2003; Haridas et al. 2006).
Schematic diagram of the experimental setup. Subjects walked on a motorized treadmill while receiving backward pulls at the waist. Reflex responses evoked from electrical stimulation of two different nerves serving the right hand were recorded during four conditions combining VISION (eyes open, eyes closed) and TOUCH (touch, no touch). The right arm was positioned so that the elbow angle was 90°, while the forearm was horizontal. Perturbations were delivered randomly throughout the step cycle. Electrical stimuli to elicit interlimb cutaneous reflexes were delivered at heel-strike
Interlimb cutaneous reflexes were examined by delivering electrical stimulation to two nerves in the right arm: (1) the median nerve (MED) which innervates the palmar surface and the distal thumb, the index and middle finger, and (2) the superficial radial nerve (RAD) which innervates the dorsum of the thumb, index and middle finger. Subjects visited the lab on two different occasions, one for each nerve stimulation protocol. The order of presentation of the nerve stimulation protocols was randomized between subjects. The influence of touch sensory cues on cutaneous reflexes in the legs was evaluated both in the presence and absence of vision. For this purpose, two TOUCH conditions (TOUCH: t, NO TOUCH: n) were combined with two VISION conditions (eyes open: EO, eyes closed: EC). On each visit, subjects were exposed to the four different conditions: eyes open without touch (EOn), eyes open with touch (EOt), eyes closed without touch (ECn) and eyes closed with touch (ECt). A Latin square design was used to randomize the order of presentation of the condition to each subject before beginning the study. Subjects were allowed to rest between conditions if needed.
The touch reference was provided by a rigid rod (i.e., touch surface) positioned in front of the subject’s right arm, with a 2.5 cm × 2.5 cm contact area comprised of a smooth aluminum surface. During the touch conditions, subjects were asked to maintain light touch by extending their right index finger to reach the touch surface. The surface was aligned laterally with the right shoulder of the subject, with the height set so that the elbow was held at a 90° position, while the forearm was held horizontally and aligned with the extended hand and finger. A force transducer was used to measure the vertical component of the touch force applied by the finger. Subjects were asked to maintain a vertical force of less than 1 N during the touch conditions. The touch load was monitored online by a researcher who then provided verbal cueing to the subject if the load exceeded 1 N. Typically, the vertical load varied across the step cycle with a range between 0.25 and 0.75 N. During the no touch conditions, subjects were asked to hold the arm in a position similar to the one maintained for the touch conditions. During the eyes closed conditions, subjects wore a blindfold that fully occluded their vision. No specific instructions were given for the left arm, which was free to swing naturally in all conditions.
To allow the subjects to walk comfortably on the treadmill with their eyes closed, the subjects received auditory feedback through a pair of headphones (Sony MDR-XD100 stereo headphones, Minato-ku, Tokyo, Japan). The subject’s fore–aft position on the treadmill was linked to a computer generated tone with the frequency and volume varying as the subject moved from a central reference position. The volume of the tone increased as the subjects moved further from the reference position. A higher pitched tone indicated a more forward position of the subject on the treadmill, and a lower pitched tone indicated when the subject was drifting backward (the auditory feedback setup is fully described in Forero and Misiaszek 2013). Subjects practiced walking on the treadmill with their eyes closed prior to the start of the experiment until they were comfortable. Mechanical stops were in place 20 cm fore and aft of the reference position to prevent the subject from drifting to the ends of the treadmill. The auditory positional feedback was provided for all conditions (including when the eyes were open) to minimize any potential effect in the presence of the tone might have on the results.
Electrical stimulation
Trains (5 × 1.0 ms pulses at 300 Hz) of isolated constant current (Grass S88 stimulator with SIU5 and CCU1 isolation and constant current units, Warwick, RI) stimulation were delivered to the MED or RAD of the right arm. A pair of flexible 2 cm × 2 cm disposable Ag/AgCl surface electrodes (NeuroPlus A10040) was placed on the ventral surface of the forearm just proximal to the crease of the wrist joint to deliver stimuli to the MED. The electrodes for the RAD were placed on the dorsal surface of the forearm just proximal to the distal radial head and the crease of the wrist. Stimulus intensity was expressed as a multiple of the radiating threshold (RT). RT was estimated as the lowest current necessary for the subject to perceive a clear radiating cutaneous paresthesia into the innervation area of the stimulated nerve (MED, palmar surface of the hand; RAD, dorsum of the hand). A non-noxious stimulus intensity was established for each subject, which varied between 2 and 3 × RT. RT was checked between conditions to verify consistency of the applied stimulus.
Balance corrective responses to pulls at the waist during treadmill walking have been shown to be most pronounced at heel-strike (Misiaszek 2003). Previously, we showed that light touch further enhances these responses at heel-strike (Forero and Misiaszek 2013). Consequently, we tested the interlimb cutaneous reflexes for this study at right heel-strike. A computer was used to randomly deliver a stimulus once every three to five steps and to accurately control the timing of the stimulus (triggered from a foot contact sensor, see data acquisition). The first stimulus was delivered 1 min after data recording started, and stimuli were delivered for five more minutes. This technique resulted in 25–39 stimuli to be analyzed for each subject and each condition.
Data acquisition
A force-sensitive sensor was placed at the heel of the insole of right foot to detect right heel-strike (Interlink Electronics, Camarillo, CA, USA). Touch force was recorded from a force transducer used to measure the vertical component of the force applied by the finger. In addition, electromyographic (EMG) recordings were obtained from tibialis anterior (TA), soleus (SOL), vastus lateralis (VL) and biceps femoris (BF) of the right leg. A pair of Ag/AgCl surface electrodes was placed over the bellies of each muscle, 2 cm apart and aligned with the predicted direction of the muscle fibers. A common ground electrode was placed over the tibia. Raw EMG signals were band-pass filtered (30 Hz–1 kHz) and variably amplified (500–5000×) using Grass P511 amplifiers (Astro-Med, Inc., West Warwick, RI, USA). All signals were digitized online at 4 kHz and stored to hard drive using a custom-written LabVIEW v8.20 data acquisition routine and a National Instruments data acquisition card (National Instruments PCI-MIO-16E-4, Austin, TX, USA).
Data analysis
Postprocessing of the signals was done offline using custom-written LabVIEW v8.20 analysis routines. Force signals were digitally low-pass filtered at 10 Hz (zero-lag second-order Butterworth filter), and EMG signals were first digitally full-wave rectified and then low-pass filtered at 150 Hz (zero-lag fourth-order Butterworth filter).
The data stream from each trial was divided into sweeps representing individual steps. Each sweep was 300 ms in duration, starting 50 ms before right heel-strike. If an electrical stimulation was delivered in that step, the sweep was defined as a stimulated sweep. The step preceding a stimulated sweep was taken as the corresponding control sweep. In this study, we wished to test the interlimb cutaneous reflexes during steady-state walking. The pulls to the waist were intended to create an unstable environment, but we did not wish to test cutaneous reflexes during a balance correction. Therefore, the stimulated sweep and its corresponding control sweep were removed from further analysis if a pull to the waist had been delivered within three steps before an electrical stimulation. Stimulated and control traces were then calculated from the stimulated and control sweeps. Average control traces and their 95 % confidence bands were calculated from the control sweeps. Subtracted traces were then calculated by subtracting the average control traces from the individual stimulated sweeps.
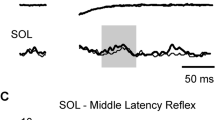
Cutaneous reflexes were calculated from the subtracted traces. Average subtracted traces were calculated separately for each muscle, subject and condition. A reflex response was identified and included in the analysis if the average subtracted trace fell outside the 95 % confidence band for more than five continuous milliseconds. Figure 2a shows examples of the average subtracted traces for TA and SOL for conditions for one subject recorded following stimulation of the MED. From these traces, a clear reflex response can be seen in TA and SOL at the middle latency windows (80–120 ms). As can be seen, interlimb cutaneous reflexes within the middle latency window often demonstrated both positive and negative deflections (Duysens et al. 1990; Yang and Stein 1990; Haridas et al. 2005). Therefore, reflex amplitudes were quantified as the root mean square (RMS) value within the time window of 80–120 ms, instead of the mean value, to obtain a better estimate of the middle latency reflex amplitude. Reflex amplitudes were then expressed as a percentage of the reflex amplitude for the EOn condition for each subject.
a Subtracted electromyographic (EMG) traces for TA and SOL after median nerve (MED) stimulation for a single subject following electrical stimuli delivered at heel-strike. The top pair of traces in each grouping represents the response traces for the eyes open (EO) conditions, and the bottom pair of traces represents the eyes closed (EC) conditions. The thin traces represent average traces for the NO TOUCH conditions and the thick traces for the TOUCH conditions. The shaded rectangular boxes indicate the poststimulus time window in which the middle latency reflex was analyzed. The vertical dashed line in each column is aligned to stimulus onset. Stimulus artifacts were digitally removed from the subtracted traces for display purposes. b Means and standard errors of the middle latency cutaneous reflexes arising from MED stimulation delivered at heel-strike for TA and SOL for each combination of TOUCH and VISION. The data were standardized to the normal condition for each subject. Open bars represent the data for the NO TOUCH conditions. Solid bars represent the data for the TOUCH conditions. The hashes indicate significant simple effect differences (Tukey’s HSD) between average response amplitudes (TA tibialis anterior, SOL soleus)
Background EMG activity was estimated from the control traces for each muscle, subject and condition. Background EMG amplitude was calculated as the RMS value over the same period of time that would have been analyzed for the stimulated traces (i.e., a window covering 80–120 ms after heel-strike). These values were then normalized to the average peak EMG amplitude for that muscle obtained during unperturbed walking in the normal condition (i.e., EOn).
Statistics
Statistical comparisons were made using the standardized values for the cutaneous reflex amplitudes and the background EMG amplitudes. Separate two-factor repeated measures ANOVAs (TOUCH [2] × VISION [2]) were used to determine whether there was an effect of touch and vision on the amplitude of the reflex response or the background activity for each nerve stimulation protocol and each muscle analyzed. If a significant interaction between TOUCH and VISION was found, Tukey’s Honestly Significant Difference (HSD) post hoc comparisons were used to test the simple effects of TOUCH and VISION. All comparisons were made using α = 0.05.
Results
Electrical stimulation of the MED and RAD nerves at heel-strike of treadmill walking evoked significant reflex responses in TA and SOL in all subjects. In contrast, significant responses in VL were observed from only seven subjects and from only four subjects in BF. Given the inconsistent expression of interlimb cutaneous reflexes in VL and BF, the focus of this paper will be on describing the effects of TOUCH and VISION on the consistent interlimb reflexes observed in TA and SOL.
MED stimulation protocol
Electrical stimulation of the MED resulted in clear middle latency responses in TA and SOL. Figure 2a shows example traces from a single subject for each condition. The thin traces represent the NO TOUCH conditions (EOn and ECn), and the thick traces represent the TOUCH conditions (EOt and ECt). The top pair of traces in each grouping represents the EO conditions, and the bottom pair of traces represents the EC conditions. The gray boxes represent the window over which the middle latency reflex amplitudes were measured. For the data shown in Fig. 2a, an obvious suppression of the middle latency reflex in SOL is seen for TOUCH, compared with NO TOUCH in the EC condition. In contrast, in the EO condition, the middle latency reflex was larger during TOUCH, compared with NO TOUCH. It is also apparent from these data that the middle latency reflexes during NO TOUCH are substantially larger in the EC condition than in the EO condition. Similar descriptions can be applied to the reflexes evoked in TA, although for this subject, the differences between conditions were less apparent for the middle latency reflex in TA. A pronounced late latency reflex emerged in TA during the ECn condition for this subject. However, the response of interest for this paper was the middle latency reflex, and therefore, late latency responses were not analyzed further.
Figure 2b shows group-averaged data of the amplitudes of the middle latency reflex from stimulation of the MED. As described above for the single subject’s data traces in Fig. 2a, the largest responses in both TA and SOL were observed during the ECn condition, while the smallest were observed during the EOn. It can also be seen that there is an interaction between TOUCH and VISION as TOUCH in the EO condition resulted in generally larger reflexes compared to NO TOUCH, whereas in the EC condition, the reflexes with TOUCH were smaller compared to NO TOUCH. The interaction effect was identified as significant by the ANOVA for both TA (F = 5.08, p = 0.024) and SOL (F = 15.10, p < 0.001). Tukey’s post hoc comparisons revealed that in the absence of TOUCH, reflexes in both TA and SOL were significantly larger when the eyes were closed (EOn < ECn). In addition, in the absence of VISION, reflexes were significantly smaller with TOUCH (ECn > ECt). However, the provision of TOUCH when walking with the eyes open resulted in no significant effect on reflexes in TA (EOn = EOt), while a significant increase in amplitude was found in SOL (EOn < EOt). Moreover, for both TA and SOL, VISION had no significant effect on reflex amplitude in the presence of TOUCH (EOt = ECt).
RAD stimulation protocol
Electrical stimulation of the RAD resulted in visible middle latency responses in TA and SOL. Figure 3a shows the average response traces from a single subject. The same convention used for the data presented in Fig. 2a is used in Fig. 3a. The data for the subject depicted in Fig. 3a show that for both TA and SOL TOUCH in the NO VISION resulted in smaller middle latency reflex amplitudes. It can also be seen that middle latency reflexes tended to be larger in the NO VISION conditions for both TOUCH and NO TOUCH.
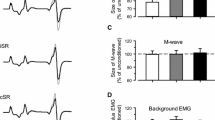
a Subtracted electromyographic (EMG) traces for TA and SOL after radial nerve (RAD) stimulation for a single subject following electrical stimuli delivered at heel-strike. The top pair of traces in each grouping represents the response traces for the eyes open (EO) conditions, and the bottom pair of traces represents the eyes closed (EC) conditions. Thin traces represent average traces for the NO TOUCH conditions and thick traces for the TOUCH conditions. The shaded rectangular boxes indicate the poststimulus time window in which the middle latency reflex was analyzed. The vertical dashed line in each column is aligned to the stimulus onset. Stimulus artifacts were digitally removed from the subtracted traces for display purposes. b Means and standard errors of the middle latency cutaneous reflexes arising from RAD stimulation delivered at heel-strike for TA and SOL for each combination of TOUCH and VISION. The data were standardized to the normal condition for each subject. Open bars represent the data for the NO TOUCH conditions. Solid bars represent the data for the TOUCH conditions. The asterisks indicate significant main effect differences in average response amplitudes between conditions, whereas the hashes indicate significant simple effect differences (Tukey’s HSD)
The group-averaged data for the RAD middle latency reflex amplitudes are shown in Fig. 3b. As can be seen, the largest reflexes in both TA and SOL were found during the ECn condition, whereas the smallest reflexes occurred during the EOt condition. For TA, the ANOVA revealed a significant main effect of touch (F = 9.66, p = 0.001) and a significant main effect of vision (F = 31.04, p < 0.001) with no significant interaction between these two factors (F = 0.18, p = 0.670). Reflex responses in TA were larger when subjects walked with their eyes closed. In addition, allowing the subjects to touch a stable surface while walking resulted in smaller reflexes. A similar trend was observed in SOL where the reflexes with the eyes closed tended to be larger and the provision of touch resulted in smaller reflexes. However, the ANOVA revealed a significant interaction effect between the touch and vision conditions (F = 29.83, p < 0.001). Tukey’s post hoc comparisons revealed that the significant interaction effect arose from the EO comparison as the modest decrease in the average reflex amplitude that is seen in EOt compared to EOn was not significant. Otherwise, like TA, reflexes in SOL were larger when the eyes were closed ((EOn < ECn and EOt < ECt). In addition, the provision of touch resulted in a significant reduction in the amplitude of the response when the eyes were closed (ECn > ECt). In general, the removal of vision resulted in larger middle latency responses in both TA and SOL, and the provision of touch resulted in smaller responses.
Background activity
In Fig. 4, the group-averaged background EMG amplitudes for TA and SOL are shown. From the group averages, there is an apparent effect of both VISION and TOUCH on the level of EMG activity produced during walking. The ANOVAs for TA and SOL showed significant main effects for both TOUCH (F = 13.63, p < 0.001 and F = 13.41, p < 0.001) and VISION (F = 20.15, p < 0.001 and F = 40.27, p < 0.001), with no significant interaction effects (F = 0.85, p = 0.354 and F = 3.35, p = 0.066). Therefore, closing the eyes led to an increase in the background EMG of both TA and SOL, whereas the provision of light touch led to a reduction in the activity on both muscles.
Means and standard errors of the background EMG for TA and SOL. The background EMG was calculated from the control traces as the RMS value over the same period that would be analyzed for the stimulated traces (80–120 ms after heel-strike). The data were standardized to the maximum EMG amplitude observed during normal undisturbed walking. Open bars represent the data for the NO TOUCH conditions. Solid bars represent the data for the TOUCH conditions. The asterisks indicate significant main effect differences in average amplitudes between conditions
Discussion
The primary purpose of this study was to investigate whether sensorimotor pathways linking the hand to the legs are facilitated when sensory cues from the hand (light touch) would be relevant for maintaining stability on a treadmill. To do so, we used interlimb cutaneous reflexes from MED and RAD stimulation while subjects walked on a motorized treadmill and received periodic balance disturbances. To enhance the relevance of the light touch cue, subjects walked with their eyes closed. Perhaps contrary to our hypothesis, the largest interlimb reflexes were seen when the eyes were closed and the subjects were not using touch. Provision of touch ameliorated the effect of walking with the eyes closed, returning the amplitude of the interlimb reflexes to close to those seen when walking normally. Although this marked effect of removing vision and then adding touch was observed for interlimb reflexes derived from both MED and RAD stimulation, the modulation of the reflexes from these two sources did not mirror each other completely. Rather, interlimb reflexes from MED stimulation were facilitated, but interlimb reflexes from RAD were suppressed, when subjects were touching a stable support. Taken together, these findings suggest that provision of light touch in this study contributed two separate effects on the amplitude of the interlimb reflexes: (1) a generalized effect related to the uncertainty introduced by walking on a motorized treadmill with the eyes closed and (2) a nerve-specific facilitation of the interlimb reflex arising from the skin area used during the light touch.
Light touch and postural uncertainty
The greatest influence on the amplitude of the interlimb reflexes during walking in this study appears to be the level of uncertainty created by the tasks. Interlimb reflexes in TA and SOL from both MED and RAD stimulation showed a marked increase in amplitude when vision was occluded, but this was largely ameliorated when the visually deprived subjects were able to touch a stable reference. Walking on a treadmill is a challenging task that becomes harder when performed in the absence of vision (Dickstein and Laufer 2004; Forero and Misiaszek 2013, 2014a). The threat was further amplified in this study by application of periodic and unpredictable pulls to the waist (Forero and Misiaszek 2013; Haridas et al. 2005). The fear of drifting backward and falling off the back of the treadmill when walking with the eyes closed could result in a general increase in postural threat, hence resulting in a heightened level of anxiety. The link between anxiety and adaptation of postural control has been studied during standing (Adkin et al. 2000; Carpenter et al. 2001, 2004) and walking (Brown et al. 2002; Delbaere et al. 2009; Rietdyk and Patla 1997). For instance, Brown et al. (2002) found that environmental constraints that heightened anxiety resulted in increased activation of distal leg muscles (i.e., tibialis anterior and gastrocnemius) during walking. Similarly, Rietdyk and Patla (1997) showed that subjects permitted to run their hands over parallel rails showed a reduction in stance limb EMG activity during overground walking. Accordingly, the data from the current experiment, together with the data presented by Forero and Misiaszek (2013), showed that the removal of vision (increased anxiety) resulted in a significant increase in the level of activation in TA and SOL. Conversely, the provision of touch resulted in a significant reduction in the activity in TA and SOL. Together, these results suggest that anxiety modifies muscle activity and that touch assists in regulating this anxiety. The interlimb reflexes from MED and RAD stimulation tended to mirror the changes in the muscle activity in the eyes closed condition, and consequently were largest when subjects were walking in the most threatening condition.
Postural anxiety during walking will typically lead to the use of a more cautious gait (Hallemans et al. 2009). Changes to behavior to accommodate the specific contextual demands of the task reflect a change in the postural set (Prochazka 1989). Therefore, the generalized increase in interlimb reflex amplitude, for both the MED and RAD reflexes, observed when the eyes were closed likely reflects a generalized change in descending control to the spinal motor system associated with a change in the postural set. Consequently, in the absence of vision, the excitability of the motor neuron pool (as reflected in the background EMG activity) was increased contributing to the increase in reflex amplitude (Matthews 1986). When touch was provided in the absence of vision, the postural anxiety was reduced, restoring the postural set. The findings from this study indicate that light touch provides a supplemental spatial reference in the absence of vision that is then incorporated into the selection of the appropriate postural set. In this study, participants were always walking in conditions of relative postural threat as periodic pulls to the waist were applied during all conditions. Therefore, it is possible that our results revealed influences of touch that would only be relevant in the context of very threatening conditions, that is, walking on a treadmill with the eyes closed while being periodically pulled at the waist. However, this seems unlikely as Rietdyk and Patla (1997) demonstrated that subjects reduced stance limb EMG activity when permitted to run their hands over parallel rails during the comparatively nonthreatening task of walking overground with eyes open without perturbations. Therefore, postural anxiety during walking appears to be reflected in the level of background EMG activity, and the contribution of light touch to regulating anxiety would also be expected to be evident in less threatening conditions than what was experienced in this study.
Nerve-specific modulation of interlimb cutaneous reflexes
As described above, the changes in amplitude observed in the interlimb cutaneous reflexes evoked in TA and SOL by stimulating the MED and RAD can be related to changes in the level of postural anxiety and the associated changes in background EMG activity. In particular, the reflexes evoked by stimulating the RAD directly mirrored the changes in background EMG. In contrast, reflexes evoked by stimulating the MED can only be partly explained by the changes in background EMG. For instance, the amplitude of the MED interlimb reflex in SOL was significantly increased during TOUCH with the eyes open (Fig. 2b), despite a significant decrease in the background SOL activity (Fig. 4). A similar result is seen in TA, whereby the significant decrease in TA activity (Fig. 4) is not reflected by a decrease in MED reflex amplitude, but rather no significant difference and a trend for an increase in reflex amplitude. These results suggest that interlimb reflexes from MED stimulation do not simply follow the level of background EMG activity and are subject to a level of control independent of that related to the effect of general anxiety. Nerve-specific modulation of cutaneous reflexes in the arm (Zehr and Kido 2001; Forero and Misiaszek 2014a) and leg (Zehr et al. 1997, 1998) during walking has been shown previously. It has been argued that cutaneous reflexes from afferents serving different areas of the skin will be modulated according to the role the area of the skin plays for the task being performed. For example, light touch of a stable surface facilitated cutaneous reflexes in the arm if they transport sensory information from the skin in contact with the surface (i.e., MED, Forero and Misiaszek 2014a). Arguably, when touch cues are not relevant (i.e., RAD), the reflexes are regulated by general anxiety, mirroring the level of background EMG activity. However, when the touch cues are relevant (i.e., MED) the sensorimotor channel is facilitated relative to the EOn condition. The implication is that when tactile feedback associated with the touch surface is available, these interlimb sensorimotor pathways are facilitated, suggesting a functional integration within the ongoing locomotor activity of the legs.
Functional considerations
There is ample evidence that light touch from the hands participates in balance control during standing (Jeka 1997; Dickstein et al. 2001, 2003; Johannsen et al. 2007; Wing et al. 2011). There is now growing evidence that light touch also participates in balance control during walking (Dickstein and Laufer 2004, Forero and Misiaszek 2013). For example, light touch has been shown to scale balance corrective reactions during treadmill walking (Forero and Misiaszek 2013). The results from the current study suggest that touch also has a profound influence on the amplitude of interlimb reflexes during walking. There is strong evidence that upper and lower limb movements are coordinated during walking (Dietz 2002). Interlimb cutaneous reflexes are argued to be functionally relevant in assisting with this coordination (Haridas and Zehr 2003). In particular, Haridas and Zehr (2003) showed that functionally relevant reflex pathways exist from nerves in the arms onto muscles in the legs, capable of altering ankle joint kinematics during walking. Thus, the results from the current study suggest that sensory cues from light touch are integrated to coordinate and regulate the activity in the legs and participate in balance control during walking. Possibly, with the increase in spatial cues associated with the contact of a stable surface, the central nervous system would be able to better estimate the location of the body in space and allow for more efficient balance responses during walking. It could be argued that interlimb reflexes might be a rapid means of coordinating a whole body integrated corrective response. For instance, oscillation of a touch surface entrains postural sway during standing (Wing et al. 2011). Therefore, touch could provide a sensory cue during a disturbance if the arms are engaged in a balance functional task. For example, Forero and Misiaszek (2014b) showed that during walking, responses in the legs are activated when perturbations are applied at the arms. Activation of interlimb cutaneous reflexes linking the skin of the hand to the muscle activity of the legs would potentially provide a rapid means of initiating a correction to a balance disturbance that is induced through the arms.
References
Adkin AL, Frank JS, Carpenter MG, Peysar GW (2000) Postural control is scaled to level of postural threat. Gait Posture 12:87–93
Brown LA, Gage WH, Polych MA, Sleik RJ, Winder TR (2002) Central set influences on gait. Age-dependent effects of postural threat. Exp Brain Res 145:286–296
Carpenter MG, Frank JS, Silcher CP, Peysar GW (2001) The influence of postural threat on the control of upright stance. Exp Brain Res 138:210–218
Carpenter MG, Frank JS, Adkin AL, Paton A, Allum JHJ (2004) Influence of postural anxiety on postural reactions to multi-directional surface rotations. J Neurophysiol 92:3255–3265
Delbaere K, Sturnieks DL, Crombez G, Lord SR (2009) Concern about falls elicits changes in gait parameters in conditions of postural threat in older people. J Gerontol A Biol Sci Med Sci 64:237–242
Dickstein R, Laufer Y (2004) Light touch and center of mass stability during treadmill locomotion. Gait Posture 20:41–47
Dickstein R, Shupert CL, Horak FB (2001) Fingertip touch improves postural stability in patients with peripheral neuropathy. Gait Posture 14:238–247
Dickstein R, Peterka RJ, Horak FB (2003) Effects of fingertip touch on postural responses in subjects with diabetic neuropathy. J Neurol Neurosurg Psychiatry 74:620–626
Dietz V (2002) Do human bipeds use quadrupedal coordination? Trends Neurosci 25:462–467
Duysens J, Trippel M, Horstmann GA, Dietz V (1990) Gating and reversal of reflexes in ankle muscles during human walking. Exp Brain Res 82:351–358
Feldman F, Robinovitch SN (2007) Reducing hip fracture risk during sideways falls: evidence in young adults of the protective effects of impact to the hands and stepping. J Biomech 40:2612–2618
Forero J, Misiaszek JE (2013) The contribution of light touch sensory cues to corrective reactions during treadmill locomotion. Exp Brain Res 226:575–584
Forero J, Misiaszek JE (2014a) The effect of light touch on the amplitude of cutaneous reflexes in the arms during treadmill walking. Exp Brain Res 232:2967–2976
Forero J, Misiaszek JE (2014b) Balance-corrective responses to unexpected perturbations at the arms during treadmill walking. J Neurophysiol 112:1790–1800
Hallemans A, Beccu S, Van Loock K, Ortibus E, Truijen S, Aerts P (2009) Visual deprivation leads to gait adaptations that are age- and context-specific: II. Kinematic parameters. Gait Posture 30:307–311
Haridas C, Zehr EP (2003) Coordinated interlimb compensatory responses to electrical stimulation of cutaneous nerves in the hand and foot during walking. J Neurophysiol 90:2850–2861
Haridas C, Zehr EP, Misiaszek JE (2005) Postural uncertainty leads to dynamic control of cutaneous reflexes from the foot during human walking. Brain Res 1062:48–62
Haridas C, Zehr EP, Misiaszek JE (2006) Context-dependent modulation of interlimb cutaneous reflexes in arm muscles as a function of stability threat during walking. J Neurophysiol 96:3096–3103
Holden M, Ventura J, Lackner JR (1994) Stabilization of posture by precision contact of the index finger. J Vestib Res 4:285–301
Jeka JJ (1997) Light touch contact as a balance aid. Phys Ther 77:476–487
Jeka J, Lackner JR (1994) Fingertip contact influences human postural control. Exp Brain Res 100:495–502
Johannsen L, Wing AM, Hatzitaki V (2007) Effects of maintaining touch contact on predictive and reactive balance. J Neurophysiol 97:2686–2695
Kouzaki M, Masani K (2008) Reduced postural sway during quiet standing by light touch is due to finger tactile feedback but not mechanical support. Exp Brain Res 188:153–158
Maki BE, McIlroy WE (2006) Control of rapid limb movements for balance recovery: age-related changes and implications for fall prevention. Age Ageing 35(Suppl 2):ii12–ii18
Marigold DS, Misiaszek JE (2009) Whole-body responses: neural control and implications for rehabilitation and fall prevention. Neuroscientist 15:36–46
Matthews PB (1986) Observations on the automatic compensation of reflex gain on varying the pre-existing level of motor discharge in man. J Physiol 374:73–90
Misiaszek JE (2003) Early activation of arm and leg muscles following pulls to the waist during walking. Exp Brain Res 151:318–329
Misiaszek JE, Krauss EM (2004) Compensatory arm reactions when holding a stable support during walking. Program No. 180.4. 2004 Neuroscience Meeting Planner. San Diego, Ca: society For Neuroscience, 2004. Online
Misiaszek JE, Krauss EM (2005) Restricting arm use enhances compensatory reactions of leg muscles during walking. Exp Brain Res 161:474–485
Misiaszek JE, Stephens MJ, Yang JF, Pearson KG (2000) Early corrective reactions of the leg to perturbations at the torso during walking in humans. Exp Brain Res 131:511–523
Prochazka A (1989) Sensorimotor gain control: a basic strategy of motor systems? Prog Neurobiol 33:281–307
Rietdyk S, Patla AE (1997) Context-dependent reflex control: some insights into the role of balance. Exp Brain Res 119:251–259
Rogers MW, Wardman DL, Lord SR, Fitzpatrick RC (2001) Passive tactile sensory input improves stability during standing. Exp Brain Res 136:514–522
Roos PE, McGuigan MP, Kerwin DG, Trewartha G (2008) The role of arm movement in early trip recovery in younger and older adults. Gait Posture 27:352–356
Sakamoto M, Endoh T, Nakajima T, Tazoe T, Shiozawa S, Komiyama T (2006) Modulations of interlimb and intralimb cutaneous reflexes during simultaneous arm and leg cycling in humans. Clin Neurophysiol 117:1301–1311
Wing AM, Johannsen L, Endo S (2011) Light touch for balance: influence of a time-varying external driving signal. Philos Trans R Soc Lond B Biol Sci 366:3133–3141
Yang JF, Stein RB (1990) Phase-dependent reflex reversal in human leg muscles during walking. J Neurophysiol 63:1109–1117
Zehr EP, Duysens J (2004) Regulation of arm and leg movement during human locomotion. Neuroscientist 10:347–361
Zehr EP, Haridas C (2003) Modulation of cutaneous reflexes in arm muscles during walking: further evidence of similar control mechanisms for rhythmic human arm and leg movements. Exp Brain Res 149:260–266
Zehr EP, Kido A (2001) Neural control of rhythmic, cyclical human arm movement: task dependency, nerve specificity and phase modulation of cutaneous reflexes. J Physiol 537:1033–1045
Zehr EP, Komiyama T, Stein RB (1997) Cutaneous reflexes during human gait: electromyographic and kinematic responses to electrical stimulation. J Neurophysiol 77:3311–3325
Zehr EP, Stein RB, Komiyama T (1998) Function of sural nerve reflexes during human walking. J Physiol 507:305–314
Zehr EP, Collins DF, Chua R (2001) Human interlimb reflexes evoked by electrical stimulation of cutaneous nerves innervating the hand and foot. Exp Brain Res 140:495–504
Acknowledgments
This work was funded by a Natural Sciences and Engineering Research Council (Canada) grant to JEM.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Forero, J., Misiaszek, J.E. The amplitude of interlimb cutaneous reflexes in the leg is influenced by fingertip touch and vision during treadmill locomotion. Exp Brain Res 233, 1773–1782 (2015). https://doi.org/10.1007/s00221-015-4250-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-015-4250-8