Abstract
Closing-in (CI) is the tendency to act very close to the model in tasks such as drawing, 3D construction, gesture imitation, or writing. Closing-in is observed in degenerative and focal brain diseases, but also in normally developing children. In the present paper, three experiments were conducted to evaluate whether CI can be triggered during a copying task in normal young adults by increasing stimulus complexity and attentional load. Participants were required to copy complex lines in one of three conditions: without interfering activities (baseline), during counting, or during execution of a 2-back short-term memory task. In Experiment 1, participants were required to reproduce horizontally aligned stimuli, starting from a dot placed below each stimulus and proceeding from left to right; in Experiment 2, stimuli were again horizontally aligned, but the starting dot was placed above each stimulus, and writing proceeded from right to left; in Experiment 3, stimuli were aligned vertically and copying proceeded in upward direction. Results from all experiments showed that when normal young adults are engaged in an attentional-demanding concurrent activity, they tend to approach to the model, whereas the effect of stimulus complexity disappeared with unusual writing direction (Experiments 2 and 3). These findings demonstrate that even in normal young adults, a reduction in available attentional resources can release an attraction toward the model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Closing-in (CI) is the tendency to act very close to the model in tasks such as drawing, 3D construction, gesture imitation, or writing (McIntosh et al. 2008). This phenomenon is observed in childhood (Ambron et al. 2009b; Gainotti 1972), in degenerative brain diseases (Ambron et al. 2009a; Conson et al. 2009; Gainotti et al. 1992; Grossi et al. 1978), and, more rarely, after focal brain lesions (Gainotti 1972; Grossi et al. 1996; Septien et al. 1992; Vereecken 1958).
CI has often been described in early years of normal development. Gainotti (1972) and Ambron et al. (2009b) assessed the incidence of CI in children ranging from 2 to 6 years. The authors reported that frequency of CI is high (among 75 %) in 2–3-year-old children and progressively decreases. Three developmental stages of copying ability have been described: occupation of the model (drawing on the model; about 2–4 years), utilization of the model (drawing on some parts of the model; about 4–5 years), and separation of the copy from the model (by 5th year; Mendilaharsu et al. 1970). On this basis, it has been claimed that CI during drawing tasks in demented patients may be considered as a regression to a childhood level of drawing development (Ambron et al. 2009b). In a longitudinal study, Ambron et al. (2009c) found that Alzheimer disease (AD) patients tend to deviate toward the model (Near-CI) in earlier stages of the disease, whereas they tend to draw on top of the model (Overlap-CI) as severity of AD increases.
Two main hypotheses have been proposed to account for CI. According to the compensation hypothesis, this phenomenon is the result of a “compensatory strategy” adopted to overcome visuospatial or working memory deficits (Lee et al. 2004). According to this hypothesis, immature (in children) or impaired (in demented patients) visuospatial processing, hampering analysis, and retention of the model lead subjects to draw near it. In this perspective, CI would be boosted by model complexity because more complex stimuli pose higher demands on visuospatial processing and working memory than simpler models (Lee et al. 2004). Recently, in a retrospective neuropsychological study comparing AD patients with or without CI, Serra et al. (2010) observed that AD patients showing CI had poorer visual-spatial abilities, in line with such “compensatory” account.
The attraction hypothesis, recently elaborated by McIntosh et al. (2008), foresees that CI could represent a primitive, “default” behavior in which the acting hand is drawn toward the focus of visual attention. According to this point of view, CI might be related to attentional and/or executive deficits associated with frontal lobe dysfunction that involve a failure to inhibit automatic actions toward salient stimuli in the workspace (Ambron et al. 2009b; McIntosh et al. 2008), independently from impairments in the visuospatial domain. Ambron et al. (2009a) compared the frequency of CI in patients with AD and fronto-temporal dementia and found that CI is affected by stimuli’s visuospatial complexity in AD patients only. The authors conclude that the two hypothesized mechanisms of CI may apply to different samples since visuospatial dysfunction could be responsible for CI in AD patients, whereas executive dysfunction would release the primitive and automatic attraction toward the stimuli in patients with fronto-temporal dementia. Consistently, Conson et al. (2009) described a patient with probable corticobasal degeneration who showed CI in drawing, notwithstanding relatively spared visuospatial abilities; the authors ascribed their patient’s CI to a pathological attraction toward the focus of attention released by frontal damage.
Some studies suggested that reduced availability of attentional resources can play a role in the genesis of CI in typically developing children. In a study on pre-school children, Ambron et al. (2009b) found a stronger migration toward the focus of the attention (visually presented animals) in dual-task conditions (drawing task with simultaneous naming of animals), suggesting that even in children reduced availability of attentional resources can enhance the approach behavior, as foreseen by the attraction account. Such a natural tendency to act toward the focus of attention has been demonstrated to affect both drawing and reaching responses (Ambron et al. 2012).
Since Ambron et al. (2009b) have found that dividing attentional resources between two tasks can magnify the presence of CI in children, then it is possible to argue that CI-related behaviors can be induced in normal adult subjects too. However, no studies are available on CI in young adults. The tendency to show CI has been evaluated in small samples of healthy elderly subjects who served as controls in two studies on AD patients (Kwak 2004; Lee et al. 2004). In copying lines, Lee et al. (2004) found that elderly subjects showed a tendency to deviate toward the model in copying figures, independently from their complexity. Kwak (2004) found a CI-related phenomenon only in a few elderly subjects (2/22) and suggested that aging might be associated with an increase in the incidence of the CI.
In the present paper, we aimed to ascertain whether, and by which experimental manipulations, CI can be induced in healthy young adults. In particular, we assessed copying of linear stimuli and simultaneously assessed whether visual complexity and concurrent attentional load can veer graphic productions toward the model. Concurrent attentional load was manipulated by means of two dual-task conditions, differing in their requirements of attentional resources. By these means, we directly tested the hypothesis that a high attentional load in young healthy subjects may induce CI-related phenomena. Such findings would be particularly relevant for interpretation of CI, since they would demonstrate that a specific manipulation of attentional resources is sufficient to release an attraction toward a relevant stimulus even in unimpaired subjects who have reached full development of visuospatial and executive functions.
Stimulus complexity was manipulated as it has been made in studies on CI on brain-damaged patients, by increasing the number and the type of elements inscribed in the linear stimuli. Although it has been suggested that stimulus complexity adds both a visuospatial (Lee et al. 2004) and an attentional load (McIntosh et al. 2008) in drawing tasks, a significant complexity effect in young subjects would be in line with hypotheses about the visuospatial genesis of CI and demonstrate an analogy between mechanisms leading to CI in healthy controls and AD patients.
The simultaneous manipulation of stimulus complexity and attentional load, which has not been investigated in previous studies, would allow us to obtain cues to comprehend the effect of complexity itself. An enhancement of the complexity effect in conditions of concurrent attentional load would support the idea that it is related to greater requirements of attentional resources.
Experiment 1
The stimuli for the experimental tasks were derived from Luria’s figures (Luria 1966), whose laterally extensive characteristic is thought suitable to elicit CI during copying (McIntosh et al. 2008; Lee et al. 2004). Three levels of stimulus complexity were operationally defined. To manipulate concurrent attentional load, we assessed copying in three experimental conditions: (1) baseline, in which subjects performed the copying task only; (2) during an automatic verbal task (counting) that mainly involves articulatory mechanisms and a few attentional resources; (3) during a controlled 2-back verbal memory task that requires verbal working memory and monitoring processes, with high attentional load (Kirchner 1958).
Methods
Participants
Sixty-two undergraduate students (46 females; ranging from 20 to 29 years) gave their written informed consent to take part in the experiment. All subjects were right-handed, had normal or corrected-to-normal vision, and were naive to the purposes and predictions of the experiment.
Materials and procedure
The stimuli used for the experiment consisted in linear figures composed by different basic units, such as squares or triangles. All basic units were 1 cm long and 1 cm high, and the line that connected each element was .5 cm long. All stimuli included 15 elements, for a total length of 23 cm. Both lines and elements were .1 cm thick.
Three levels of stimulus complexity were obtained by varying the number of different basic units enclosed in each array: three stimuli were composed by two to four different elements (complexity level 1), three stimuli were composed by five to seven different elements (complexity level 2), and three stimuli were made by eight to ten different elements (complexity level 3). The higher the number of different units in an array, the higher the requirement of visuospatial analysis and constructional planning required to reproduce the model. Within each stimulus, elements were alternated in a pseudorandom order (Fig. 1).
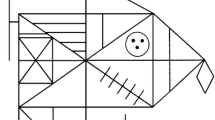
Experiment 1. Examples of stimuli of different complexity (levels 1–3 corresponding to a–c, respectively), to be copied from the left to the right starting from the dot printed below the stimulus. Under each stimulus, one example of participants’ responses is reported: the reproduction of the simplest stimulus (a) refers to the baseline condition, where the largest attempts to self-correction were observed, whereas the reproductions of the other levels (b and c) refer to the n-back condition (small self-corrections)
Stimuli were printed in a black ink on A3 (29.7 × 42 cm) white sheets, centered in the upper part of the page: the left edge of the stimulus was located 5.2 cm from the upper margin and 9.5 cm from the left margin. In the lower part of the sheet, a black dot (.1 cm diameter) was printed, aligned with the left edge of the stimulus at a distance of 7.2 cm from it.
The stimuli were presented one at a time on a tabletop, with their medial vertical axis aligned to subjects’ midsagittal plane; this position was maintained throughout the experiment. The simplest stimulus was administered as a practice trial and was not included in the analysis; presentation order of the remaining stimuli was randomized across subjects. Task instructions required to copy each item as fast and accurately as possible, starting from the black dot printed on each page and proceeding horizontally from left to right; subjects were explicitly instructed not to raise the tip of the pen from the paper while copying.
Participants were randomly assigned to one of three groups. Twenty participants were simply required to copy each stimulus (control group). Twenty-one participants performed the same drawing task while counting aloud from 1 onward until the end of each copying (counting group). The remaining 21 participants completed the copying task while performing a 2-back verbal memory task (2-back group): subjects were auditorily presented with a continuous sequence of digits, and as soon as they heard a target digit (7), they had to report the digit presented two positions before the target in the sequence. Performance on the secondary tasks was monitored online; in case of failure to comply with test instructions, or of errors on the secondary task, subjects were excluded from analysis.
Measures and statistical analysis
For each stimulus, we computed the minimum distance in centimeters between the stimulus and the copy; the lower the numeric values of such measure, the stronger the tendency to close on to the model. Since in pilot trials we observed that participants can become aware of their drift toward the model and try to compensate for it, we also computed the distance in centimeters between the stimulus and the copy at the end of the copying process (final distance). We called tweaking index the difference between final and minimum distances; the minimum value for the tweaking index was 0 (no self-correction), and higher values meant stronger tendency to self-correction during copying.
Minimum distance and tweaking index (after root square transformation) were analyzed separately by means of mixed 3X3 analyses of variance (ANOVA) with experimental condition (simple copying, automatic verbal task, controlled verbal memory task) as a between-subject factor and figure complexity (levels 1–3) as within-subject factor. Bonferroni test was used for post hoc contrasts (p < .05).
Results
All subjects performed the secondary task accurately and none was excluded from analysis.
As regards minimum distance (Table 1), the main effects of experimental condition (F(2,59) = 15.86; p < .0001; η 2p = .35) and of task complexity (F(2,118) = 20.13; p < .0001; η 2p = .25) were significant. The interaction between complexity and experimental condition was not significant (F(4,118) = .21; p = .93; η 2p = .007).
Post hoc comparisons to analyze the main effect of the experimental condition showed significantly shorter distances (i.e., greater CI-related phenomenon) in both dual-task groups versus the control group, and significantly shorter distances in the 2-back group versus the counting group (all p < .05). The complexity effect was due to significantly shorter distances for most complex stimuli with respect to stimuli of the two remaining levels (both p < .05).
Analysis of variance on (square-root transformed) tweaking index showed no effect of task complexity (F(2,118) = 1.7; p = .187; η 2p = .03), whereas the main effect of experimental condition (F(2,58) = 4.7; p = .013; η 2p = .14) and the complexity X experimental condition interaction (F(4,116) = 4.49; p = .002; η 2p = .13) were significant. Post hoc comparisons showed a significantly larger tweaking index (i.e., self-correction) in the control group for complexity level 1 (p < .05) with respect to the remaining complexity levels. In the other experimental groups, the tweaking index did not significantly differ across complexity levels.
Comment
The present findings showed that young normal adults are prone to drift toward the model when they are engaged in concurrent activities, particularly when such activities pose high attentional demands. Moreover, the deviation toward the model was enhanced by stimulus complexity. Normal young subjects tended to overcome this drift, but this attempt at self-correction was particularly evident only with the simplest stimuli in the baseline condition and was significantly disrupted when subjects’ attention was overloaded by a demanding secondary task (see Fig. 1).
These results are consistent with the view that reduction in available attentional resources and, to a lesser extent, stimulus complexity can release a tendency to be attracted toward the model even in normal young adults. However, such results might be related to the specific experimental setting and, for instance, could be ascribed to a generic tendency to move the copying arm toward the top of the sheet. As Lee et al. (2004) suggested when normal people write a sentence from left to right, they tend to deviate the script gradually upward from the baseline, and this could mimic CI phenomenon if the model is placed above the copying space. As argued by Lee et al. (2004), this hypothesis would hardly explain the specific effects of figure complexity and of dual-task conditions. Nonetheless, to exclude the possibility that our findings were generated by any generic motor bias, we performed a control experiment in which we modified model position.
Experiment 2
The most direct way to verify whether a generic motor bias could explain findings of Experiment 1 was to place the model in the lower part of the stimulus sheet and to require subjects to produce their copy above it. However, we considered that right-handed participants writing from the left to the right would have had their own arm upon the stimulus to be copied, exposing it gradually as the copy proceeded. This might have decreased stimulus saliency (and its possible “attractive” power). To overcome this problem, we could have required participants to draw with their arm extended forward and their elbow flexed, or to copy ‘open-loop’, looking at the model in a mirror with their hand invisible. For sake of simplicity, we required participants to produce a copy of the horizontally aligned model placed below the starting dot writing from the right to the left.
Methods
Participants
A novel sample of 42 undergraduate students (26 females; ranging from 19 to 34 years) was enrolled for the experiment. All subjects were right-handed, had normal or corrected-to-normal vision, and were naive to the purposes and predictions of the experiment. All subjects gave their written informed consent to take part in the experiment.
Materials and procedure
The stimuli and the procedure were the same of the Experiment 1, but in this case the stimulus was printed in the lower part of the A3 sheet, and the black starting dot in the upper part of the sheet, at 7.2 cm above the right edge of the stimulus (Fig. 2).
Experiment 2. Examples of stimuli of different complexity (levels 1–3 corresponding to a–c, respectively), to be copied from the right to the left starting from the dot printed above the stimulus. Above each stimulus, one example of participants’ responses is reported: the reproduction of the simplest stimulus (a) refers to the baseline condition, whereas the reproductions of the other levels (b and c) refer to the n-back condition
Also in this experiment, subjects were randomly assigned to one of three conditions as above: (1) baseline drawing task; (2) drawing task associated with counting task; (3) drawing task associated with 2-back task. As above, performance on the two secondary tasks was monitored online, in order to exclude subjects who did not comply with test instructions or made errors in the secondary task.
Measures and statistical analysis
As above, two mixed 3X3 analyses of variance (ANOVA) with experimental condition (simple copying, automatic verbal task, controlled verbal memory task) as a between-subject factor and figure complexity (levels 1–3) as within-subject factor were conducted on minimum distance and tweaking index (after root square transformation). Bonferroni test was used for post hoc contrasts (p < .05).
Results
All subjects performed the secondary tasks accurately and none was excluded from analysis.
ANOVA on minimum distance showed (Table 1) a significant main effect of the experimental condition (F(2,39) = 5.45; p < .01; η 2p = .22) and a significant interaction between complexity and experimental condition (F(4,78) = 2.7; p < .05; η 2p = .12). The effect of task complexity was not significant (F(2,78) = 2.4; p = .09; η 2p = .06).
Post hoc comparisons showed significantly shorter distances (i.e., greater CI-related phenomenon) in n-back and counting groups versus the control group (p < .05), whereas the n-back and counting groups did not differ between each other. Furthermore, the counting group showed a significant decrease in minimum distance with the most complex stimuli (complexity 3), significantly differing from control group and the other complexity levels. The 2-back group showed CI-effect independently from stimulus complexity.
ANOVA on the square root of the tweaking index showed no significant effects of experimental condition (F(2,39) = 2.67; p = 08; η 2p = .12) or task complexity (F(2,78) = 1.18; p = .31; η 2p = .03); the interaction between complexity and experimental condition was not significant (F(4,78) = .87; p = .48; η 2p = .04).
Comment
The results of Experiment 2 confirmed that subjects tended to approach the model in the dual-task conditions, with a downward drift of the graphic productions. Therefore, such findings would exclude that in Experiment 1, the upward drift was due to a tendency to draw toward the top of the sheet rather than to a CI-related effect, although in the Experiment 2 the effect of dual task was not dependent on the amount of attentional resources required by the secondary task. Further discrepancies in results of the two experiments were related to the lack of the main effect of figure complexity in Experiment 2 (here only the counting group was affected by stimulus complexity) and to the fact that in the latter Experiment, the attempts at self-correction (as expressed by the tweaking index) were not affected by experimental condition or task complexity.
In synthesis, the main finding of the Experiment 1 (i.e., a concurrent attentional load induced the subjects to deviate toward the stimulus) was confirmed by Experiment 2, but here the changes in the experimental setting determined some divergences in results, with substantial fading of the complexity effect. This observation could suggest that the effect of stimuli’s visual complexity is not as robust as that exerted by reduction in available attentional resources. However, it has to be taken into account that in the Experiment 2, participants were required to draw from the right to the left, in order to prevent a possible bias introduced by the fact that by drawing in the natural left-to-right direction, they would have covered the stimulus with their arm. This choice allowed us to confirm that the effect of attentional load is independent from stimulus position or writing direction, but also likely rendered more complex graphic reproduction of all stimuli, masking the visual complexity effect in Experiment 2. The unusual writing condition also determined more variability in the attempts to self-correct the drift toward the model (as expressed by the tweaking index), and thus, no significant effect was found, although the tweaking index was larger in the baseline condition than in the two dual-task conditions.
However, since in Experiment 2 we changed the writing direction, an alternative explanation for the present findings was plausible. The observation is that in both experiments, the participants showed a deviation of their copying movements toward the model, but in opposite directions (upward and downward, respectively), as a function of model position; this could still be interpreted as the result of a motor bias. Actually, if participants draw with their elbow in a relatively fixed position flanking their body, it remains possible that their arm tended upward in abductive movements and downward in adductive movements. To test this hypothesis, we performed a third experiment with further changes in model position and writing direction.
Experiment 3
To explore possible generalization of the effect of concurrent attentional load observed in drawing in horizontal direction (Experiments 1 and 2) and to verify the alternative hypothesis that a concurrent attentional load might enhance a simple motor bias, we presented subjects with a vertically aligned model to be reproduced drawing in vertical (radial) direction.
Methods
Participants
A novel sample of thirty-one undergraduate students (18 females; ranging from 20 to 30 years) was enrolled for the experiment. All subjects were right-handed, had normal or corrected-to-normal vision, and were naive to the purposes and predictions of the experiment. All subjects gave their written informed consent to take part in the experiment.
Materials and procedure
The stimuli and the procedure were the same of the Experiment 1, but in this case we presented the A3 stimulus sheets in portrait orientation (i.e., 90° counterclockwise rotation with respect to Experiment 1; Fig. 3).
Experiment 3. Examples of stimuli of different complexity (levels 1–3, corresponding to a–c, respectively); the same stimuli as in Experiment 1 were presented in vertical orientation (i.e., rotated by 90° counterclockwise with respect to Experiment 1). On the right of each stimulus, one example of participants’ responses is reported: the reproduction of the simplest stimulus (a) refers to the baseline condition, whereas the reproductions of the other levels (b and c) refer to the n-back condition
Participants were required to copy the stimuli starting from the black dot located in the bottom part of the sheet (in fact, participants were required to draw in radial direction away from their body). Also in this experiment, subjects were randomly assigned to one of three conditions as above: (1) baseline drawing task (n = 10); (2) drawing task associated with counting task (n = 10); (3) drawing task associated with 2-back task (n = 11). As above, performance on the two secondary tasks was monitored online, in order to exclude subjects who did not comply with test instructions or made errors in the secondary task.
Measures and statistical analysis
As above, for each stimulus, we computed the minimum distance between the stimulus and the copy and the tweaking index (difference between minimum and final distances) in centimeters.
Minimum distance and tweaking index (after root square transformation) were analyzed separately by means of mixed 3X3 analyses of variance (ANOVA) with experimental condition (simple copying, automatic verbal task, controlled verbal memory task) as a between-subject factor and figure complexity (levels 1–3) as within-subject factor. Bonferroni test was used for post hoc contrasts (p < .05).
Results
Only one subject did not perform the secondary task (the n-back task) accurately and had to be excluded from analysis. The statistical analysis was then performed on data from 30 participants, 10 per group.
ANOVA performed on minimum distance (see Table 2) showed a significant main effect of experimental condition (F(2,27) = 16.90; p < .001; η 2p = .556), whereas the effect of task complexity (F(2,54) = 2.60; p = .08; η 2p = .088) and the interaction between complexity and experimental condition (F(4,54) = 1.09; p = .36; η 2p = .075) were not significant.
Post hoc comparisons showed significantly shorter values (i.e., greater CI-related phenomenon) in n-back task group versus the counting and control groups (all p < .05), whereas the counting and control groups did not differ between each other.
ANOVA on the square root of the tweaking index showed a significant effect of experimental condition (F(2,27) = 5.23; p < .05; η 2p = .18), whereas the effect of task complexity (F(2,54) = 2.56; p = .09; η 2p = .07) and the interaction between complexity and experimental condition (F(4,54) = .93; p = .45; η 2p = .04) were not significant. Post hoc contrasts showed a significantly smaller tweaking index in the n-back task group with respect to the control group (baseline condition; p < .05).
Comment
In Experiment 3, participants tended to approach the model in the dual-task conditions, as in the previous Experiments. Therefore, such findings demonstrated that this phenomenon was not specific of horizontal writing directions and can likely be observed in all drawing tasks. As in Experiment 2, no effect of figure complexity was observed in Experiment 3. This observation would confirm that the effect of visual complexity is weaker and more susceptible to changes of the experimental setting (drawing in a direction different from the natural left-to-right direction) than that exerted by reduction in available attentional resources. It is worth underlining that also in Experiment 3, the subjects tried to self-correct their drift toward the model, but this was possible only when they were not engaged in demanding concurrent activities (see Fig. 3).
It is noteworthy that the leftward deviation (toward the relevant visual stimulus) in Experiment 3 allowed us to exclude the hypothesis according to which an attentional-demanding concurrent task could enhance a simple motor bias. Actually, if one posits that subjects drew with their elbow flanking their body (as if they pivoted their arm at the elbow), then a rightward shift would have been expected in drawing from the lower part to the upper part of the response sheet (radially, in fact), a drift opposite to what was observed. Therefore, even though in our Experiments we did not manipulate stimulus position only, but also changed writing direction, we could gather converging evidence to exclude that our findings were related to a simple motor bias.
Discussion
The present study aimed at verifying whether stimulus complexity and dual-task conditions can determine a tendency to deviate the graphic productions toward the model in healthy young adults. Results from the three experiments consistently demonstrated that normal young subjects are attracted toward a model to be reproduced and that the resulting drift is particularly enhanced when participants are concurrently engaged in a dual-task condition implying strong attentional load, as it is the case for n-back task. Moreover, in all the experiments, normal subjects tried to overcome such a drift toward the model, but their attempt at self-correction was significantly disrupted when subjects’ attention was overloaded by a demanding secondary task. The effect of stimulus complexity was significant in Experiment 1, but disappeared in Experiments 2 and 3 when participants were engaged in a drawing task with greater graphomotor complexity with respect to the natural left-to-right direction. Taken together, the present results appear to support the hypothesis that a reduction in available attentional resources releases automatic attraction toward the focus of attention (Ambron et al. 2009b).
Recent research demonstrated that during copying of complex lines, continuous shifts of eye gaze and (plausibly) attention occur between the drawing hand and the model to be reproduced (Tchalenko and Miall 2009). Moreover, several neurofunctional investigations of drawing in normal subjects have reported that frontal regions are involved in copying tasks, likely for the purposes of planning and monitoring the graphic productions (Miall et al. 2009; Ogawa and Inui 2009). In the present study, subjects were required to divide their attentional resources between two tasks. Classical studies demonstrated that in such cases, cognitive resources must be distributed between the tasks with a consequent performance deterioration (Navon and Miller 1987; Sperling and Dosher 1986; Wickens et al. 1983). Our dual-task conditions implied different cognitive burden: counting is an automatic task requiring limited attentional and monitoring resources, whereas the n-back task strongly involves working memory and attention that are subtracted from the drawing task.
In the first experiment, stimuli’s visual complexity also determined a tendency to close-into the model. It has been claimed that complex models require subjects to compare the target with their own copy more frequently than simple stimuli; for this reason, patients with visuospatial deficits would tend to draw near to or on the model (Lee et al. 2004). Therefore, CI for more complex stimuli could be explained as due to both visuospatial and working memory burden, consistent with the hypothesis suggesting that the CI may represent a compensative attempt at coping with visuospatial disorders in patients. Other authors (McIntosh et al. 2008) have argued, instead, that the complexity effect is compatible with both the compensation hypothesis and the attraction hypothesis. The present findings would be better compatible with the idea that the complexity effect is not as robust as the effect of reduced availability of attentional resources, since it disappeared when subjects were engaged in an unusual drawing task (Experiments 2 and 3). Moreover, in Experiment 1, a significant interaction between task complexity and experimental condition was found on the normal subjects’ attempts to correct their tendency to approach to the model: the smallest tweaking index was observed in the baseline group, for the simplest visual stimuli. The same interaction was also observed in Experiment 2, where participants assigned to one dual-task condition showed the smallest distance from the model for most complex stimuli. Although such interactions were observed only in a few analyses, they could suggest that the effect of visual complexity was not completely independent from the effect related to the division of attentional resources on two tasks. This observation seems to reinforce the idea that the main determinant of the tendency to move the acting hands toward current attentional focus in normal young subjects is a reduction in resources devoted to monitor task execution, as in normally developing children (Ambron et al. 2009b). The clinical counterpart of this phenomenon can be found in patients with dysexecutive/frontal impairments who show CI (McIntosh et al. 2008; Conson et al., 2009; Lepore et al. 2005).
It remains entirely possible that different factors may underlie the CI in normal childhood and in patients with degenerative diseases, since the “attentional hypothesis” and the “compensatory hypothesis” are not mutually exclusive. However, the present results demonstrated that increasing concurrent attentional load determined a significant attraction toward the model in a line-copying task, and such findings cannot be accommodated within the “compensatory account”. If we also consider that the simultaneous manipulation of visual complexity did not determine an analogously robust effect (it disappeared in Experiments 2 and 3, where participants wrote in an unusual direction), we have to conclude that there is no room to support the “compensation hypothesis” to explain CI-related phenomena in cognitively unimpaired young individuals who achieved the full development of their visuospatial and executive functions.
In conclusion, we found that a tendency to draw close to the model (that may be considered as a CI-related phenomenon) can be triggered even in healthy young subjects during demanding dual-task conditions, particularly in reproducing complex visual stimuli. We could thus demonstrate that reduced attentional resources determine a behavior that may be considered as an attraction toward the model even in normal adults. Although in brain-damaged patients the “compensation” and the “attentional” hypotheses might not be mutually exclusive, our results are consistent with the view that a dysexecutive impairment in brain-lesioned patients can impair their ability to keep their hands far from the spatial location where their attention is focused on.
References
Ambron E, Allaria F, McIntosh RD, Della Sala S (2009a) Closing-in behaviour in fronto-temporal dementia. J Neurol 256:1004–1006
Ambron E, Della Sala S, McIntosh RD (2009b) Animal magnetism: evidence for an attraction account of closing-in behaviour in pre-school children. Cortex 45:278–284
Ambron E, McIntosh RD, Allaria F, Della Sala S (2009c) A large-scale retrospective study of closing-in behavior in Alzheimer’s disease. J Int Neuropsychol Soc 15:787–792
Ambron E, Della Sala S, McIntosh RD (2012) Closing-in behaviour and motor distractibility. Neuropsychologia 50:419–425
Conson M, Salzano S, Manzo V, Grossi D, Trojano L (2009) Closing-in without severe drawing disorders: the ‘‘fatal’’ consequences of pathological attraction. Cortex 45:285–292
Gainotti G (1972) A quantitative study of the ‘‘closing-in’’ symptom in normal children and in brain-damaged patients. Neuropsychologia 10:429–436
Gainotti G, Parlato V, Monteleone D, Carlomagno S (1992) Neuropsychological markers of dementia on visuospatial tasks: a comparison between Alzheimer’s type and vascular forms of dementia. J Clin Exp Neuropsychol 14:239–252
Grossi D, Orsini A, De Michele G (1978) The copying of geometric drawings in dementia: an experimental study of 218 subjects. Acta Neurol 33:355–360
Grossi D, Correra G, Calise C, Trojano L (1996) Selective constructional disorders after right subcortical stroke. A neuropsychological premorbid and follow-up study. Ital J Neurol Sci 14:23–33
Kirchner WK (1958) Age differences in short-term retention of rapidly changing information. J Exp Psychol 55:352–358
Kwak YT (2004) ‘Closing-in’ phenomenon in Alzheimer’s disease and subcortical vascular dementia. BMC Neurol 4:3
Lee BH, Chin J, Kang SJ, Kim E, Park KC, Na DL (2004) Mechanism of the closing-in phenomenon in a figure copying task in Alzheimer’s disease patients. Neurocase 10:393–397
Lepore M, Conson M, Grossi D, Trojano L (2005) Multidirectional transpositions suggesting pathologic approach behavior after frontal stroke. Neurology 64:1615–1617
Luria AR (1966) Human brain and psychological process. Harper and Row, New York
McIntosh RD, Ambron E, Della Sala S (2008) Evidence for an attraction account of closing-in behaviour. Cogn Neuropsychol 25:376–394
Mendilaharsu C, Delfino de Cultelli I, Sapriza de Correa S (1970) Evolución de la conducta de la copia de las figures geometricas en el niňo. Acta Neurol Latinoam 16:192–213
Miall RC, Gowen E, Tchalenko J (2009) Drawing cartoon faces—a functional imaging study of the cognitive neuroscience of drawing. Cortex 45:394–406
Navon D, Miller J (1987) Role of outcome conflict in dual-task interference. J Exp Psychol Hum Percept Perform 13:435–448
Ogawa K, Inui T (2009) The role of the posterior parietal cortex in drawing by copying. Neuropsychologia 47:1013–1022
Septien L, Giroud M, Sautreaux JL, Dumas R (1992) Corpus callosotomy for intractable seizures in the pediatric age group: influence on frontal syndrome. Child Nerv Syst 8:2–3
Serra L, Fadda L, Perri R, Caltagirone C, Carlesimo GA (2010) The closing-in phenomenon in the drawing performance of Alzheimer disease patients: a compensation account. Cortex 46:1031–1036
Sperling G, Dosher BA (1986) Strategy and optimization in human information processing. In: Boff K, Kaufman L, Thomas J (eds) Handbook of perception and performance, vol 1. Wiley, New York, pp 1–85
Tchalenko J, Miall RC (2009) Eye-hand strategies in copying complex lines. Cortex 45:368–376
Vereecken PJ (1958) New symptoms of closing-in and their interpretation. J Nerv Ment Dis 127:42–50
Wickens CD, Sandry D, Vidulich M (1983) Compatibility and resource competition between modalities of input, output, and central processing. Hum Factors 25:227–248
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sagliano, L., D’Olimpio, F., Conson, M. et al. Inducing closing-in phenomenon in healthy young adults: the effect of dual task and stimulus complexity on drawing performance. Exp Brain Res 225, 409–418 (2013). https://doi.org/10.1007/s00221-012-3381-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-012-3381-4