Abstract
The repetition of nociceptive stimuli of identical modality, intensity and location at short (1 s) and constant inter-stimulus interval (ISI) determines a strong habituation of the corresponding electroencephalographic (EEG) responses. To understand what determines this response habituation, we (1) examined the effect of introducing a selective change in the spatial location of the repeated stimulus (i.e., without altering its modality, intensity and timing), and (2) dissected the relative contribution of bottom-up, stimulus-driven spatial changes and top-down, cognitive expectations of such a change. Multichannel EEG was recorded while participants received a triplet of stimuli (S1–S2–S3) delivered to the hand dorsum at 1-s ISI. S3 was delivered either to the same hand as S1 and S2 or to the other hand, and participants were either explicitly informed or not informed of the location of S3. We found that, unlike the introduction of a change in the sensory modality of the repeated stimulus (Valentini et al. in J Cogn Neurosci 23:2822–2837, 2011), introducing a change in its spatial location did not produce a significant dishabituation of the laser-evoked N1, N2 and P2 peaks, but only a small amplitude increase following the P2 peak, maximal on the hemisphere contralateral to the stimulated hand. Furthermore, the magnitude of the elicited responses was not significantly affected by cognitive expectations. Altogether, these results indicate that bottom-up, stimulus-driven novelty resulting from a change in stimulus spatial location does not revert the habituation caused by repetition suppression, but determines a small increase of neural activity over the contralateral central-parietal cortex, likely reflecting shifts in spatial attention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brief laser heat pulses selectively activate Aδ and C skin nociceptors (Bromm and Treede 1984) and elicit transient responses (laser-evoked potentials, LEPs) in the ongoing electroencephalogram (EEG) (Carmon et al. 1976; Mouraux et al. 2003), mediated by type-II Aδ mechano-heat nociceptors (II-AMH) and spinothalamic neurons (Treede 2003). The largest LEP deflection, a negative–positive complex (N2–P2) maximal at the scalp vertex (Bromm and Treede 1984), is preceded by a smaller negative deflection, the N1 wave, maximal over the temporal-central region contralateral to the stimulated hand (Treede et al. 1988; Valentini et al. 2012). These waves result from the activity of several cortical sources, including the bilateral operculoinsular, the anterior cingulate and the contralateral primary sensory cortices (Garcia-Larrea et al. 2003). Besides eliciting such event-related potentials (ERPs), nociceptive stimuli also induce transient modulations of ongoing oscillatory EEG activities, revealed when single-trial waveforms are analyzed in the time–frequency domain (Mouraux et al. 2003).
Although LEPs are increasingly used to investigate the peripheral and central processing of nociceptive inputs (Treede 2003), a full understanding of their functional significance remains to be achieved. By recording the psychophysical and EEG responses elicited by triplets of identical laser stimuli (S1–S2–S3) delivered to the hand at 1-s interval, we showed that stimulus repetition strongly decreases the ERP magnitude without affecting the perception of pain (Iannetti et al. 2008), which indicates that the amplitude of the LEP response does not reflect subjective pain perception. However, the fact that LEP amplitude can be clearly dissociated from perceived pain intensity does not necessarily imply that the neural activity sampled by LEPs does not contribute to the perception of pain (see Legrain et al. 2011; Iannetti and Mouraux 2010).
Characterizing what causes the response reduction induced by stimulus repetition is important to understand the functional significance of laser-evoked EEG responses. This response reduction reflects habituation, which is defined as a response decrement that results from repeated stimulation and that is distinct from sensory adaptation and sensory fatigue (Thompson and Spencer 1966). The habituation of the LEP amplitude could be explained by the fact that S2 and S3 were identical to S1 in the four basic attributes defining a sensory stimulus (modality, location, intensity and timing; Gardner and Martin 2000), and hence that S2 and S3 were less novel than S1 (bottom-up stimulus-driven changes), but also by the fact that subjects knew that S2 and S3 would be identical to S1, and hence that S2 and S3 were more certain than S1 (top-down cognitive expectations). Therefore, with the paradigm used in Iannetti et al. (2008), we could not (1) determine which sensory attributes are important for causing the ERP habituation, and (2) dissect the respective contribution of the lack of bottom-up stimulus-driven changes, and top-down cognitive expectations of such a lack of change.
We recently showed that bottom-up novelty resulting from a change in stimulus modality (nociceptive vs auditory, i.e., what) is important in causing the ERP dishabituation (Valentini et al. 2011). In the theoretical framework of the ‘dual process’ theory of habituation (Groves and Thompson 1970), which proposes that two distinct and independent processes govern the behavioral response to repetitive stimulation (an incremental process defined as sensitization and a decremental process defined as habituation), this observation would support the view that a change in the modality of the repeated stimulus is able to contrast the loss of stimulus saliency and, thereby, to promote sensitization.
Here we aimed to define the role of another important stimulus attribute, that is, its spatial location (where), by examining the effect of introducing a selective change in the spatial location of the repeated stimulus. In this context ‘selective’ means that we introduced a change only in where the stimulus was delivered, without altering its modality (as in Valentini et al. 2011), intensity and timing. Participants were either explicitly informed or not informed of the location of S3, to examine the interaction between stimulus-driven changes in spatial location and the cognitive expectations of such a change in causing response habituation. This study, besides characterizing the functional significance of LEPs, contributes to the understanding of how the brain processes spatial and temporal patterns of somatosensory stimuli, a question that has been also addressed in behavioral experiments (e.g., exploring the saltation illusion using repeated laser stimuli, Trojan et al. 2006).
Materials and methods
Subjects
Eleven healthy subjects (6 men and 5 women; 10 right-handed and one left-handed) aged 21–60 years (mean ± SD: 29.8 ± 11.4) participated in this study. All participants gave their written informed consent. The study conformed to the standards required by the Declaration of Helsinki and was approved by the local ethics committee.
Nociceptive stimulation
Noxious radiant stimuli were generated by an infrared neodymium yttrium aluminum perovskite (Nd:YAP) laser with a wavelength of 1.34 μm (Electronical Engineering, Florence, Italy). At this wavelength, the skin is transparent to the laser beam, and hence, passive heat propagation is not needed to reach the depth where Aδ- and C-fiber nociceptive terminals are located (Baumgartner et al. 2005; Iannetti et al. 2006). The laser beam was transmitted through an optic fiber, and its diameter was set at approximately 8 mm (~50 mm2) by focusing lenses. The duration of the laser pulses was 4 ms. Laser pulses were directed at the dorsum of the right or the left hand, on a squared area (5 × 5 cm) defined prior to the beginning of the experimental session. To familiarize subjects with the nociceptive stimulus and to ascertain that the sensation elicited was not different between the two hands, a small number of laser pulses were delivered to the right and left hand dorsum. The energy of the laser stimulus was then adjusted individually using the method of limits, in order to elicit a verbally reported clear pricking pain sensation (3.0 ± 0.5 J), related to the activation of Aδ nociceptors (Treede et al. 1995).
Experimental design
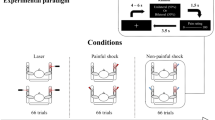
A schematic illustration of the experimental design is shown in Fig. 1. Stimuli were delivered in four different blocks. Their order was counterbalanced across subjects, to avoid any effect of long-term habituation. In each block, trains of laser stimuli were delivered. Each train consisted of three stimuli of identical energy (S1–S2–S3, a triplet) delivered to the hand dorsum at a constant inter-stimulus interval (ISI) of 1 s. The time interval between each triplet was 8–12 s (rectangular distribution). While S1 and S2 were always delivered to the same hand, S3 was either also delivered to the same hand (triplet same) or to the other hand (triplet other). Approximately 3 s before the onset of each triplet subjects were verbally informed of the side on which S1 and S2 would be delivered. The number of triplets starting with right or left hand stimulation was balanced within each block. In two out of four blocks the participants were also informed of the spatial location of the incoming S3 (triplet certain), whereas in the remaining two, they were not (triplet uncertain). Within each uncertain block, the occurrence of same and other triplets was balanced and pseudo-randomized. The maximum number of consecutive triplets belonging to the same condition (i.e., same or other) was three. Before starting the recording, subjects were instructed to relax and equally attend all stimuli of each triplet, independently of experimental condition and stimulated hand. The choice of using trains of three stimuli was driven by previous evidence (e.g., Fruhstorfer 1971; Iannetti et al. 2008) that habituation is already maximal after the second stimulus, and it does not further increase. The decision of delivering only three stimuli had also the advantage of limiting the total number of delivered noxious stimuli.
Experimental design. Laser-evoked potentials (LEPs) were recorded in four different blocks (top right), whose order was counterbalanced across subjects. In each block, laser stimuli were presented on the dorsum of either the right (blue) or the left hand (red). Stimuli were delivered in trains. Each train consisted of three stimuli (S1–S2–S3, a triplet) delivered at a constant inter-stimulus interval (ISI) of 1 s (bottom right). While S1 and S2 were always delivered on the same hand, S3 was delivered either to the same hand (triplet same) or to the other hand (triplet other). In two out of four blocks, participants were explicitly informed of the spatial location of S3 (condition certain), whereas in the remaining two blocks, they were not (condition uncertain). Within each uncertain block, the occurrence of same and other triplets was balanced and pseudo-randomized. This 2 × 2 design allowed us to dissect the effects of ‘space change’ from the effect of ‘uncertainty’ of such a change in determining the habituation and dishabituation of the elicited EEG responses (left)
In each block we delivered 30 triplets, for a total of 120 triplets in the whole experiment. To avoid nociceptor fatigue and sensitization, the location of the irradiated spot was shifted after each stimulus. The spot location was controlled by a computer that used two servo-motors (HS-422; Hitec RCD; angular speed, 60°/160 ms) to orient the laser beam along two perpendicular axes (see Lee et al. 2009 for details). Between S1 and S2 of all triplets, and between S2 and S3 of triplets same, the target of the laser beam was displaced by approximately 2 cm along a proximal–distal axis on the hand dorsum (see previous paragraph for details). The direction of this displacement was balanced in each block (15 stimuli in the proximal and 15 in the distal direction) (Iannetti et al. 2008). This procedure aimed to minimize the variation in thickness and innervation of the irradiated skin and, consequently, the intensity of the nociceptive somatosensory input (Schlereth et al. 2001). Between S2 and S3 of triplets other, the target of the laser beam was automatically displaced to the dorsum of the other hand. Because variations in baseline skin temperature could bias results (Baumgartner et al. 2005), an infrared thermometer was used to ensure that no significant variations occurred in baseline skin temperature throughout the blocks.
At the end of each block participants were asked to provide an across-trials average rating of the intensity of the sensation elicited by the first (S1), the second (S2) and the third (S3) stimulus composing each type of presented triplets, using a numerical scale ranging from 0 (no pain) to 10 (pain as bad as it could be) (Jensen et al. 1989). Thus, we obtained three separate ratings (S1–S2–S3), one representing the average perceived intensity of S1, one representing the average perceived intensity of S2 and one representing the average perceived intensity of S3.
EEG recording
Participants were seated on a comfortable chair in a silent, temperature-controlled room. They were asked to place their hands on a desk, and to keep their eyes open and gaze slightly downwards. A screen in front of the participants blocked the vision of both hands. The EEG was recorded using 21 Ag–AgCl electrodes, placed on the scalp according to the International 10–20 system and referenced to the nose. Acoustic isolation was ensured using earplugs and headphones. The electro-oculogram (EOG) was recorded using two surface electrodes, one placed over the right lower eyelid, the other placed lateral to the outer canthus of the right eye. Signals were amplified and digitized using a sampling rate of 1,024 Hz and a conversion of 12 bit, giving a resolution of 0.195 μV (SD32, Micromed, Treviso, Italy).
EEG analysis
Preprocessing
EEG data were preprocessed and analyzed using Letswave (http://amouraux.webnode.com) (Mouraux and Iannetti 2008) and EEGLAB (Delorme and Makeig 2004). EEG data were segmented into epochs using a time window ranging from 1 s before the first stimulus (S1) to 1 s after the third stimulus (S3) of each triplet (total epoch duration: 4 s). Each epoch was baseline corrected using the prestimulus interval from −1 to 0 s as reference. The baseline correction was done by subtracting the mean of the signal of the prestimulus interval so that the mean of the baseline was equal to zero after baseline correction. EEG epochs were band-pass filtered from 1 to 30 Hz using a fast Fourier transformation filter. EOG artifacts were subtracted using a validated method based on independent component analysis (ICA; Jung et al. 2000). In all datasets, ICs related to eye movements had a large EOG channel contribution and a frontal scalp distribution. Since frequency filtering and ICA changed the EEG signals, a second baseline correction was performed using the same −1 to 0 s reference interval, thus ensuring that the average signal in the prestimulus interval was equal to zero. Finally, epochs with amplitude values exceeding ±100 μV (i.e., epochs likely to be contaminated by an artifact) were rejected. These epochs constituted the 3.2 ± 3.6% of the total number of epochs.
Analysis in the time domain (N1, N2 and P2 waves of LEPs)
In each experimental condition, EEG epochs were divided in two groups (right and left) according to the hand to which S3 was delivered (see Fig. 1). Epochs belonging to each of these two groups were averaged, time-locked to the onset of S1 of each triplet. This procedure yielded eight average waveforms for each subject (one ‘right S3’ and one ‘left S3’ waveform for each of the four experimental conditions, that is, ‘certain same’, ‘certain other’, ‘uncertain same’ and ‘uncertain other’). For example, in the condition ‘certain other’, one average waveform was calculated using the trials in which S3 was delivered to the right hand and was preceded by S1 and S2 delivered to the left hand, and the other average waveform was calculated using the trials in which S3 was delivered to the left hand and was preceded by S1 and S2 delivered to the right hand. The N2 and P2 waves were identified at the vertex (Cz) referenced to the nose. The N2 wave was defined as the most negative deflection after stimulus onset. The P2 wave was defined as the most positive deflection after stimulus onset. The N1 wave was identified at the contralateral temporal electrode (Tc), referenced to Fz (Treede et al. 2003). The N1 wave was defined as the negative deflection preceding the N2 wave. Using such Tc–Fz montage, the N2 is seen as a positive deflection following the N1. The amplitude of all ERP waves was measured from baseline to peak. For plotting scalp topographies of laser-evoked responses, channel locations of the ERPs elicited by S3 delivered to the right hand were flipped on the x-axis (right-left), and this flipped ‘right S3’ ERPs were subsequently averaged together with the corresponding ‘left S3’ ERPs, thus yielding one average scalp topography for each of the four experimental conditions, as if all S3 were delivered to the left hand.
Analysis in the time–frequency domain
For each EEG epoch, a continuous wavelet transform was used to obtain an estimate of the amplitude of oscillatory activity as a function of time and frequency. Because this estimate is a time-varying expression of oscillation amplitude regardless of its phase, averaging these estimates across trials discloses both phase-locked and non-phase-locked modulations of signal amplitude, provided that these modulations are both time-locked to the onset of the event and consistent in frequency (i.e., the latency and frequency at which they occur are reproducible across trials). The wavelet transform was performed using a Morlet wavelet, which consists in a complex exponential function localized in time by a Gaussian envelope. The initial spread of the Gaussian envelope was set to 2.5/πω0 (ω0 being the central frequency of the wavelet—for details of the method, see Mouraux et al. 2003; Mouraux and Iannetti 2008). Across-trial averaging of the obtained time–frequency matrices produced a spectrogram of the average EEG oscillation amplitude as a function of time and frequency, which was used to identify non-phase-locked, laser-induced modulations of ongoing EEG rhythms (event-related synchronization and desynchronization, ERS and ERD). For each estimated frequency, results were expressed as an increase or decrease of oscillation amplitude relative to a prestimulus reference interval (−1 to −0.1 s relative to the onset of S1), using the following formula: ER% t,f = [A t,f − R f ]/R f , where A t,f is the signal amplitude at a given time t and at a given frequency f, and R f is the signal amplitude at the frequency f, averaged within the prestimulus reference interval (Pfurtscheller and Lopes da Silva 1999).
Quantitative analysis of time–frequency spectrograms
To explore the differences between the brain responses elicited in the four different experimental conditions, three time–frequency regions of interest (ROIs) were defined in the spectrograms obtained at Cz. The time–frequency limits of these time–frequency ROIs were defined based on previous work from our group (Iannetti et al. 2008), as follows: phase-locked LEP (2–8 Hz and 100–500 ms), non-phase-locked ERS (10–20 Hz and 100–500 ms) and non-phase-locked ERD (7–13 Hz and 400–900 ms). Within each time–frequency ROI, ER% values were extracted to compute the mean of the 20% of points displaying the highest increase (LEP and ERS) or decrease (ERD). This ‘top 20%’ summary measure reflects the higher ER% values within each window of interest, with the aim of reducing the noise introduced by including all points of the spectrogram, some of which may display little or no response (Mouraux and Iannetti 2008). This approach has been successfully used to analyze both ERP (Iannetti et al. 2008; Valentini et al. 2011) and blood oxygen level-dependent functional magnetic resonance imaging data (Iannetti et al. 2005; Mitsis et al. 2008).
Statistical analyses
In order to confirm the previously observed repetition suppression of LEPs (Iannetti et al. 2008; Valentini et al. 2011), a paired t test was performed to compare, for each condition, the N2 and P2 peak amplitudes elicited by S1 with those elicited by S2.
A two-way repeated-measure ANOVA was used to explore the main effect of the factors ‘space change’ (two levels: ‘same hand’, ‘other hand’) and ‘uncertainty’ (two levels: ‘certain’, ‘uncertain’), as well as the possible interaction between them, on the following perceptual and electrophysiological measures: (1) average intensity ratings elicited by S3; (2) N1, N2 and P2 peak amplitudes elicited by S3; (3) ER% summary measure of each ROI of the response elicited by S3. These statistical comparisons were performed using SPSS 16.0 (SPSS Inc. Chicago).
Furthermore, to disclose the time course of the possible effects of ‘space change’ and ‘uncertainty’, we performed the same repeated-measures ANOVA, but using each time point of the averaged ERP waveforms, as implemented in LetsWave (http://amouraux.webnode.com) (Mouraux and Iannetti 2008). This analysis yielded two waveforms expressing the significance of the effect, one for each of the two experimental factors across time. A consecutivity threshold of 40 ms was chosen to account for multiple comparisons.
Results
Quality and intensity of perception
In all subjects, laser stimuli elicited a clear pinprick sensation, related to the activation of Aδ fibers (Bromm and Treede 1984). There was no main effect of either the factor ‘space change’ (P = 0.14) or the factor ‘uncertainty’ (P = 0.16) on the intensity of the sensation elicited by S3. There was no significant interaction between the two factors (P = 0.92).
Laser-evoked N1, N2 and P2 waves
Grand average waveforms of LEPs obtained in the four different conditions are shown in Fig. 2 (left panel), together with the single-subject average amplitudes of the N2 and P2 waves of S1-, S2- and S3-LEPs (right panel).
Left panel Group-level average LEPs. While S1 and S2 were always delivered to the same hand, S3 was delivered either to the same hand of S1 and S2 (triplet same, black and green waveforms) or to the other hand (triplet other, red and blue waveforms). The spatial location of S3 was either certain (black and red waveforms) or uncertain (blue and green waveforms). Displayed signals were recorded at Cz (nose reference). x-axis: time (s); y-axis: amplitude (μV). The vertical dashed lines mark the onset of the three stimuli. Right panel Single-subject and group-level average peak amplitudes of the N2 and P2 waves elicited by S1, S2 and S3. x-axis: stimulus number (S1–S3); y-axis: amplitude (μV). Horizontal lines represent the group averages
Effect of stimulus repetition
In all conditions, there was significant reduction of the N2 and P2 peak amplitudes elicited by S2 compared with those elicited by S1 (‘certain same’: N2: P = 0.002; P2: P = 0.03; ‘certain other’: N2: P = 0.006; P2: P = 0.0006; ‘uncertain same’: N2: P = 0.001; P2: P = 0.002; ‘uncertain other’: N2: P = 0.0005; P2: P = 0.001). This finding confirms the previously observed repetition suppression of LEPs when identical laser stimuli are repeated at constant ISI of 1 s (Iannetti et al. 2008; Valentini et al. 2011).
Effect of ‘space change’
There was no significant main effect of the factor ‘space change’ on the amplitude of the N1, N2 and P2 waves elicited by S3 (N1: P = 0.83; N2: P = 0.16; P2: P = 0.62). The S3-LEP amplitudes were not larger when S3 was delivered to the hand opposite to the one where S1 and S2 were delivered (triplet other) than when S3 was delivered to the same hand (triplet same) (Fig. 3, left panel).
Main effect of ‘space change’ and ‘uncertainty’ of such a change on LEPs. Superimposition of orange and green waveforms represents the main effect of ‘space change’ (left panel). Superimposition of full and dashed black waveforms represents the main effect of the ‘uncertainty’ (right panel). The vertical dashed lines mark the onset of S3. Average peak amplitudes and scalp maps are shown in the insets
Effect of ‘uncertainty’
There was no significant main effect of the factor ‘uncertainty’ on the amplitude of the N1, N2 and P2 waves elicited by S3 (N1: P = 0.55; N2: P = 0.28; P2: P = 0.79). The S3-LEP amplitudes were not larger when the subject was uncertain about the side where S3 was delivered (triplet uncertain) than when the subject was certain about the side where S3 was delivered (triplet certain) (Fig. 3, right panel).
Interaction between the factors ‘space change’ and ‘uncertainty’
Finally, there was no significant interaction between the factors ‘space change’ and ‘uncertainty’ on the amplitude of the N1, N2 and P2 waves elicited by S3 (N1: P = 0.61; N2: P = 0.90; P2: P = 0.88).
Time course of the effect of ‘space change’ and ‘uncertainty’
To follow the effect of these two experimental factors across time, in addition to peak amplitude analysis, we computed a two-way repeated-measures ANOVA for each time point of the average S3-LEP waveforms. At electrode Cz, the factor ‘space change’ was a significant source of variation of the ERP amplitude in the time interval immediately following the P2 peak (361–409 ms) (Fig. 4). Within this time interval, the ERP amplitude was greater when S3 was delivered to the hand opposite to the one where S1 and S2 were delivered (triplet other) than when S3 was delivered to the same hand (triplet same). This amplitude increase was localized over the hemisphere contralateral to the stimulated hand (Fig. 4, top panel). The interaction between the two experimental factors across time was not significant.
Whole-waveform ANOVA and scalpmap timeseries. Top panel to assess the time course of the effects of ‘space change’ and ‘uncertainty’ on LEPs, we performed a repeated-measures ANOVA using each time point of the averaged waveforms at Cz (nose reference). Below each waveform the F values for each time point are represented. The time interval in which the effect was significant is highlighted in blue (consecutivity threshold = 40 ms). Note that the factor ‘space change’ significantly modulated the ERP waveforms in a short time interval following the P2 peak (361–409 ms). Bottom panel scalpmap time series in the time window following the P2 peak, and containing the significant effect of the factor ‘space change’. Scalpmaps of the two levels of this experimental factor, as well as of the difference between the two factors, are plotted every 20 ms
Laser-induced ERS and ERD
Grand average spectrograms of time–frequency EEG responses in the four different conditions are shown in Fig. 5.
Main effect of ‘space change’ (top panel) and ‘uncertainty’ of such a change (bottom panel) on laser-induced ERS and ERD. Plots depict the time–frequency representation of the stimulus-induced modulations of EEG oscillations at Cz (nose reference). x-axis: time (s); y-axis: frequency (Hz). The vertical dashed lines mark the onset of the S3. The color scale represents the average increase (ERS%) or decrease (ERD%) of oscillation amplitude, relative to the S1 prestimulus interval. Three time–frequency regions of interest (ROIs) were defined. For each region of interest, a summary measure was obtained by averaging the top 20% time–frequency points displaying the highest signal increase (regions of interest ‘LEP’ and ‘ERS’) or decrease (‘ERD’)
Effect of ‘space change’
There was no significant main effect of the factor ‘space change’ on the ER% summary values of all three time–frequency responses elicited by S3 (‘LEP’: P = 0.13; ‘ERS’: P = 0.53; ‘ERD’: P = 0.62) The magnitudes of the ER% summary values were not significantly larger in triplets other than in triplets same (Fig. 5, top panel).
Effect of ‘uncertainty’
There was no significant main effect of ‘uncertainty’ on the amplitude of the ER% summary values of all three time–frequency responses elicited by S3 (‘LEP’: P = 0.48; ‘ERS’: P = 0.08; ‘ERD’: P = 0.54). The magnitudes of the ER% summary values were not significantly larger when the subject was uncertain about the side where S3 was delivered (triplet uncertain) than when the subject was certain about the side where S3 was delivered (triplet certain) (Fig. 5, bottom panel).
Interaction between ‘space change’ and ‘uncertainty’
There was no significant interaction between the factors ‘space change’ and ‘uncertainty’ on the amplitude of the ER% summary values of all three time–frequency responses elicited by S3 (‘LEP’: P = 0.74; ‘ERS’: P = 0.59; ‘ERD’: P = 0.24).
Discussion
In this study, we aimed to dissect the selective contribution of (1) the occurrence of a change in the stimulus location and (2) the uncertainty of such a change in causing the habituation of the EEG responses elicited by laser stimuli of identical intensity repeated at a short and constant 1-s inter-stimulus interval (Iannetti et al. 2008). We observed three main findings. First, a selective change of the spatial location of S3 did not produce a significant dishabituation of the N1, N2 and P2 waves of LEPs as well as of the laser-induced ERS and ERD. This indicates that, unlike the introduction of a change in the sensory modality of the stimulus (Valentini et al. 2011), the introduction of a change in its spatial location does not induce a strong dishabituation of the corresponding EEG response. Second, the lack of explicit knowledge of the spatial location of S3 and, hence, the uncertainty of a change in the spatial location of the stimulus, did not modulate the peak amplitude of the N1, N2 and P2 waves of LEPs nor did it modulate the magnitude of the laser-induced ERS and ERD. This supports the view that top-down cognitive expectations are not major determinants of the EEG response habituation induced by stimulus repetition. Third, the introduction of a change in the spatial location of S3 determined an increase in the neural activity contralateral to the stimulated hand, in a time window following the P2 peak (361–409 ms). This activity may reflect neural processes related to bottom-up shifts of spatial attention toward stimuli delivered to a novel location (Legrain et al. 2002; Indovina and Macaluso 2004; Brignani et al. 2009).
These results, together with our previous findings (Valentini et al. 2011), contribute to disentangle the different contributions of the four basic attributes of information defining a sensory stimulus (modality, location, intensity and timing; Gardner and Martin 2000) in determining the habituation and dishabituation of sensory ERPs (Fruhstorfer et al. 1970; Fruhstorfer 1971; Fuji et al. 1994; Kekoni et al. 1997). Specifically, the present results indicate that the spatial location of the delivered stimulus is not a major determinant of the habituation and dishabituation of the EEG responses elicited by nociceptive stimulation.
Bottom-up effects of introducing a change in the spatial location of the stimulus
When we described the habituation of the ERPs elicited by three repeated laser stimuli, each identical in terms of modality, location, intensity and timing information (Iannetti et al. 2008), we were able to show that these responses habituate strongly to stimulus repetition, but we were unable to determine which attributes defining the sensory stimulus are important to cause this habituation. In the present experiment, we used a similar paradigm (Fig. 1) to characterize the selective effect of introducing a change in stimulus location and found that such a change does not significantly modulate the magnitude of the corresponding ERP (Fig. 3).
The finding that introducing a change in the stimulus spatial location does not significantly revert the observed repetition suppression is important. Indeed, when such finding is contrasted with our previous results showing that introducing a change in the sensory modality of the repeated stimulus induces a strong response recovery and dishabituation (Valentini et al. 2011), it contributes to building up the understanding of the functional significance of LEPs, by indicating that a change in what the sensory stimulus is, is far more important than a change in where the stimulus is delivered, in determining the magnitude of the corresponding cortical response. It is important to note that this statement only refers to the occurrence of a change in what or where the stimulus is, while the information about the actual spatial location of the nociceptive stimulus is clearly encoded in the topographical distribution of the N1 wave of the LEPs (Valentini et al. 2012).
We have recently proposed (Iannetti et al. 2008; Mouraux and Iannetti 2009; Valentini et al. 2011) that LEPs reflect cortical processes largely related to the detection and reaction to salient changes in the sensory environment (Downar et al. 2000, 2002; Mouraux and Iannetti 2009). The present results indicate that, unlike the occurrence of a change in sensory modality, the occurrence of a selective change in the spatial location of a nociceptive stimulus does not enhance its saliency sufficiently to induce a significant dishabituation of the corresponding ERP response (Figs. 3, 4), that is, what the dual process theory defines as sensitization (Groves and Thompson 1970).
The lack of a significant effect of a change in the spatial location of the laser stimulus might appear, at a first glance, in contrast with studies showing that the magnitude of the N1 and N2 waves of LEPs elicited by laser stimuli delivered to the dorsum of the hand has a larger amplitude than that of LEPs elicited by preceding laser stimuli delivered to the dorsum of the opposite hand (Legrain et al. 2002, 2003). However, there are important differences between the objectives and, consequently, the experimental designs of the two studies. Legrain et al. (2002, 2003) aimed to characterize the effect of spatial attention on the magnitude of LEPs, and not the phenomenon of the ERP dishabituation. Indeed, by explicitly asking to maintain the focus of spatial attention on one of the two stimulated hands, they showed that laser stimuli applied at the attended hand elicit N1 and N2 waves of significantly greater magnitude than laser stimuli applied at the unattended hand. In the present study, participants were not asked to selectively attend one of the two hands. This allowed us to examine the specific bottom-up effect of the occurrence of a change in stimulus location without a top-down contribution of spatial attention. Furthermore, in our paradigm, the change in spatial location of the laser stimulus was either always present (condition certain other) or happening in 50% of the trials (conditions uncertain) (Fig. 1), whereas the increase of LEP magnitude observed by Legrain et al. (2002, 2003), when the stimulated hand was changed, could have been caused not only by the change in the spatial location of the stimulus itself, but also by the fact that displaced stimuli were infrequent (10 vs 90%). Lastly, we tested the response dishabituation obtained using a short train of three stimuli delivered at a constant inter-stimulus interval of 1 s (Iannetti et al. 2008), whereas in Legrain et al. (2002, 2003), a long sequence of 50 nociceptive stimuli was presented, at a longer inter-stimulus interval of 3 s.
The significantly larger ERP amplitude following the P2 peak (Fig. 4, top panel), observed when S3 was delivered to the novel location (triplet other), does not reflect a recovery from habituation of the main LEP peaks. Indeed, the N1, N2 and P2 peak amplitudes were remarkably similar to those elicited by S3 in the triplets same (i.e., the triplets showing the expected repetition suppression; Iannetti et al. 2008) (Fig. 2). This increase of amplitude following the P2 peak likely reflects the bottom-up attentional shift toward sensory stimuli delivered to a novel spatial location (Legrain et al. 2002; Indovina and Macaluso 2004; Brignani et al. 2009). Importantly, the observation that this effect has a scalp topography contralateral to the stimulated hand is compatible with neural activity arising from the frontoparietal network involved in the control of spatial attention, including the reorienting of attention to a novel spatial location (Gitelman et al. 1999; Bisley and Goldberg 2010).
While the functional significance of the N1, N2 and P2 waves of LEPs is progressively being clarified (e.g., Legrain et al. 2005; Iannetti et al. 2008; Legrain et al. 2009; Mouraux and Iannetti 2009), the functional significance of laser-induced ERS (Mouraux et al. 2003) has not been investigated to the same extent. Here we provide evidence that the occurrence of a change in the spatial location of the stimulus does not exert any significant modulation on the laser-induced ERS. This finding, combined with previous findings having shown that the laser-induced ERS is similarly modulated by various experimental factors as the laser-evoked N2 and P2 waves, further suggests that these different EEG responses have a fundamentally similar functional significance.
Top-down effects of uncertainty of change in the spatial location of the stimulus
We did not observe any significant effect of the top-down cognitive expectation of a change in the spatial location of the stimulus (Figs. 2, 3). This finding is similar to the results obtained in our previous study, in which we examined the effect of introducing a change in sensory modality, and showed that explicit knowledge of the occurrence of a change contributed only marginally to the modulation of the elicited brain responses (Valentini et al. 2011). The finding that the expectedness or unexpectedness of the occurrence of a change in the stimulus has only a limited effect on the amplitude of the elicited LEP is important because it suggests that the magnitude of LEPs is largely independent of explicit cognitive expectations.
Conclusion
Unlike the occurrence of a selective change in the sensory modality of the stimulus (Valentini et al. 2011), the occurrence of a change in the spatial location of a nociceptive stimulus repeated at short and constant 1-s inter-stimulus interval does not induce a significant dishabituation of the elicited EEG responses. Considering the fact that the ERPs elicited by sensory stimuli might reflect the activity of a saliency-detection system, necessary to prioritize relevant information to prompt appropriate behavioral responses (Legrain et al. 2011), these results suggest that changes in the spatial location of a repeated stimulus (i.e., in where the stimulus is) do not enhance the saliency of the stimulus as much as changes in its sensory modality (i.e., in what a stimulus is).
References
Baumgartner U, Cruccu G, Iannetti GD, Treede RD (2005) Laser guns and hot plates. Pain 116:1–3
Bisley JW, Goldberg ME (2010) Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci 33:1–21
Brignani D, Lepsien J, Rushworth MF, Nobre AC (2009) The timing of neural activity during shifts of spatial attention. J Cogn Neurosci 21:2369–2383
Bromm B, Treede RD (1984) Nerve fibre discharges, cerebral potentials and sensations induced by CO2 laser stimulation. Hum Neurobiol 3:33–40
Carmon A, Mor J, Goldberg J (1976) Evoked cerebral responses to noxious thermal stimuli in humans. Exp Brain Res 25:103–107
Delorme A, Makeig S (2004) EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134:9–21
Downar J, Crawley AP, Mikulis DJ, Davis KD (2000) A multimodal cortical network for the detection of changes in the sensory environment. Nat Neurosci 3:277–283
Downar J, Crawley AP, Mikulis DJ, Davis KD (2002) A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. J Neurophysiol 87:615–620
Fruhstorfer H (1971) Habituation and dishabituation of the human vertex response. Electroencephalogr Clin Neurophysiol 30:306–312
Fruhstorfer H, Soveri P, Jarvilehto T (1970) Short-term habituation of the auditory evoked response in man. Electroencephalogr Clin Neurophysiol 28:153–161
Fuji M, Yamada T, Aihara M, Kokubun Y, Noguchi Y, Matsubara M, Yeh M (1994) The effects of stimulus rates upon median, ulnar and radial nerve somatosensory evoked potentials. Electroencephalogr Clin Neurophysiol 92:518–526
Garcia-Larrea L, Frot M, Valeriani M (2003) Brain generators of laser-evoked potentials: from dipoles to functional significance. Neurophysiol Clin 33:279–292
Gardner EP, Martin JH (2000) Coding of sensory information. In: Kandel ER, Schwartz JH, Jessell TM (eds) Principles of neural science, 4th edn. Elsevier Science, pp 411–429
Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Kim YH, Meyer JR, Mesulam M (1999) A large-scale distributed network for covert spatial attention: further anatomical delineation based on stringent behavioural and cognitive controls. Brain 122(Pt 6):1093–1106
Groves PM, Thompson RF (1970) Habituation: a dual-process theory. Psychol Rev 77:419
Iannetti GD, Mouraux A (2010) From the neuromatrix to the pain matrix (and back). Exp Brain Res 205:1–12
Iannetti GD, Niazy RK, Wise RG et al (2005) Simultaneous recording of laser-evoked brain potentials and continuous, high-field functional magnetic resonance imaging in humans. Neuroimage 28:708–719
Iannetti GD, Zambreanu L, Tracey I (2006) Similar nociceptive afferents mediate psychophysical and electrophysiological responses to heat stimulation of glabrous and hairy skin in humans. J Physiol 577:235–248
Iannetti GD, Hughes NP, Lee MC, Mouraux A (2008) Determinants of laser-evoked EEG responses: pain perception or stimulus saliency? J Neurophysiol 100:815–828
Indovina I, Macaluso E (2004) Occipital-parietal interactions during shifts of exogenous visuospatial attention: trial-dependent changes of effective connectivity. Magn Reson Imaging 22:1477–1486
Jensen MP, Karoly P, O’Riordan EF, Bland F Jr, Burns RS (1989) The subjective experience of acute pain. An assessment of the utility of 10 indices. Clin J Pain 5:153–159
Jung TP, Makeig S, Westerfield M, Townsend J, Courchesne E, Sejnowski TJ (2000) Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clin Neurophysiol 111:1745–1758
Kekoni J, Hamalainen H, Saarinen M, Grohn J, Reinikainen K, Lehtokoski A, Naatanen R (1997) Rate effect and mismatch responses in the somatosensory system: ERP-recordings in humans. Biol Psychol 46:125–142
Lee MC, Mouraux A, Iannetti GD (2009) Characterizing the cortical activity through which pain emerges from nociception. J Neurosci 29:7909–7916
Legrain V, Guerit JM, Bruyer R, Plaghki L (2002) Attentional modulation of the nociceptive processing into the human brain: selective spatial attention, probability of stimulus occurrence, and target detection effects on laser evoked potentials. Pain 99:21–39
Legrain V, Guerit JM, Bruyer R, Plaghki L (2003) Electrophysiological correlates of attentional orientation in humans to strong intensity deviant nociceptive stimuli, inside and outside the focus of spatial attention. Neurosci Lett 339:107–110
Legrain V, Bruyer R, Guerit JM, Plaghki L (2005) Involuntary orientation of attention to unattended deviant nociceptive stimuli is modulated by concomitant visual task difficulty. Evidence from laser evoked potentials. Clin Neurophysiol 116:2165–2174
Legrain V, Perchet C, Garcia-Larrea L (2009) Involuntary orienting of attention to nociceptive events: neural and behavioral signatures. J Neurophysiol 102:2423–2434
Legrain V, Iannetti GD, Plaghki L, Mouraux A (2011) The pain matrix reloaded: a salience detection system for the body. Prog Neurobiol 93:111–124
Mitsis GD, Iannetti GD, Smart TS, Tracey I, Wise RG (2008) Regions of interest analysis in pharmacological fMRI: how do the definition criteria influence the inferred result? Neuroimage 40:121–132
Mouraux A, Iannetti GD (2008) Across-trial averaging of event-related EEG responses and beyond. Magn Reson Imaging 26:1041–1054
Mouraux A, Iannetti GD (2009) Nociceptive laser-evoked brain potentials do not reflect nociceptive-specific neural activity. J Neurophysiol 101:3258–3269
Mouraux A, Guerit JM, Plaghki L (2003) Non-phase locked electroencephalogram (EEG) responses to CO2 laser skin stimulations may reflect central interactions between A partial partial differential- and C-fibre afferent volleys. Clin Neurophysiol 114:710–722
Pfurtscheller G, Lopes da Silva FH (1999) Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 110:1842–1857
Schlereth T, Magerl W, Treede R (2001) Spatial discrimination thresholds for pain and touch in human hairy skin. Pain 92:187–194
Thompson RF, Spencer WA (1966) Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev 73:16–43
Treede RD (2003) Neurophysiological studies of pain pathways in peripheral and central nervous system disorders. J Neurol 250:1152–1161
Treede RD, Kief S, Holzer T, Bromm B (1988) Late somatosensory evoked cerebral potentials in response to cutaneous heat stimuli. Electroencephalogr Clin Neurophysiol 70:429–441
Treede RD, Meyer RA, Raja SN, Campbell JN (1995) Evidence for two different heat transduction mechanisms in nociceptive primary afferents innervating monkey skin. J Physiol 483(Pt 3):747–758
Treede RD, Lorenz J, Baumgartner U (2003) Clinical usefulness of laser-evoked potentials. Neurophysiol Clin 33:303–314
Trojan J, Stolle A, Kleinböhl D, Mørch C, Arendt-Nielsen L, Hölzl R (2006) The saltation illusion demonstrates integrative processing of spatiotemporal information in thermoceptive and nociceptive networks. Exp Brain Res 170:88–96
Valentini E, Torta DM, Mouraux A, Iannetti GD (2011) Dishabituation of laser-evoked EEG responses: dissecting the effect of certain and uncertain changes in stimulus modality. J Cogn Neurosci 23:2822–2837
Valentini E, Hu L, Chakrabarti B, Hu Y, Aglioti SM, Iannetti GD (2012) The primary somatosensory cortex largely contributes to the early part of the cortical response elicited by nociceptive stimuli. Neuroimage 59:1571–1581
Acknowledgments
M Liang is supported by a grant from the Biotechnology and Biological Sciences Research Council (BBSRC). GD Iannetti is University Research Fellow of The Royal Society and acknowledges the support of the BBSRC and El.En. Group. A Mouraux is supported by an EFIC-Grünenthal Grant (EGG), as well as by a Marie Curie European Reintegration Grant. All authors are grateful to Dr Chiara Sambo and the members of the GAMFI Project for insightful comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
D. M. Torta and M. Liang contributed equally to this article.
Rights and permissions
About this article
Cite this article
Torta, D.M., Liang, M., Valentini, E. et al. Dishabituation of laser-evoked EEG responses: dissecting the effect of certain and uncertain changes in stimulus spatial location. Exp Brain Res 218, 361–372 (2012). https://doi.org/10.1007/s00221-012-3019-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-012-3019-6