Abstract
We first explored whether the ability of subjects to detect the direction of slow ramp imposed movements may be improved by the application of mechanical noise to muscle tendons. Movements were plantar/dorsal flexion of the ankle at 0.04°/s, and the amplitude was just sub-threshold for each subject. A white noise signal (random vibration), low-pass filtered to 100 Hz and distributed uniformly in amplitude, was applied to both the extensor and the flexor ankle muscle tendons with four different mean amplitudes (20, 30, 100, 280 μm). The population of subjects was observed to exhibit clear stochastic-type behaviour: their ability to determine the direction of sub-threshold movements significantly increased when the two lower levels of noise were added and subsequently decreased when the noise magnitude was enhanced. Second, using microneurography, we explored the response of 9 primary muscle spindle afferents and 8 cutaneous afferents to the same imposed movements with and without noise application. While these conditions of ankle mobilisation were too small to induce a response in most of the recorded afferents, two muscle afferents exhibited responses that were characteristic of aperiodic stochastic resonance behaviour: the unit movement response was either triggered or improved by the application of an optimal level of noise. All cutaneous afferents were unresponsive to the imposed movements with or without noise application. We conclude that ankle movement sense can be significantly improved by adding an optimal level of mechanical noise to ankle muscle tendons and discuss the optimisation of the response of movement-encoding receptors that may account for this improvement. The application of a mechanical noise on ankle muscle tendons may constitute a means of improving postural stability in subjects with sensory deficits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kinaesthesia, the sense of limb position and movement, relies on the activity of peripheral receptors, muscle spindles, and skin stretch receptors (Proske and Gandevia 2009). With regard to muscle receptors, both animal and human studies have shown that the quality of information is enhanced when muscle afferent activities are noisy. Thus, messages are improved when variability in the spindle instantaneous frequency of discharge is introduced by an active fusimotor drive, which produces asynchronous contractions of intrafusal muscle fibres (Inbar et al. 1979; Bergenheim et al. 1995; Tock et al. 2005). For example, it has been shown in the cat that ensembles of spindle afferents are more effective at discriminating different movement amplitudes than a single afferent only when the fusimotor system is at work, introducing variation in the individual response profiles (Bergenheim et al. 1995). In humans, an increase in the variability of the spindle instantaneous firing rate has been observed to accompany an increase in a subject’s attention, permitting the identification of passive movements forming different letters (Hospod et al. 2007). These studies support the assumption that the fusimotor system constitutes a beneficial intrinsic source of noise for the muscle proprioceptive system (Cordo et al. 1996; Fallon et al. 2004). This assumption is consistent with observations in other sensory systems, such as the visual system, in which synaptic noise is a prerequisite for neurons to encode two parameters, orientation and contrast, simultaneously (Anderson et al. 2000).
Numerous studies have shown that noise can also be extrinsic. External input noise with optimal amplitude can improve the performance of nonlinear systems by increasing their response to weak input signals. This is known as the stochastic resonance phenomenon that has been originally demonstrated for periodic and later for aperiodic input signals (Chow et al. 1998). This phenomenon has been described in a variety of physiological systems, such as the visual, auditory, or somatosensory and motor systems (reviews: Moss et al. 2004; McDonnell and Ward 2011). As an example, noise has been demonstrated to enhance a subject’s ability to detect sub-threshold tactile stimuli (Collins et al. 1997; Dhruv et al. 2002; Liu et al. 2002), and consequently, applied on the plantar foot sole of standing subjects, noise improves balance control in elderly people and in patients with stroke or diabetic neuropathy (Gravelle et al. 2002; Priplata et al. 2003, 2006). Stochastic resonance is due to optimal noise, which leads to small membrane potential fluctuations that cause the receptor to reach threshold for stimuli that were initially sub-threshold (Volgushev and Eysel 2000). In the somatosensory system, increases in receptor sensitivity due to mechanical noise have been demonstrated for cutaneous receptors in the rat (Collins et al. 1996b; Ivey et al. 1998), for golgi tendon afferents and muscle primary and secondary endings in the cat (Fallon et al. 2004), and for spindle afferents in humans (Cordo et al. 1996). However, the latter study did not mention whether such noise-induced changes in muscle receptor activities were accompanied by changes in movement perception.
The aim of the present study was to explore the ability of subjects to detect the direction of movements imposed on the ankle joint with and without the application of mechanical noise on the muscle tendons and to compare the activity of movement-encoding receptors in exactly the same conditions of stimulation.
Methods
The subjects gave informed written consent to the experimental conditions, and the study was approved by the local ethics committee (Comité de Protection des Personnes Sud-Méditerranée I).
Experimental set-up
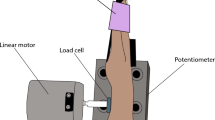
The subjects were seated in a semi-reclined armchair with their legs positioned in cushioned grooves, so that a standardised position could be maintained without muscle activity. The knee joint was at an angle of approximately 120–130°. The right foot was laid on a stationary plate, and the left foot was laid on a rotating pedal connected to a computer-controlled machine that permitted the imposition of ramp-and-hold ankle movements. The subjects were blindfolded and wore white noise headphones to eliminate any auditory cues that might have been triggered by the starting of the computer-controlled machine.
The subjects were asked to remain relaxed. The absence of any muscle activity was monitored throughout the experiments by recording surface electromyographic activity (EMG). Two pairs of surface electrodes were placed over the tibialis anterior (TA) and gastrocnemius soleus (GS) muscle bellies. The EMGs were band-pass filtered (3–3,000 Hz), recorded with a high gain (5000), and sampled at 10 kHz.
Two very light vibrating elements (C-2 tactor, Engineering Acoustics, Winter Park, FL, USA) were strapped over the distal tendon of the TA and GS muscles with soft elastic tape. The noise was simultaneously delivered on both the TA and the GS muscle tendons to eliminate any additional cue on movement direction that would have been available if vibrations were only applied on the tendon of the stretched muscle. However, the vibration was only effective in the stretched muscle because of a slackening of the shortened muscle (Roll et al. 1989). The noise generator consisted of a single-chip record/playback device, in which a digitised white noise signal, low-pass filtered at 100 Hz, distributed uniformly in amplitude with a mean of 20, 30, 100, or 280 μm, was stored (Priplata et al. 2003). Noises at the two lower levels were not sensed, and those at the two highest levels were slightly to clearly perceivable.
Psychophysical experiment
Ten healthy subjects (six females and four males, mean age, 28 years, range 20–38) participated in this study that consisted in analysing their ability to determine the direction of slow ramp movements imposed at the ankle joint.
The initial position of the ankle was slightly plantar flexed (100°), and either a dorsal flexion or a plantar flexion was imposed followed by a hold phase, and the foot was then returned to its initial position. Movement velocity was 0.04°/s, its magnitude was fixed, and total displacement corresponded to each subject’s sub-threshold amplitude (see below) and the hold phase lasted for 3 s.
One experimental session consisted of five dorsal flexions, five plantar flexions, and five “no-movements” performed in random order. The latter consisted of equal time windows during which no-movement was imposed. The entire experiment consisted of five sessions: one control session without noise and four sessions with noise of different mean amplitudes (20, 30, 100, or 280 μm).
Subjects had to report the direction of any movement they perceived just prior to the end of the hold phase only when they could report it with certainty. When the subject was uncertain of his/her response, the response was qualified as “undetermined.” Independent of the subject’s performance, the instructions were frequently repeated to minimise the number of incorrect responses.
Experimental protocol and data processing
On the first day, the subjects came for familiarisation with the task and establishment of her/his movement sub-threshold amplitude. The movement was initially imposed with an amplitude set at 0.6° (Refshauge and Fitzpatrick 1995). This amplitude was considered the subject’s sub-threshold if the percentage of correct responses was 40–60 %. When fewer or more correct responses were given, we increased or decreased the movement amplitude by 0.1°, respectively. The 60 % level was determined using the binomial test with the assumption that the three events (dorsal flexion, plantar flexion, and no-movement) were equally likely.
Only the subjects who remained totally relaxed during the preliminary tests conducted on the first day participated in the experiment, that is, the inclusion criterion was the absence of any involuntary activity of the dorsal or plantar ankle flexor muscles, activity that may occur notably when focusing attention on the ankle joint in order to determine the direction of movement. Of the ten subjects satisfying the criteria, three were tested with a movement amplitude of 0.5°, six subjects with 0.6°, and one subject with 0.7°.
On the second day, the subjects participated in five recording sessions (control, 20 μm noise, 30 μm noise, 100 μm noise, 280 μm noise), which were performed in random order. No feedback regarding the subject’s performance was given, and each session lasted for approximately 15 min. These sessions were separated by 10 min rest periods during which the subject was allowed to move his/her feet. The duration of the entire experiment was approximately 2 h.
The data are expressed as the percentage of correct responses. A correct response corresponded to the reporting of the actual movement direction or the correct absence of movement [% correct = (N correct/N total)*100, with N total = 10]. Friedman and post hoc Wilcoxon tests were used to evaluate the statistical differences in the % correct data.
Microneurographic experiment
Afferent activity was recorded with an insulated tungsten microelectrode (impedance 0.3–1 MΩ tested at 1 kHz, tip diameter about 5–8 μm, length 30 mm, Frederick Haer, Bowdoinham, ME), which was inserted into the common peroneal nerve at the level of the popliteal fossa in seven healthy subjects (7 males, mean age 24 years, range 20–31). To be included in the present population, in addition to the ability to stay completely relaxed during the entire experiment duration, subjects had to be comfortable with needle insertion and to present a common peroneal nerve that could be located by tactile exploration. Only one subject in the psychophysical experiment satisfied these criteria and was enrolled in the microneurographic experiment.
Neural activity was recorded with a high gain (100000), band-pass filtered (300–3,000 Hz), and sampled at 20 kHz. Recordings were made from nine muscle spindle primary afferents and eight cutaneous afferents responsive to movements (see: Aimonetti et al. 2007). The primary classification of muscle afferents was deduced from the response to passive ramp-and-hold (5°, 8°/s) and sinusoidal movements (5°, 0.5 Hz), during which the afferents exhibited a high dynamic sensitivity to muscle stretching and silence during muscle shortening (Edin and Vallbo 1990). Three afferents belonged to the TA, five to the extensor digitorum longus (EDL), and one to the extensor hallucis longus (EHL) muscles. With regard to cutaneous afferents, the skin surface was probed with Semmes–Weinstein nylon monofilaments delivering a force of 0.2–147 mN in order to map the receptive fields and determine the force thresholds. Receptor adaptation properties were determined by pressing a monofilament delivering four times the force threshold onto the hot spot, and keeping it there for several seconds. Units showing sustained activity in response to these maintained pressures were classified as slowly adapting (SA) and the others as fast adapting (FA) units. Lastly, type I units with small receptive fields and clearly defined boundaries were distinguished from type II units with larger receptive fields and obscure boundaries (Vallbo and Johansson 1984). One SAI, one FAI, five SAII, and one FAII units were found to be responsive to movements, that is, they also responded to passive ramp-and-hold (5°, 8°/s) and sinusoidal movements (5°, 0.5 Hz). The receptive fields of these units were located on the PL muscle belly (3 SAII), TA muscle belly (1 SAII, 1 FAII), EDL muscle belly (1 SAII) muscles, PL muscle tendon (1 SAI, 1 FA1).
Experimental protocol and data processing
The ankle starting position was set at 100° but might have changed slightly during the attempts to ensure that the movement was sub-threshold for the monitored afferent. When occurring, the changes never exceeded ±2° to maintain similar experimental conditions to those used in the psychophysical experiments. Next, plantar/dorsal flexion movements were imposed with 0.5° amplitude, 0.04°/s velocity, and 1 s hold phase. Five movements were successively imposed in a random order: one control movement without noise and four movements with random noise (20, 30, 100, or 280 μm), which were applied on both the TA and the GS muscle tendons.
The data were processed offline with the software “Spike 2” (Cambridge Electronic Design, Cambridge, England). Each unit response was filtered by using a 0.1 s unit-area symmetric Hanning window. Next, a cross-correlation between the stimulus (S) and response (R) was performed. The maximum value of the cross-correlation function (CS.R.) characterised the coherence of the unit response with the aperiodic input signal (Collins et al. 1996a).
Results
Psychophysical experiment
The subject’s ability to detect the direction of the ankle-imposed movements was determined without (control condition) or with the application of mechanical noise to the ankle muscle tendons.
The percentage of correct responses (correct movement direction + correct no-movement) for each subject is shown in Fig. 1. The movements were previously defined as sub-threshold, that is, the amplitude was selected, so that it led to a percentage of correct responses that did not significantly differ from that expected by chance (≤60 %). The movements were also sub-threshold when the control session was randomly mixed with noise sessions, with the exception of one participant (subject 8) who performed better (%correct = 66.7) in the control session during the recording day. Then, in all subjects, the percentage of correct responses significantly increased, reaching a peak with the application of 20 μm noise (3/10 subjects) or 30 μm noise (7/10), and then decreased during application of the two highest levels of noise, that is, for most subjects the performance was below the level of significance (<60 %). Finally, for all sessions in all subjects, the percentage of false-positive responses, that is, reports of the wrong direction or reports of direction during no-movements, was 5 % for dorsal flexion, 7 % for plantar flexion, and 8 % for no-movements.
Individual percentage of correct responses for each level of noise (from 0 to 280 μm). A correct response corresponds to a report of the actual direction of the imposed ankle ramp-and-hold movement or to a correct report of the absence of movement. The line at 60 % corresponds to the level of significance. This level was determined using the binomial test with the assumption that the three events (dorsal flexion, plantar flexion, and no-movement) were equally likely. Values above this level indicate that the subject’s percentage of correct responses was significantly better than that expected by chance (p < 0.05)
The averaged data obtained from the 10 subjects are illustrated in Fig. 2a. The percentage of correct responses significantly differed from the one characterising the control condition when 20 μm noise was applied (p < 0.05), and it increased to a maximum rate with a 30 μm noise (p < 0.01). Next, the performance returned to a level similar to that of the control with a 100 μm noise and was significantly altered with a 280 μm noise (p < 0.05). The correct perception of movement/no-movement with a 30 μm noise was accompanied by a significant decrease in the percentage of movement for which the direction was impossible for the subjects to determine (p < 0.01; Fig. 2b). Furthermore, the decrease in performance in the 100 and 280 μm noise conditions was accompanied by a decrease in the ability to identify the absence of movement (Fig. 2c).
Mean results of the psychophysical experiment (mean ± standard error). a Percentage of correct responses for all subjects. b Percentage of movements in which the direction was impossible for the subjects to determine. c Percentage of no-movements in which the subjects wrongly report a direction. Comparison with the control data, *p < 0.05; **p < 0.01
Microneurographic experiment
Microneurographic recordings were acquired under conditions that were strictly identical to those employed in the psychophysical study: the unitary responses to 0.5° ramp-and-hold movements imposed at 0.04°/s at the starting position of 100 ± 2° in the presence or absence of noise applied to both the TA and the GS muscle tendons were compared.
Of the nine muscle afferents recorded, two presented responses during the application of noise of different amplitudes that were characteristic of aperiodic stochastic resonance behaviour, that is, increased response to a weak aperiodic input signal (Chow et al. 1998). The first muscle afferent, a primary spindle afferent that belonged to the TA muscle, was silent at the starting position. This afferent did not respond to the imposed plantar/dorsal flexion movement in the control situation (Fig. 3a). Conversely, a response to the same movement was triggered when a low amplitude level of noise (20 μm) was simultaneously applied on the TA and GS muscle tendons (Fig. 3b). Furthermore, noise with increased amplitude (from 30 to 280 μm) triggered a response by itself prior and during the imposed movement (Fig. 3c–e). The cross-correlation between the stimulus and response gave rise to a very good coefficient of correlation only when a 20 μm noise was applied (CS.R. ~0.8). All other levels of noise degraded the coherence between the stimulus and response, that is, the coefficient decreased for all levels (Fig. 3f).
Effect of noise on the sensitivity of a spindle afferent that was unresponsive to a 0.5° ramp-and-hold movement in the control trial. a–e Microneurographic recordings. Represented from the bottom to top: the EMG of the gastrocnemius soleus (GS) and tibialis anterior (TA) muscles, the 0.5° plantar/dorsal flexion movement, the unitary response with the corresponding instantaneous frequency histogram. Movement was imposed without noise (a) or with the simultaneous application of a 20, 30, 100, or 280 μm noise (b–e). PF: Plantar flexion. f Cross-correlations performed between the stimulus and response to plantar/dorsal flexion movements recorded during the application of a noise of 20 (red), 30 (blue), 100 (green), or 280 μm (grey)
The second afferent, which belonged to the EDL muscle, was spontaneously active regardless of the starting position (100 ± 2°, see Fig. 4a). This afferent was slightly responsive to the plantar/dorsal flexion movement imposed in the control situation (Fig. 4b), as shown by a small coefficient of correlation. For this unit, the response slightly increased with a 20 μm noise, as corroborated by an increase in the coefficient of correlation, and then decreased with increasing noise levels (Fig. 4c).
Effect of noise on the sensitivity of a spindle afferent that was slightly responsive to a 0.5° ramp-and-hold movement in the control trial. a Looking for the starting position, around the pre-defined 100°, that was more appropriate for the plantar/dorsal flexion to be sub-threshold in the control condition. Represented from bottom to top: the ankle position, that is, 0.5° plantar/dorsal flexion movements are imposed on different starting positions from 98° to 102°, and the unitary recording with its corresponding instantaneous frequency curve. b The afferent response was nearly imperceptible in the control condition. Same mode of representation as shown above. c Cross-correlations performed between the stimulus and response to plantar/dorsal flexion movements recorded during trials without (control) and with the application of a noise of 20, 30, 100, or 280 μm
The remaining seven muscle afferents (2 TA, 4 EDL, 1 EHL), which did not present (N = 3) or did present (N = 4) spontaneous activities, were unresponsive to the 0.5°–0.04°/s ramp-and-hold movement, independent of the change in starting position, and no-movement response was triggered regardless the level of noise simultaneously applied on the ankle muscle tendons. With regard to cutaneous afferents, none of them presented any spontaneous activity, and they were unresponsive to the imposed movements with or without noise application.
Discussion
While noise interferes with the perception of supra-threshold stimuli, it may aid in the perception of sub-threshold stimuli; this has been demonstrated in particular for the detection of tactile stimuli (Collins et al. 1997). In the present study, we showed that the application of mechanical noise on the ankle muscle tendons improves the detection of the direction of imposed movements that were initially sub-threshold. Furthermore, all subjects exhibited clear stochastic resonance-type behaviour: their ability to determine the direction of sub-threshold imposed movements was significantly enhanced by adding a particular level of noise and further increases in the noise amplitude degraded the subjects’ performances. More specifically, the improved performance yielded by the optimal noise level consisted of an increased ability to determine the direction of movements as well as to detect the absence of movement, as shown by the significant decrease in the percentage of movement for which the direction was impossible for the subjects to determine (undetermined responses). By contrast, increasing the noise amplitude degraded the subjects’ ability to detect the absence of movements. This increased number of no-movement errors might be seen as resulting from the induction of illusory movements that are known to occur with supra-threshold tendon vibration (Roll and Vedel 1982). The co-vibration of two antagonist muscles at the same frequency is known to induce a sensation of stabilised position and not an illusion of movement (Gilhodes et al. 1986). However, a vibratory stimulation may be more effective when applied on a stretched muscle and, conversely, it may be less effective when applied on a relaxed muscle. In our experimental conditions, the initial position of the foot was slightly plantar flexed, and this might have made the TA vibration more effective. The fact that the rates at which subjects identified the wrong direction (false-positive) were higher both for plantar flexion (7 %) and for no-movement (8 %) when compared to dorsal flexion (5 %) supports the hypothesis that some of the errors might have been due to the induction of illusory movements. Thus, as a whole, as the input noise amplitude increased, the movement acuity significantly peaked and subsequently decreased. This inverted-U function characterises the stochastic resonance effect that is demonstrated in this study in the case of movement sense and has been previously reported to occur from tactile sensations (Collins et al. 1996c, 1997) to sensorimotor performances (Mendez-Balbuena et al. 2012).
The origin of this improvement in movement perception should be an increase in the response of sensory neurons and, more precisely, a decrease in their threshold in the presence of a particular level of noise (Collins et al. 1996b; Cordo et al. 1996). This hypothesis led us to record the unitary activity of muscle and cutaneous afferents under stimulation conditions identical to those used in the psychophysical experiment. Unfortunately, we only rarely observed changes in the responses of muscle afferents and never in the case of cutaneous afferents. In one TA muscle afferent, we observed that a plantar/dorsal flexion movement that was sub-threshold became encoded during the application of an optimal level of noise on the ankle muscle tendons. More specifically, as expected for all muscle afferents of the ankle flexor muscles, the firing rate of the afferent increased during the plantar flexion and decreased during the dorsal flexion. In a second afferent (EDL), for which the imposed plantar/dorsal flexion movement was just at threshold in the control condition, an optimal level of noise caused a slight improvement in the response. In both afferents, increasing the input noise level degraded the movement encoding.
In fact, in the present study, the microneurographic experiments were not designed to suit the analysis of the stochastic resonance phenomenon, but to exactly copy the psychophysical conditions. Consequently, 0.5° and 0.04°/s were the parameters of the movements that characterised the subjects’ perception threshold, but these parameters were probably too small to investigate afferent activities. In addition, the leg muscles are long, and this results in more frequent damping of small and slow movements as well as mechanical noises, depending on the receptor location, and less opportunity to observe the phenomenon compared to short muscles of the wrist (Cordo et al. 1996). Finally, the ankle starting position was such that most of the tested muscle afferents remained active, which was not a condition favourable to studying stochastic resonance in muscle receptors. It is better to adjust muscle length to just less than that required to produce a maintained discharge (Fallon et al. 2004). Therefore, we chose here to analyse both movement sense and the sensitivity of the receptors in exactly the same conditions of stimulation, and both aims were difficult to achieve because each experimental technique required specific designs/constraints. Other studies that specifically analysed the effect of mechanically induced noise on afferent encoding observed stochastic resonance phenomena in more numerous afferents. This was the case in the study by Cordo et al. (1996), where stochastic resonance was observed in six out of eight muscle spindle afferents in human wrist muscles. In the latter study, noise was applied on the tendon of the unit’s parent muscle only, while in the present study, the ago/antagonist ankle muscles were both submitted to mechanical noise. The co-vibration was chosen here to eliminate any additional cue on movement direction that would have been available if vibrations were only applied on the tendon of the stretched muscle. However, during imposed movement, vibration was only effective in the stretched muscle because of a slackening of the shortened muscle. Therefore, the vibratory conditions can be considered as similar in both studies and thus do not explain the difference in observations.
With regard to cutaneous receptors, the only studies that specifically analysed the eventual changes in the receptors’ encoding properties induced by noise performed their experiments with tactile stimuli. The increased ability of cutaneous mechanoreceptors to detect sub-threshold pressure stimuli has been shown to be responsible for the enhancement of tactile sensations by noise (Collins et al. 1996b). These observations lead us to suppose that the sensitivity of the cutaneous receptors responsive to movements (Edin and Johansson 1995; Aimonetti et al. 2007, 2012) likely also increases by adding an optimal level of noise. To observe the phenomenon, it was necessary to record cutaneous afferents that were not only responsive to movements but also located on muscle tendons, as these receptors stop responding to mechanical vibration as soon as the probe is moved away from the receptive field (Vedel and Roll 1982). We did record cutaneous afferents that were movement sensitive, but none of them was located under the vibrating probe, which likely explains the absence of noise effect on these afferents. Along the same lines, even if tendon vibration more easily activates muscle afferents, their response depends on the spindle location inside the muscle (Roll et al. 1989). This may explain why the 30 μm noise amplitude alone caused a response in both responding muscle afferents, thereby degraded the unit response to movement, while the same noise level caused the greatest increase in ability to identify the direction of imposed movements. This observation indicates that while the 30 μm noise activated the tested afferents, it might remain sub-threshold for other afferents.
The fact that very few changes were observed in the response of muscle receptors under the same stimulation conditions as those observed to lead to a significantly improved perceptual performance may signify that a small increase in the number of units responding suffices to shift the threshold of perception. This may also account for cutaneous afferents, as the number of cutaneous afferents responding should be limited to those around the vibrating site. One should also consider that even if the changes affect only a small part of muscle and cutaneous afferents, the resulting perceptual effect may be magnified, as shown from psychophysiological experiments that use the paradigm of illusory movements by stimulating populations of cutaneous and muscle receptors both separately and together (Collins et al. 2005; Blanchard et al. 2011). These studies show that when the two modalities are simultaneously and congruently activated, the resulting sensations of movement increase when compared with those induced by the stimulation of a single sensory modality.
To conclude, in the present study, we showed that movement sense can be significantly improved by the application of an optimal level of mechanical noise to the ankle muscle tendons. This may constitute a means of improving postural stability, as demonstrated with electrically induced noise in subjects with functional ankle instability (Ross 2007).
References
Aimonetti JM, Hospod V, Roll JP, Ribot-Ciscar E (2007) Cutaneous afferents provide a neuronal population vector that encodes the orientation of human ankle movements. J Physiol 580:649–658
Aimonetti JM, Roll JP, Hospod V, Ribot-Ciscar E (2012) Ankle joint movements are encoded by both cutaneous and muscle afferents in humans. Exp Brain Res 221:167–176
Anderson JS, Lampl I, Gillespie DC, Ferster D (2000) The contribution of noise to contrast invariance of orientation tuning in cat visual cortex. Science 290:1968–1972
Bergenheim M, Johansson H, Pedersen J (1995) The role of the gamma-system for improving information transmission in populations of Ia afferents. Neurosci Res 23:207–215
Blanchard C, Roll R, Roll JP, Kavounoudias A (2011) Combined contribution of tactile and proprioceptive feedback to hand movement perception. Brain Res 1382:219–229
Chow CC, Imhoff TT, Collins JJ (1998) Enhancing aperiodic stochastic resonance through noise modulation. Chaos 8:616–620
Collins JJ, Chow CC, Capela AC, Imhoff TT (1996a) Aperiodic stochastic resonance. Physical Review E 54:5575–5584
Collins JJ, Imhoff TT, Grigg P (1996b) Noise-enhanced information transmission in rat SA1 cutaneous mechanoreceptors via aperiodic stochastic resonance. J Neurophysiol 76:642–645
Collins JJ, Imhoff TT, Grigg P (1996c) Noise-enhanced tactile sensation. Nature 383:770
Collins JJ, Imhoff TT, Grigg P (1997) Noise-mediated enhancements and decrements in human tactile sensation. Phys Rev E 56:923–926
Collins DF, Refshauge KM, Todd G, Gandevia SC (2005) Cutaneous receptors contribute to kinesthesia at the index finger, elbow, and knee. J Neurophysiol 94:1699–1706
Cordo P, Inglis JT, Verschueren S, Collins JJ, Merfeld DM, Rosenblum S, Buckley S, Moss F (1996) Noise in human muscle spindles. Nature 383:769–770
Dhruv NT, Niemi JB, Harry JD, Lipsitz LA, Collins JJ (2002) Enhancing tactile sensation in older adults with electrical noise stimulation. NeuroReport 13:597–600
Edin BB, Johansson N (1995) Skin strain patterns provide kinaesthetic information to the human central nervous system. J Physiol 487(Pt 1):243–251
Edin BB, Vallbo AB (1990) Classification of human muscle stretch receptor afferents: a Bayesian approach. J Neurophysiol 63:1314–1322
Fallon JB, Carr RW, Morgan DL (2004) Stochastic resonance in muscle receptors. J Neurophysiol 91:2429–2436
Gilhodes JC, Roll JP, Tardy-Gervet MF (1986) Perceptual and motor effects of agonist-antagonist muscle vibration in man. Exp Brain Res 61:395–402
Gravelle DC, Laughton CA, Dhruv NT, Katdare KD, Niemi JB, Lipsitz LA, Collins JJ (2002) Noise-enhanced balance control in older adults. NeuroReport 13:1853–1856
Hospod V, Aimonetti JM, Roll JP, Ribot-Ciscar E (2007) Changes in human muscle spindle sensitivity during a proprioceptive attention task. J Neurosci 27:5172–5178
Inbar G, Madrid J, Rudomin P (1979) The influence of the gamma system on cross-correlated activity of Ia muscle spindles and its relation to information transmission. Neurosci Lett 13:73–78
Ivey C, Apkarian AV, Chialvo DR (1998) Noise-induced tuning curve changes in mechanoreceptors. J Neurophysiol 79:1879–1890
Liu W, Lipsitz LA, Montero-Odasso M, Bean J, Kerrigan DC, Collins JJ (2002) Noise-enhanced vibrotactile sensitivity in older adults, patients with stroke, and patients with diabetic neuropathy. Arch Phys Med Rehabil 83:171–176
McDonnell MD, Ward LM (2011) The benefits of noise in neural systems: bridging theory and experiment. Nat Rev Neurosci 12:415–426
Mendez-Balbuena I, Manjarrez E, Schulte-Monting J, Huethe F, Tapia JA, Hepp-Reymond MC, Kristeva R (2012) Improved sensorimotor performance via stochastic resonance. J Neurosci 32:12612–12618
Moss F, Ward LM, Sannita WG (2004) Stochastic resonance and sensory information processing: a tutorial and review of application. Clin Neurophysiol 115:267–281
Priplata AA, Niemi JB, Harry JD, Lipsitz LA, Collins JJ (2003) Vibrating insoles and balance control in elderly people. Lancet 362:1123–1124
Priplata AA, Patritti BL, Niemi JB, Hughes R, Gravelle DC, Lipsitz LA, Veves A, Stein J, Bonato P, Collins JJ (2006) Noise-enhanced balance control in patients with diabetes and patients with stroke. Ann Neurol 59:4–12
Proske U, Gandevia SC (2009) The kinaesthetic senses. J Physiol 587:4139–4146
Refshauge KM, Fitzpatrick RC (1995) Perception of movement at the human ankle: effects of leg position. J Physiol 488(Pt 1):243–248
Roll JP, Vedel JP (1982) Kinaesthetic role of muscle afferents in man, studied by tendon vibration and microneurography. Exp Brain Res 47:177–190
Roll JP, Vedel JP, Ribot E (1989) Alteration of proprioceptive messages induced by tendon vibration in man: a microneurographic study. Exp Brain Res 76:213–222
Ross SE (2007) Noise-enhanced postural stability in subjects with functional ankle instability. Br J Sports Med 41:656–659
Tock Y, Inbar GF, Steinberg Y, Ljubisavljevic M, Thunberg J, Windhorst U, Johansson H (2005) Estimation of muscle spindle information rate by pattern matching and the effect of gamma system activity on parallel spindles. Biol Cybern 92:316–332
Vallbo AB, Johansson RS (1984) Properties of cutaneous mechanoreceptors in the human hand related to touch sensation. Hum Neurobiol 3:3–14
Vedel JP, Roll JP (1982) Response to pressure and vibration of slowly adapting cutaneous mechanoreceptors in the human foot. Neurosci Lett 34:289–294
Volgushev M, Eysel UT (2000) Neuroscience. Noise makes sense in neuronal computing. Science 290:1908–1909
Acknowledgments
The authors thank Guy Escoffier for technical support, the American Journal Experts and, particularly, Dr. Michele Avissar-Whiting for correcting the English writing, and the CNRS and INSERM for supporting this study.
Conflict of interest
The authors have no conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ribot-Ciscar, E., Hospod, V. & Aimonetti, JM. Noise-enhanced kinaesthesia: a psychophysical and microneurographic study. Exp Brain Res 228, 503–511 (2013). https://doi.org/10.1007/s00221-013-3581-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-013-3581-6