Abstract
The cerebral integration of somatosensory inputs from multiple sources is essential to produce adapted behaviors. Previous studies suggest that bilateral somatosensory inputs interact differently depending on stimulus characteristics, including their noxious nature. The aim of this study was to clarify how bilateral inputs evoked by noxious laser stimuli, noxious shocks, and innocuous shocks interact in terms of perception and brain responses. The experiment comprised two conditions (right-hand stimulation and concurrent stimulation of both hands) in which painful laser stimuli, painful shocks and non-painful shocks were delivered. Perception, somatosensory-evoked potentials (P45, N100, P260), laser-evoked potentials (N1, N2 and P2) and event-related spectral perturbations (delta to gamma oscillation power) were compared between conditions and stimulus modalities. The amplitude of negative vertex potentials (N2 or N100) and the power of delta/theta oscillations were increased in the bilateral compared with unilateral condition, regardless of the stimulus type (P < 0.01). However, gamma oscillation power increased for painful and non-painful shocks (P < 0.01), but not for painful laser stimuli (P = 0.08). Despite the similarities in terms of brain activity, bilateral inputs interacted differently for painful stimuli, for which perception remained unchanged, and non-painful stimuli, for which perception increased. This may reflect a ceiling effect for the attentional capture by noxious stimuli and warrants further investigations to examine the regulation of such interactions by bottom–up and top–down processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nociception is the encoding of noxious stimuli by nociceptors (Sherrington 1906; Woolf and Ma 2007). These nociceptive inputs are then processed in the spinal cord and may produce nociceptive reflexes that are independent of volition (Sandrini et al. 2005; Willer 1977). Nociceptive inputs are then processed in the brain, which may result in pain and defensive responses that prevent tissue damage (Sandrini et al. 2005; Sherrington 1910; Willer 1977). Further cortical processing and integration of nociceptive inputs lead to elaborated behaviors that are adapted to the context and environment (Tabor et al. 2017), which may be particularly needed when noxious stimuli are complex or occur in a dynamic environment.
Sensory integration of concurrent stimuli has been explored for visual (Beume et al. 2015; Girard et al. 2013; Liu et al. 2009; Saija et al. 2017), auditory (Bidet-Caulet et al. 2007a, b), tactile (Girard et al. 2013; Hoechstetter et al. 2001; Kakigi and Jones 1986; Ragert et al. 2011; Simões et al. 2002) and vibrotactile (D'Amour and Harris 2014, 2016; Harris et al. 2006; Kuroki et al. 2017; Tame et al. 2015) modalities. For example, integration of different vibration signals from distinct locations leads to assimilation or averaging effects, indicating that tactile perception can be global although information arises from different sensory channels (Kuroki et al. 2017). Accordingly, tactile stimulation on two locations produced suppression of cortical responses in the somatosensory cortex, which was proposed to contribute to better coordination of manipulative actions (Tame et al. 2015). Recent studies also indicate that concurrent bilateral noxious inputs lead to modulation of pain and electroencephalographic (EEG) responses compared with unilateral inputs, although these effects seem to vary depending on stimulus properties (Northon et al. 2019; Rustamov et al. 2019).
Noxious stimuli are intrinsically salient and threatening and thus capture attention (Legrain et al. 2011). But what happens when more than one noxious stimulus occurs? Concurrent noxious stimuli may be more salient and their information may interact and lead to the perception of greater threat compared with a single stimulus. Such interaction of noxious inputs from different locations also implies coordination to produce an adapted behavior. In a recent EEG study, a sub-additive increase of N100 amplitude and gamma oscillation power was reported for bilateral painful shocks applied to sural nerves compared with a unilateral shock (Rustamov et al. 2019). In contrast, a reduction of vertex potentials and gamma oscillation power was reported for bilateral laser stimuli applied to the hands compared with unilateral stimulation (Northon et al. 2019). Thus, bilateral noxious inputs may lead to different responses, but it remains unclear whether the direction of these effects (increase vs. decrease) are due to stimulus properties (laser heat pain vs. shock pain), stimulus location (hand vs. ankle) or other factors such as individual differences, which may vary between studies.
The objective of this study was to examine the cortical integration of noxious and innocuous inputs. In a first experiment, event-related potentials (ERPs) and event-related spectral perturbations (ERSPs) were compared between unilateral and bilateral hand stimulation (painful laser stimuli, noxious shocks and innocuous shocks). In a second experiment, which was a replication of a previous study (Northon et al. 2019), ERPs and ERSPs were compared between unilateral and bilateral stimulation for painful laser stimuli and noxious shocks. A within-subjects design was used to prevent the influence of individual differences between stimulus modalities. Based on previous reports (Northon et al. 2019; Rustamov et al. 2019), we expected that the interactions resulting from bilateral stimulation would produce opposite effects for painful shocks and painful laser stimuli (increase and decrease, respectively). Considering that these differences may be due to individual differences between studies and that painful shocks and laser stimuli may activate different brain regions (Dowman 2004), this may result in different cortical responses between stimulus modalities. In addition, considering that the behavioral responses related to painful and non-painful stimuli are different (e.g., withdrawal reflex for painful, but not non-painful stimuli), we examined whether bilateral non-painful shocks would produce an increase in cortical responses, similarly to what is observed for painful shocks.
Methods
Experiment 1
Participants
Twenty-five participants were recruited on the campus of Université du Québec à Trois-Rivières. Participants gave their written informed consent and acknowledged their right to withdraw at any time without prejudice. Procedures were accepted by the institutional ethical committee and were in accordance with the declaration of the revised version of Helsinki. Participants were included if they were right-handed and aged between 18 and 45 years old. They were excluded if they had chronic pain, a diagnosed neurologic or psychiatric disorder, or if they were taking medication two weeks prior to the experiment. Three participants were excluded because of technical issues leaving a total of 22 participants (11 females, 11 males; age range 20–38 years; mean ± SD: 25 ± 5.3).
Experimental procedures
Room temperature was kept constant at 23 °C. Participants were seated comfortably in a chair, with both arms on an armrest with hands in pronation. The inter-limb distance was 70 cm and the viewing distance from the computer monitor was approximately 100 cm. Both participants and experimenters wore safety glasses designed for a 1340 nm wavelength laser, during the entire experiment.
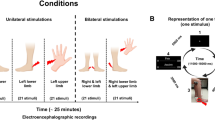
The experimental paradigm is illustrated in Fig. 1. Before the experiment, participants were instructed to refrain from excessive head and body movement and were instructed to look at a fixation cross. The experiment included three counterbalanced conditions containing one modality each: painful laser stimulation, painful transcutaneous electrical stimulation and non-painful transcutaneous electrical stimulation. Each condition included a total of 66 stimuli with a 9–11 s inter-stimulus interval. The fixation cross appeared 4–6 s before a stimulus was delivered. Following the stimulus, a visual numerical rating scale appeared on the screen 1.5 s later. After 3.5 s, the fixation cross reappeared. A rest period of up to 120 s was allowed every 21–23 stimuli. Participants were informed that stimuli would be delivered unilaterally on the right hand or bilaterally on both hands with a 50:50 ratio. There were no more than two identical stimuli in a row.
Experimental paradigm. The three modalities are presented. Condition order was counterbalanced. The pictograms in the first row represent the unilateral conditions (one input) for each modality, and in the second row, the bilateral conditions (two inputs). The red circle overlying the hand is the side on which attention had to be deployed. Top-right: temporal depiction of a single trial. Participants were informed that stimuli would be delivered unilaterally on the right hand or bilaterally on both hands with a 50:50 ratio. Pain (for laser and painful shocks) or tactile intensity (for non-painful shocks) were rated 1.5 s after each stimulus. EEG electroencephalography. Figure was created using Microsoft PowerPoint 2013
Pain and tactile intensity ratings
Participants were instructed to pay attention to the right hand and to rate tactile intensity (for non-painful shock) or pain intensity (for painful shocks and laser) perceived on the right hand, following each stimulus. This rating ensured that participants attended the right hand prior to each stimulation. They were prompted to report pain verbally on a 0–100 numerical rating scale that appeared on the screen 1.5 s after each stimulus. The scale anchors were “no pain” and “most intense pain imaginable”, respectively. For non-painful electrical stimuli, participants were asked to report the intensity of the stimulation between 0 (no sensation) and 100 (intense enough to be painful). This ensured that the stimulation was always perceived, but not painful. During each rest period, participants also provided an average rating for the unattended left-hand stimuli to ensure that the stimuli were perceived. Following each rest period, participants were reminded to attend and rate the right hand.
Noxious and innocuous transcutaneous electrical stimulation
Noxious and innocuous transcutaneous electrical stimulation was delivered as train pulses (10 × 1 ms pulses at 333 Hz) from an isolated DS7A constant current stimulator (Digitimer Ltd., Welwyn Garden City, Hertfordshire, UK). The device was triggered by a Grass S88 train generator (Grass Medical Instruments, Quincy, MA, USA) and controlled by a computer with a stimulus presentation program (E-Prime2, Psychology Software Tools, Sharpsburg, PA, USA). The skin in the innervation territory of the left and right dorsal ulnar nerve was stimulated using a pair of custom-made surface electrodes (1 cm2) separated by 2 cm.
Pain thresholds were determined for the left and right hands using the staircase method beginning from a low stimulus intensity and increasing progressively. Thresholds were defined as the lowest stimulus intensity evoking pain. To confirm the reliability of the pain threshold, the intensity that first produced pain was applied 5 times. Stimulus intensity was then adjusted individually to 120% or 70% of pain threshold for noxious and innocuous electrical stimuli, respectively. A series of five stimuli were then delivered to ensure that the selected intensity produced a stable and tolerable sensation. Perception was matched between hands by slightly increasing or decreasing one of the two stimulus intensities. Innocuous shocks were rated using a non-painful scale, and all participants confirmed that the stimuli produced a tactile sensation and did not evoke pain.
Painful laser stimulation
Painful radiant heat stimuli were produced by laser pulses using two infrared neodymium yttrium aluminum perovskite lasers (Nd:YAP, DEKA 1340; Electronical Engineering, Florence, Italy) each held by one experimenter. Nd:YAP laser pulses have been shown to activate Aδ and C fiber nociceptors selectively (Plaghki and Mouraux 2003). Laser beams were transmitted through a 10 m fiber-optic cable. The pulse duration was set at 4 ms and the diameter at 5 mm (≈ 20 mm2 area). Following safety recommendations for repeated laser stimuli to a 0.4 mm ink-marked skin, a maximal output was fixed at 3.75 J (maximal fluence < 20 J/cm2) (Madden et al. 2016). The lasers were controlled by a computer with a stimulus presentation program (E-Prime2, Psychology Software Tools, Sharpsburg, PA, USA). Ink markers were drawn on the superficial radial nerve territory of each hand dorsum to avoid stimulating the same area more than once before each pause. The in-built helium neon laser was used for aiming purposes and the mounting guides allowed a constant stimulating distance.
Pain thresholds were determined for each hand separately using the staircase method. Participants were instructed to focus on the pinprick sensation and to report pain intensity on the 0–100 scale. Laser energy output was set at 0.5 J and was increased by 0.25 J increments until pain was reported, or up to the 3.75 J upper limit. To confirm the reliability of the pain threshold, stimulation at the energy that first elicited a painful pinprick was repeated 5 times. To obtain a sharp and painful pinprick sensation for the experiment, stimulation was adjusted individually by increasing the output by one or two increments. For each hand, a sequence of 5 consecutive stimuli at the selected intensity was delivered to familiarize participants. Pain intensity was then compared between hands and laser intensity was adjusted to match perception between hands. Hand temperature was monitored for both hands using an infrared thermometer before and after the threshold and was within a range of 27–34 °C.
Electroencephalographic recordings
Brain activity was measured with electroencephalography (EEG). EEG was recorded with 64 active electrodes positioned on the scalp in accordance with the international 10–20 system (Brain Products, Gilching, Germany). The ground electrode was set at FPz and all electrodes were nose-referenced. The signals were digitized at 2000 Hz with a hardware band-pass filter set from 0.01 to 500 Hz. Eye blinks and movements were monitored using electrooculography (EOG) with electrodes placed on the right suborbital ridge and region lateral to the outer canthus.
Event-related potentials
EEG data were exported to Matlab and processed in EEGLab v14.1.0 to examine event-related potentials (ERPs) and event-related spectral perturbations (ERSPs). For ERPs, data were processed with a finite impulse response band-pass filter (0.5–30 Hz), down sampled to 500 Hz, and re-referenced to the common average. Prior to re-referencing, noisy channels were interpolated using the spherical interpolation method available in EEGLab. Data were time-locked to the stimulus and segmented in epochs from − 100 to 700 ms. The − 100-to-0 window was used for baseline correction. Epochs were screened for non-stereotyped artifacts and removed if necessary. An Infomax independent component analysis (Runica function) was used to identify and remove components that were associated with noise based on their spectral, temporal and topographical characteristics (eye blinks or movements, muscle and cardiac artifacts). Epochs were averaged separately for bilateral and unilateral stimuli and for each modality.
ERP components were examined for each modality, by extracting their peak amplitude (laser stimulation) or mean amplitude within a fixed time window (electrical stimulation) as in previous studies (Dowman 1994a, b; Perchet et al. 2008; Rustamov et al. 2019). The peak and mean amplitude were calculated for both modalities and lead to similar results. Thus, only the peak amplitude is reported, consistent with the current literature. For laser ERPs, this included the N2 peak (first major negative deflection maximum at Cz between 160 and 280 ms) and P2 peak (first major positive deflection maximum at Cz between 250 and 400 ms) (Perchet et al. 2008). Following re-referencing to the Fz electrode, the N1 peak (first negative deflection at contralateral central electrodes between 140 and 200 ms) was also extracted. Only participants that felt a clear pricking pain at or before the maximal laser fluence were retained. Due to a technical issue with the lasers, the first three participants had to be excluded (n = 19; 8 women; range 18–35 years; mean: 25.2, SD: 1.9). From these 19 subjects, 5 did not have clear N2 and P2 deflections from their average waveforms. Thus, N2 and P2 components were computed with data from the remaining 14 subjects.
For noxious and innocuous electrical stimulation, ERP components included the P45 (first positive deflection at contralateral centro-parietal electrodes from 45 to 55 ms), the N100 (first major negative deflection maximum at Cz between 90 and 120 ms), and the P260 (second positive deflection maximum at Cz between 240 and 300 ms) (Dowman 1994a, b). All 22 subjects had a clear N100 and P260 potential.
Event-related spectral perturbations
For ERSPs, data were processed with a finite impulse response band-pass filter (1–100 Hz, down sampled to 500 Hz and re-referenced to the common average. Noisy channels were interpolated using the spherical interpolation method available in EEGLab. Data were time-locked to the stimulus and segmented in epochs from − 2000 to 2000 ms. The − 700 to − 200 window was used for baseline correction. The baseline period was different from that of ERPs to avoid edge artifacts that occur at lower frequencies. Epochs were screened for non-stereotyped artifacts and removed if necessary. An Infomax independent component analysis (Runica function) was used to identify and remove noisy components. Time–frequency analysis was computed using a Morlet wavelet convolution with variable cycles for low and high frequencies (from 3 to 15 cycles). This flexibility allows a better frequency resolution at low frequencies and better temporal resolution at higher frequencies (Delorme and Makeig 2004). ERSPs were computed in decibels (dB) relative to the − 700 to − 200 ms baseline period. The ERSP values were first computed for all electrodes separately and for each trial. For each subject, all trials were averaged separately for unilateral and bilateral trials and for each modality, resulting in 6 time–frequency maps for each electrode.
From these time–frequency maps, values were extracted from predetermined time × frequency regions of interests based on previous EEG studies (Gross et al. 2007; Schulz et al. 2012; Tiemann et al. 2015, 2010; Zhang et al. 2012) [also see (Ploner et al. 2017) for a general overview]. The timing for these regions was adapted to the expected latency differences between responses to laser and electrical stimuli (Dowman 1994a, b; Perchet et al. 2008). For laser stimulation, this included the frequency content of evoked potentials (delta and theta oscillations at 2–10 Hz from 150 to 400 ms), alpha–beta oscillations (8–29 Hz from 300 to 1000 ms), low-gamma oscillations (30–60 Hz from 100 to 350 ms) and high-gamma oscillations (61–100 Hz from 150 to 350 ms). For electrical stimulation, this included oscillations in the range of 2–10 Hz (50–400 ms), 8–29 Hz (250–1000 ms), 30–60 Hz (50–350 ms) and 61–100 Hz (100–350 ms). The low-frequency (delta and theta) and gamma responses were maximal at the Cz electrode. As the 8–29 Hz response likely originates from the sensorimotor cortices bilaterally (Ploner et al. 2017), a cluster of four electrodes was created for both hemispheres (left: C3, C5, CP3 and CP5; right: C4, C6, CP4 and CP6) and the values were averaged across the four electrodes. For each subject and region of interest, the values were extracted, sorted from lowest to highest, and a mean value was obtained by taking the top 20% (for increases relative to baseline) or lowest 20% (for decreases relative to baseline). This approach allows selecting a wide time–frequency region, thus accounting for between-subject variability while minimizing the problem associated with near-zero values (Mouraux and Iannetti 2008).
Experiment 2
The similar sub-additive integration between modalities obtained in Experiment 1 was divergent from a previous study, where bilateral laser stimuli lead to a decrease in EEG responses (Northon et al. 2019). To address this issue, a separate experiment was conducted to replicate previous findings (Northon et al. 2019) and to examine if discrepancies may be explained by methodological differences. The main methodological differences were the design of the conditions (separate unilateral and bilateral conditions vs blocks with 50% unilateral 50% bilateral stimuli) and the inter-stimulus interval (fixed vs variable inter-stimulus interval), which may lead to predictability and habituation. For brevity, only the main results are presented in the manuscript and all Figures and Tables for this experiment are available in the Online Resource document.
Participants
Twenty participants were recruited at the campus of Université du Québec à Trois-Rivières. Selection criteria were the same as Experiment 1. One participant was excluded because of technical issues leaving a total of 19 participants (9 female, 10 male; age range 20–37 years; mean ± SD: 26 ± 8.3).
Experimental paradigm
The experimental paradigm of Experiment 2 is illustrated in the Online Resource (Fig. 5). Two stimulus modalities were used for this experiment: painful laser stimulation and painful electrical stimulation. Participants underwent a total of 8 conditions, including four conditions per modality. The four conditions were counterbalanced between participants and included (1) unilateral left hand, (2) unilateral right hand, (3) bilateral with attention to the left and (4) bilateral with attention to the right hand. The conditions were counterbalanced similarly between modalities. Each condition included a total of 20 stimuli with a fixed inter-stimulus interval of 6 s. Prior to each condition, participants received instructions for stimulus location, and for which hand they should direct their attention to. Participants were instructed to rate pain intensity following each stimulus. At the end of each bilateral condition, participants also provided an average rating for the unattended hand. Before each condition, participants were reminded of the hand that had to be attended.
Pain ratings and stimulus intensities
Participants fixated a cross presented on a computer monitor. They were prompted to report pain verbally on a 0–100 numerical rating scale that appeared on the screen after each stimulus. The scale anchors were “no pain” and “most intense pain imaginable”, respectively.
Painful electrical stimulation
Painful transcutaneous electrical stimulations were delivered using the same material and threshold methods as for Experiment 1.
Painful laser stimulation
Painful radiant heat stimuli were produced by the same lasers as in Experiment 1. The laser spot size was adjusted to 4 mm to replicate the methodology used in our previous study (Northon et al. 2019). As such, the maximal intensity in Joules was set at 2.25 for a maximum fluence of 20 J/cm2.
Electroencephalographic recordings
As in Experiment 1, brain activity was measured with a BrainVision EEG system comprising 64 active electrodes.
Event-related potentials
EEG data were preprocessed as described in Experiment 1. For ERPs, the epochs were averaged separately for the four conditions and for the two modalities (laser stimuli and painful electrical shocks). For laser stimuli, the 19 participants reported a clear pricking pain at or before the maximal fluence. Three did not have clear N2 and P2 deflections from their average waveforms. Thus, these components were computed on the 16 remaining participants. For painful electrical stimuli, two participants did not show clear N100 and P260 potentials, so the two components were examined in 17 participants.
Event-related spectral perturbations
Data were analyzed as described in Experiment 1, with the only difference being a shorter time window used for time–frequency transformation (from − 1500 to 1500 ms) to take into account the shorter inter-stimulus interval.
Statistical analysis
Statistical analyses were performed using Statistica v12 (Dell Inc, Tulsa, OK, USA). All results are presented as mean ± standard deviation (SD). Statistical threshold was set at P ≤ 0.05 (two-tailed). All effect sizes are expressed as partial eta-squared (η2p).
Experiment 1: Paired t tests were used to compare variables between unilateral and bilateral stimulation for each modality: ratings (pain and tactile intensity), ERPs (laser: N1, N2 and P2; electrical shocks: P45, N100, P260) and ERSPs (2–10 Hz, 8–29 Hz, 30–60 Hz, 61–100 Hz). The 8–29 Hz response was examined with an ANOVA with stimulation and hemisphere as within-subject factors, since it is observed bilaterally.
Experiment 2: Repeated-measures ANOVAs were computed with two within-subject factors: stimulation (unilateral vs bilateral conditions) and attention (attention to left-hand vs right hand). This approach was used to compare pain ratings, ERPs (laser: N1, N2 and P2; electrical shocks: P45, N100, P260) and ERSPs (2–10 Hz, 8–29 Hz, 30–60 Hz, 61–100 Hz). The hemisphere (left vs. right) factor was also included in the model for the 8–29 Hz response, since it is observed bilaterally.
It should be noted that a systematic approach was used to include participants in EEG analyses as described above; only participants with clear deflections were included. For consistency, the ERSPs were calculated using the same participants. However, since the inclusion/exclusion of participants is still a debated issue, and that it is also possible to include all participants regardless of the deflections’ observation, we also examined another possibility. For participants with no clear deflections, the most positive or negative value (depending on the deflection polarity) within a time window spanning, one standard deviation before and after the grand average peak latency was attributed as peak amplitude. Although this increased the sample size, all statistical values were lower, including the effect size. Therefore, we only report the results from the systematic approach that excludes participants from EEG analyses when no clear deflections are observable.
Results
Experiment 1
Perception and stimulus intensity
The mean ratings for unilateral and bilateral stimuli and the mean stimulus intensity for all modalities are presented in Table 1. On average, unilateral and bilateral laser stimuli evoked similar pain, with no significant difference (t(13) = 0.96, P = 0.35, η2p = 0.07). Laser intensity was not significantly different between right and left hands (t(13) = 0.71, P = 0.49, η2p = 0.04). Painful electrical stimuli evoked moderate pain, with a trend towards a significant difference between unilateral and bilateral conditions (t(21) = 2.0, P = 0.059, η2p = 0.16), while stimulus intensity was higher for the right compared with the left hand (t(21) = 2.2, P = 0.037, η2p = 0.19). Lastly, non-painful electrical stimuli evoked moderate tactile sensation and ratings were higher for the bilateral condition compared with the unilateral condition (t(21) = 4.0, P < 0.001, η2p = 0.43) while stimulus intensity was not significantly different between right and left hands (t(21) = 2.0, P = 0.064, η2p = 0.15).
These results indicate that bilateral painful stimulation (laser or electrical) did not evoke more pain compared with unilateral stimulation while tactile sensation was greater in the bilateral compared with unilateral condition. Stimulus intensity is unlikely to explain these results.
Vertex evoked potentials
Group-average ERPs and topoplots are presented in Fig. 2 and mean values are reported in Online Resource (Table 2).
Event-related potentials for unilateral and bilateral conditions for each modality. A Time course of the event-related potentials for laser (left), painful shocks (middle), and non-painful shocks (right) for unilateral and bilateral stimuli. Unilateral stimuli are depicted as dashed lines and bilateral as full lines. The lightly colored areas above and below the event-related potentials time course represent the standard deviation. The black arrows (for Noxious laser) depict the peaks used for the analysis. The gray rectangles overlying the event-related potentials for noxious and innocuous shocks depict the time windows used to calculate the mean. The conditions are identified by the pictogram in the legend. The red circle overlying the hand is the side on which attention had to be deployed. B Mean N2 and P2 (left) and N100 and P260 mean values (middle: painful shock; right: non-painful shocks) extracted for unilateral and bilateral conditions. The negative potentials are shown reversed (negative: up) for easier comparison with the positive potentials. Unilateral (Uni) are depicted as dotted bars and bilateral (Bi) as full bars. The dots represent individual data points. *, ***P < 0.05 and < 0.001 for the t test between unilateral and bilateral conditions, respectively. C Scalp topoplots for the vertex potentials (laser: N2, P2; painful and non-painful shocks: N100 and P260) for unilateral and bilateral conditions. Figure was created using Microsoft PowerPoint 2013
For N2 and P2 components of laser-evoked potentials (LEPs), the scalp distribution was centered at Cz and they occurred at expected latencies (N2: 197.9 ± 14.0 ms, P2: 319.5 ± 40.1 ms). N2 and P2 peak amplitude was greater in the bilateral compared with unilateral condition (t(13) = 4.3, P < 0.001, η2p = 0.59 and t(13) = 2.28, P = 0.04, η2p = 0.29, respectively).
For N100 and P260 components of potentials evoked by painful shocks, the scalp distribution was centered at Cz as expected. N100 amplitude was greater in the bilateral compared with the unilateral condition (t(21) = 6.39, P < 0.0001, η2p = 0.66) while P260 amplitude was not modulated significantly between conditions (t(21) = 0.53, P = 0.60, η2p = 0.01).
For N100 and P260 components of potentials evoked by non-painful shocks, the scalp distribution was centered at Cz as expected. N100 amplitude was greater in the bilateral compared with unilateral condition (t(21) = 5.42, P < 0.0001, η2p = 0.58), while P260 amplitude was not modulated significantly (t(21) = 1.84, P = 0.08, η2p = 0.14).
These results indicate that bilateral stimulation increased late ERPs for laser stimuli, while only the negative component of the late ERPs was modulated for electrical stimuli, whether they were painful or non-painful.
Lateralized evoked potentials
For laser stimuli, the lateralized N1 component was observed after re-referencing the signal to Fz. The measured temporo-central scalp distribution and latency (170.5 ± 16.0 ms) correspond to its usual characteristics (see Fig. 3, and Online Resource Table 2). The N1 was maximal at central electrodes contralateral to the stimulation (C3 for measurements) for the unilateral condition and its amplitude was not significantly different between unilateral and bilateral conditions (t(13) = 1.67, P = 0.12, η2p = 0.18).
Lateralized event-related potentials for unilateral and bilateral conditions for each modality. A Scalp topoplots for the N1 (top: laser) and P45 (middle: painful shocks; bottom: non-painful shocks) for unilateral and bilateral conditions. The condition is identified by the pictogram. The red circle overlying the hand is the side on which attention had to be deployed. The contralateral (left hemisphere, C3) and ipsilateral (right hemisphere, C4) electrodes are depicted by dots. B Time course of the lateralized evoked potentials at the contralateral and ipsilateral electrodes between unilateral and bilateral conditions for each modality (top: laser; middle: painful shocks; bottom: non-painful shocks). Unilateral condition is shown as dashed lines and bilateral as full lines. The lightly colored areas above and below the event-related potentials time course represent the standard deviation. The black arrows (for Noxious laser) depict the peak used for the analysis. The gray rectangles overlying the event-related potentials for noxious and innocuous shocks depict the time window used to calculate the mean. C Mean values for the N1 (top: laser) and P45 (middle: painful shocks, bottom: non-painful shocks) extracted from the left hemisphere electrode for unilateral and bilateral conditions. Unilateral is depicted as dotted bars and bilateral as full bars. The dots represent individual data points. Figure was created using Microsoft PowerPoint 2013
The lateralized P45 component was observed for painful and non-painful electrical shocks (see Fig. 3). The temporo-parietal scalp distribution of the earliest observable positive deflection corresponds to its characteristics. It was maximal over contralateral parietal electrodes (P3 for measurements) for the unilateral condition and was not significantly different between unilateral and bilateral conditions (t(21) = 0.37, P = 0.72, η2p = 0.01 and t(21) = 0.67, P = 0.51, η2p = 0.02 for painful and non-painful stimulation, respectively).
These results indicate that early lateralized components were not modulated by inputs arising from the ipsilateral hand, whether the stimulus was painful or not and whether it was evoked by lasers or electric shocks.
Event-related spectral perturbations
Group-average ERSPs are presented in Fig. 4A, topoplots along with mean and individual data points in Fig. 4B, and mean values are reported in Online Resource (Table 3).
Event-related spectral perturbations for unilateral and bilateral conditions for each modality. A Average event-related spectral perturbation analysis for unilateral and bilateral conditions for each modality (top: laser, middle: painful shocks, bottom: non-painful shocks). The condition is identified by the pictogram. The red circle overlying the hand is the side on which attention had to be deployed. Units are in decibels (dB) relative to the baseline. Dashed areas represent the four regions of interests. B Mean values extracted for unilateral and bilateral conditions for each modality. The dots represent individual data points. Below each graph: scalp topoplot of the time–frequency peak for each region of interest. For the 8–29 Hz region of interest the dashed area linking the bars to the topoplots represents the left and right electrode clusters. *P < 0.05; **P < 0.01, ***P < 0.001 for the t test between unilateral and bilateral conditions. ‡‡, ‡‡‡P < 0.01 and P < 0.001 for planned contrasts. ††P < 0.01 for the main effect of unilateral vs bilateral. Figure was created using Microsoft PowerPoint 2013
For laser stimuli, power was significantly greater in the bilateral compared with the unilateral condition for 2–10 Hz oscillations (t(13) = 4.4, P = 0.0007, η2p = 0.60) and 61–100 Hz oscillations (t(13) = 2.7, P = 0.02, η2p = 0.35), but not for 30–60 Hz oscillations (t(13) = 1.03, P = 0.32, ηp2 = 0.07). For the 8–29 Hz electrode clusters, oscillation power was significantly greater in the bilateral compared with unilateral condition (main effect: F1,13 = 4.62, P = 0.05, η2p = 0.26) and this effect was significantly different between hemispheres (interaction: F1,13 = 6.49, P = 0.02, η2p = 0.33). Planned comparisons revealed greater power for the bilateral compared with unilateral condition for the right (P = 0.026) but not the left (P = 0.30) electrode cluster.
For painful electrical stimuli, power was significantly greater in the bilateral compared with the unilateral condition for 2–10 Hz oscillations (t(21) = 9.66, P < 0.0001, η2p = 0.82), 30–60 Hz oscillations (t(21) = 3.42, P = 0.0025, η2p = 0.36) and 61–100 Hz oscillations (t(21) = 3.7, P = 0.0013, η2p = 0.39). The cluster analysis for 8–29 Hz power showed that oscillation power was greater in the bilateral compared with unilateral condition (main effect: F1,21 = 6.5, P = 0.019, η2p = 0.24), but this effect was not significantly different between hemispheres (interaction: F1,21 = 0.72, P = 0.41, η2p = 0.03).
Lastly, for non-painful electrical stimuli, power was significantly greater in the bilateral condition compared with the unilateral condition for 2–10 Hz oscillations (t(21) = 7.1, P < 0.0001, ηp2 = 0.71), 30–60 Hz oscillations (t(21) = 3.76, P = 0.0013, η2p = 0.40) and 61–100 Hz oscillations (t(21) = 2.2, P = 0.036, η2p = 0.19). For the 8–29 Hz cluster analysis, oscillation power was significantly greater in the bilateral compared with unilateral condition (main effect: F1,21 = 18.78, P = 0.0003, η2p = 0.47) and this effect was significantly different between hemispheres (interaction: F1,21 = 9.94, P = 0.005, η2p = 0.32). Planned comparisons revealed a stronger response (more negative) for the bilateral compared with unilateral condition for both the right (P = 0.0002) and the left (P = 0.006) electrode cluster.
These results indicate that bilateral stimulation increased the power for 2–10 Hz oscillations and 8–29 Hz oscillations, whether the stimulus was painful or not, and whether it was evoked by lasers or electric shocks. Bilateral stimulation also increased the power for 30–60 Hz and 61–100 Hz oscillations for painful and non-painful electrical shocks, but not for laser stimulation.
Experiment 2
Pain ratings and stimulus intensities
Mean pain ratings and mean stimulus intensities are reported in Online Resources (Table 4). On average, laser stimuli evoked light pain, with no difference between unilateral and bilateral conditions (main effect: F1,14 = 0.98, P = 0.34, η2p = 0.07) and this was not affected by attention (interaction F1,14 = 2.82, P = 0.11, η2p = 0.16). Laser intensity was higher for the right compared with the left hand (t(15) = 2.23, P = 0.041, η2p = 0.25). Painful electrical stimuli evoked moderate pain with a significant difference between unilateral and bilateral conditions depending on attention direction (interaction: F1,17 = 9.51, P = 0.007, η2p < 0.36). Planned comparisons revealed that pain was greater in the bilateral condition when attention was focused on the left hand compared with the unilateral left-hand stimulation (P = 0.013), but not when attention was focused on the right hand compared with the unilateral right-hand stimulation (P = 0.60). For stimulus intensity, no difference was observed between right and left hands (t(17) = 0.13, P = 0.89, η2p < 0.01).
These results indicate that bilateral laser stimulation did not evoke more pain compared with unilateral stimulation. For electrical stimulation, the effects of bilateral stimulation were not consistent between hands.
Vertex evoked potentials
Group-average ERPs and topoplots are presented in Online Resource (Fig. 6) and mean values are reported in Online Resource (Table 5).
For N2 and P2 LEPs, the scalp distribution was centered at Cz and occurred at the expected latencies (N2: 201.6 ± 25.6 ms, P2: 310.6 ± 27.8 ms).
N2 amplitude was significantly different between bilateral and unilateral conditions depending on attention direction (interaction: F1,14 = 4.76, P = 0.046, η2p = 0.25). Planned comparisons revealed an increase in N2 amplitude in the bilateral condition when attention was focused on the right hand compared with the unilateral right-hand stimulation (P = 0.002), but not when attention was focused on the left hand compared with the unilateral left-hand stimulation (P = 0.67).
P2 amplitude was significantly different between bilateral and unilateral conditions depending on attention direction (interaction: F1,14 = 14.17, P = 0.002, η2p = 0.50). Planned comparisons revealed a decrease in P2 amplitude for the bilateral condition when attention was focused on the left hand compared with the unilateral left-hand stimulation (P = 0.005). In contrast, P2 amplitude was increased in the bilateral condition when attention was focused on the right hand compared with the unilateral right-hand stimulation (P = 0.013).
For N100 and P260 components of potentials evoked by painful shocks, the scalp distribution was centered at Cz, as expected. N100 amplitude was greater in bilateral compared to unilateral condition (main effect: F1,16 = 77.66, P < 0.0001, η2p = 0.83), but this effect was not modulated by attention (interaction: F1,16 = 1.69, P = 0.21, η2p = 0.10). No significant effect was observed for the P260 (all P > 0.59).
These results indicate that bilateral stimulation increased the negative vertex potential for painful shocks. For laser stimuli, the effects of bilateral stimulation were not consistent between hands.
Lateralized evoked potentials
Group-average lateralized ERPs and topoplots are presented in Online Resource (Fig. 7) and mean values are reported in Online Resource (Table 5).
For LEPs, the temporo-central scalp distribution and latency (166.0 ± 19.0 ms) correspond to usual characteristics of the N1. N1 amplitude was maximal at central electrodes contralateral to the stimulation for the unilateral stimulation (right hand: C3 for measurements, left hand: C4 for measurements). N1 amplitude was significantly different between unilateral and bilateral conditions depending on attention direction (interaction: F1,14 = 7.91, P = 0.014, η2p = 0.36). Planned comparisons revealed that N1 amplitude decreased for the bilateral condition with attention to the left hand compared with unilateral left-hand stimulation (measured at C4) (P = 0.0013), but not for the bilateral condition with attention to the right hand compared with unilateral right-hand stimulation (measured at C3).
The P45 evoked by painful electrical stimuli was not consistently measurable between conditions, so it is shown but not further analyzed.
These results indicate that the effects of bilateral stimulation on early lateralized potentials are not consistent either for laser or for electrical stimulation.
Event-related spectral perturbations
Group-average ERSPs and topoplots are presented in Online Resource (Fig. 8) and mean values are reported in Online Resource (Table 6).
For laser stimuli, 2–10 Hz oscillation power was significantly different between bilateral and unilateral conditions depending on attention direction (interaction: F1,14 = 11.75, P = 0.004, η2p = 0.46). Planned comparisons revealed a power increase for the bilateral condition when attention was focused on to the right hand compared with unilateral right-hand stimulation (P = 0.005), but not for the bilateral condition when attention was focused on to the left hand compared with unilateral left-hand stimulation (P = 0.20). No power modulation was observed for 30–60 Hz oscillations (all P > 0.43), 61–100 Hz oscillations (all P > 0.08) or for the cluster analysis for 8–29 Hz oscillations (all P > 0.08).
For painful electrical stimulation, power was significantly increased in the bilateral compared with unilateral condition for 2–10 Hz oscillations (main effect: F1,17 = 45.25, P < 0.0001, η2p = 0.73), 30–60 Hz (main effect: F1,17 = 13.51, P = 0.002, η2p = 0.44) and 61–100 Hz (main effect: F1,17 = 36.40, P < 0.0001, η2p = 0.68), but these effects were not significantly modulated by attention (all P > 0.11). No significant modulation was observed for the 8–29 Hz cluster analysis (all P > 0.09).
These results indicate that bilateral stimulation increased power for 2–10 Hz, 30–60 Hz and 61–100 Hz oscillations evoked by painful shocks. For laser stimulation, the effects of bilateral stimulation were not consistent between hands.
Discussion
Previous work has shown that bilateral noxious inputs interact in the brain, as shown by a modulation of pain-related brain activity, although the limited number of studies led to divergent results (Northon et al. 2019; Rustamov et al. 2019). This could be explained by differences in stimulation procedures, since the two available studies used either laser or electrical stimuli. Indeed, laser stimuli activate nociceptive fibers selectively while transcutaneous electrical stimuli activate nociceptive and other sensory fibers (Garcia-Larrea 2006). Differences may also be due to stimulus location. The feet are closer to each other compared with the hands, both in space (in normal anatomical position) and in the cortical somatosensory map. In addition, the hands are closer to the face, the center of the defensive peri-personal space (Sambo et al. 2012), which may increase the modulation of defensive reflexes (Moayedi et al. 2015) and the behavioral relevance or threat (Moayedi et al. 2016), especially when bilateral stimuli are applied. In experimental settings where participants are seated, participants may not see their feet and hands in the same way, a factor that modulates the perception and cortical responses of noxious and innocuous stimuli (Kennett et al. 2001; Torta et al. 2015). In the present study, vertex LEP and SEP data indicate that the interactions following bilateral inputs are generally not affected by the type of afferents activated by the stimuli. These results are consistent with those from a previous study showing that the amplitude of the N100 was greater for bilateral compared to unilateral electrical stimulation at the ankle (Rustamov et al. 2019). However, the results contrast with those of another study showing that LEP amplitude is decreased for bilateral compared with unilateral stimulation (Northon et al. 2019). To clarify this discrepancy, a second experiment was conducted with the same experimental design as in the previous study (Northon et al. 2019). Results from this second experiment confirm that the most common effect for bilateral laser stimulation is an increase in LEP amplitude. Nevertheless, it should be noted that some participants showed the opposite effect. Response suppression, as reported previously (Kuroki et al. 2017), may contribute to this effect. Notwithstanding, the present study clarifies that bilateral noxious inputs interact in the brain, and that this interaction is generally reflected in increased amplitude of vertex ERPs and is not affected by stimulus properties (shock vs laser) or stimulus location (hands in the present study and feet in a previous study Rustamov et al. 2019). Also, since the shock and laser stimuli were grouped in separate blocks, the results confirm that this observation is not affected by differences in the anticipated perception or threat of the stimulus (shock vs laser).
In contrast to the vertex ERPs, lateralized ERPs were generally comparable between unilateral and bilateral stimulation. Lateralized ERPs measured in the present study mostly represent activity in the somatosensory cortex (Allison et al. 1996). The P45 is usually similar between painful and non-painful stimuli, as observed here. Likewise, the N1 is not different between perceived and unperceived laser stimuli (Lee et al. 2009). However, the N1 can be dissociated from pain ratings (Iannetti et al. 2008) and is sensitive to novelty and saliency (Ronga et al. 2013). Thus, the selective effects on vertex potentials cannot be fully explained by increased saliency, in which case N1 amplitude should also be increased. However, the signal-to-noise ratio of these lateralized responses is lower than that of vertex potentials and may preclude the detection of small effects.
ERSPs are other measures of interest from brain activity that were examined in the present study. Brain oscillations and how they synchronize or desynchronize are known to allow integration (Fries 2015; Mejias et al. 2016; Schnitzler and Gross 2005). Thus, they extend ERP findings and are especially well suited to study how noxious inputs interact in the brain.
Gamma oscillations are ubiquitous in the neocortex and are thought to reflect local cortical processing and long-range synchronization (Fries 2009). An increase in gamma oscillation power evoked by phasic painful stimuli was reported in several studies (Chien et al. 2014; Hauck et al. 2015; Heid et al. 2020; Northon et al. 2019; Ploner et al. 2017; Rossiter et al. 2013; Rustamov et al. 2019; Tan et al. 2019; Tiemann et al. 2010; Valentini et al. 2013; Yue et al. 2020; Zhang et al. 2012). Recent studies have shown that SI is an important contributor to the pain-related gamma oscillations evoked by laser stimuli (Heid et al. 2020; Tan et al. 2019; Yue et al. 2020). The present results show that bilateral laser stimuli did not increase pain or N1 amplitude, but increased high-gamma power, which may suggest that the interactions of concurrent bilateral inputs may be detectable at an early processing stage (SI) using ERSPs.
The degree to which gamma oscillations induced by painful laser and electrical stimuli can be compared remains unclear. It was reported that pain-related gamma oscillations in SI may reflect cortical activity that is specific to the processing of nociceptive information (Heid et al. 2020; Tan et al. 2019; Yue et al. 2020). However, SI gamma oscillations evoked by transcutaneous electrical stimuli exhibit a monotonic relationship for intensities that evoke sensations ranging from light non-painful up to strongly painful (Rossiter et al. 2013), which argues against this idea and is consistent with the present findings showing gamma ERSPs with both non-painful and painful shocks. The power increase of gamma oscillations in the bilateral condition is reproducible and consistent with our previous study (Rustamov et al. 2019). In that previous study, the increase in gamma power in the bilateral condition was accompanied by an increase in pain ratings. The dissociation between gamma power and pain in Experiment 1 suggest that the gamma power increase is independent of changes in pain perception and thus reflects more than pain perception, at least in the present experimental conditions.
Previous studies have shown that homo-segmental bilateral electrical stimulation, but not laser stimulation, increases pain compared with unilateral stimulation (Northon et al. 2019; Rustamov et al. 2019). The present results indicate that homo-segmental bilateral electrical stimulation does not necessarily increase pain perception compared with unilateral stimulation, although a trend was observed. Other reports also showed that integration can occur between dermatomes from the ipsi- and contralateral limbs when concurrent tonic heat pain is used (Defrin et al. 2010; Nielsen and Arendt-Nielsen 1997). In these studies, spatial attention modulated perception: attention focusing on one of the two stimuli decreased spatial summation of pain, whereas dividing attention between both stimuli decreased pain compared with a single stimulus. Similarly, global rating of two painful stimuli modulates pain compared to the rating of a single stimulus (Lautenbacher et al. 2007; Quevedo and Coghill 2007). However, these studies used tonic stimuli or a combination of tonic and phasic stimuli. Tonic painful stimuli activate a spino-bulbo-spinal loop that produce widespread inhibition of nociceptive activity in other body areas, a “pain-inhibits-pain” mechanism termed diffuse noxious inhibitory control (DNIC) (Le Bars et al. 1979) or conditioned pain modulation (CPM) (Yarnitsky 2010). In the present study, since two short-duration concurrent stimuli were used, this mechanism is unlikely to be involved. Indeed, nociceptive inputs are already ascending when inhibitory feedback reaches their spinal origin. Besides, dividing attention between concurrent painful and non-painful tonic stimuli also reduces pain perception (Quevedo and Coghill 2007) suggesting that spatial attention is important in modulating the perception of concurrent stimuli. In the present study, participants always rated the right hand to control spatial attention and examine the effect of a concurrent contralateral input on perception and brain responses across different modalities. It should be noted that since participants rated average pain on the left hand (unattended) during each rest period, this may have prevented a complete attentional focus on the right hand. However, participants were reminded to attend and rate the right hand before the beginning of each stimulation block to limit this potential interference. Future studies should examine if different spatial attention sets (e.g. global, divided attention) can modulate the integration of concurrent phasic inputs.
For non-painful stimuli, we observed a significant increase in tactile sensation in the bilateral condition. One possibility to explain the discrepancy between painful and non-painful stimuli is a ceiling effect for the attentional capture by painful stimuli. Accordingly, the summation effects may not be linear and applying a concurrent stimulus to a non-painful or to a painful stimulus may not lead to proportional increases in perception. This could be examined in future studies with non-painful and painful stimuli of various intensities.
While perception and event-related brain activity tend to co-vary, dissociations are common (Iannetti et al. 2008; Legrain et al. 2011). An example is the stable pain perception but strong decrease in event-related potentials when a triplet of painful stimuli is presented with a 1-s interval (Iannetti et al. 2008). This and other investigations have led to the prevailing view that vertex potentials likely reflect a multimodal detection of salient stimuli (Legrain et al. 2011). Yet, both the N1 and N2 of the laser-evoked potentials tend to retain some somatosensory specificity whereas the late, multimodal P2 potential shares striking similarities with late potentials evoked by visual, tactile and auditory stimuli.
By changing stimulus-driven parameters of the 3rd stimulus in the triplet paradigm, N2 habituation was shown to be sensitive to changes in novelty and saliency (Ronga et al. 2013). Behaviorally relevant changes in stimulus location from the foot to the hand for the 3rd stimuli can also revert N2 habituation specifically (Moayedi et al. 2016) and the trial-by-trial variability of the N2 predicts the reaction time of a defensive motor response to noxious stimuli (Moayedi et al. 2015). Thus, it was suggested that the N2 also reflects the detection of potential threats and initiation of defensive motor responses (Moayedi et al. 2016, 2015). This spatial-change effect is not observed when 3rd stimulus is displaced from one hand to the other (Torta et al. 2012). Thus, the effect observed in the present study is unlikely to be explained by the introduction of a spatial change (or addition of a spatial location) in the sensory stream. Based on these accounts, the selective effects on negative vertex potentials may be interpreted as an integration of bilateral inputs from individual stimuli into a more salient, threatening event. This may explain the similarity of results for painful and non-painful stimuli. These results would benefit from a multimodal approach combining methods that complement the high temporal but low spatial resolution of EEG such as functional magnetic resonance imagery (fMRI), to explore the brain areas in which such interactions may occur. Lastly, methodological studies on the selection of participants on the basis of the presence or absence of a physiological response are needed to clarify which approach is preferable in which context, and to standardize the methods between studies.
Conclusion
In summary, the present study clarifies that bilateral inputs lead to similar cortical interactions for noxious and innocuous stimuli, resulting in a sub-additive increase of brain responses, but lead to different perceptual effects.
Availability of data and material
The datasets generated during the current study are available from the corresponding author on reasonable request.
Code availability
The custom code generated for the current study are available from the corresponding author on reasonable request.
Abbreviations
- ERP:
-
Event-related potentials
- LEP:
-
Laser-evoked potentials
- ERSP:
-
Event-related spectral perturbations
- EEG:
-
Electroencephalography
References
Allison T, McCarthy G, Luby M, Puce A, Spencer DD (1996) Localization of functional regions of human mesial cortex by somatosensory evoked potential recording and by cortical stimulation. Electroencephalogr Clin Neurophysiol 100(2):126–140. https://doi.org/10.1016/0013-4694(95)00226-x
Beume LA, Kaller CP, Hoeren M, Kloppel S, Kuemmerer D, Glauche V, Umarova R et al (2015) Processing of bilateral versus unilateral conditions: evidence for the functional contribution of the ventral attention network. Cortex 66:91–102. https://doi.org/10.1016/j.cortex.2015.02.018
Bidet-Caulet A, Fischer C, Bauchet F, Aguera PE, Bertrand O (2007a) Neural substrate of concurrent sound perception: direct electrophysiological recordings from human auditory cortex. Front Hum Neurosci 1:5. https://doi.org/10.3389/neuro.09.005.2007
Bidet-Caulet A, Fischer C, Besle J, Aguera PE, Giard MH, Bertrand O (2007b) Effects of selective attention on the electrophysiological representation of concurrent sounds in the human auditory cortex. J Neurosci 27(35):9252–9261. https://doi.org/10.1523/jneurosci.1402-07.2007
Chien JH, Liu CC, Kim JH, Markman TM, Lenz FA (2014) Painful cutaneous laser stimuli induce event-related oscillatory EEG activities that are different from those induced by nonpainful electrical stimuli. J Neurophysiol 112(4):824–833. https://doi.org/10.1152/jn.00209.2014
D’Amour S, Harris LR (2014) Contralateral tactile masking between forearms. Exp Brain Res 232(3):821–826. https://doi.org/10.1007/s00221-013-3791-y
D’Amour S, Harris LR (2016) Testing tactile masking between the forearms. J vis Exp 108:e53733. https://doi.org/10.3791/53733
Defrin R, Tsedek I, Lugasi I, Moriles I, Urca G (2010) The interactions between spatial summation and DNIC: effect of the distance between two painful stimuli and attentional factors on pain perception. Pain 151(2):489–495. https://doi.org/10.1016/j.pain.2010.08.009
Delorme A, Makeig S (2004) EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134(1):9–21. https://doi.org/10.1016/j.jneumeth.2003.10.009
Dowman R (1994a) SEP topographies elicited by innocuous and noxious sural nerve stimulation. I. Identification of stable periods and individual differences. Electroencephalogr Clin Neurophysiol Evoked Potentials Sect 92(4):291–302. https://doi.org/10.1016/0168-5597(94)90097-3
Dowman R (1994b) SEP topographies elicited by innocuous and noxious sural nerve stimulation. II. Effects of stimulus intensity on topographic pattern and amplitude. Electroencephalogr Clin Neurophysiol 92(4):303–315. https://doi.org/10.1016/0168-5597(94)90098-1
Dowman R (2004) Topographic analysis of painful laser and sural nerve electrical evoked potentials. Brain Topogr 16(3):169–179. https://doi.org/10.1023/b:brat.0000019185.30489.ad
Fries P (2009) Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci 32(1):209–224. https://doi.org/10.1146/annurev.neuro.051508.135603
Fries P (2015) Rhythms for cognition: communication through coherence. Neuron 88(1):220–235. https://doi.org/10.1016/j.neuron.2015.09.034
Garcia-Larrea L (2006) Chapter 30 Evoked potentials in the assessment of pain. In: Cervero F, Jensen TS (eds) Handb clin neurol, vol 81. Elsevier, p 439-XI
Girard S, Pelland M, Lepore F, Collignon O (2013) Impact of the spatial congruence of redundant targets on within-modal and cross-modal integration. Exp Brain Res 224(2):275–285. https://doi.org/10.1007/s00221-012-3308-0
Gross J, Schnitzler A, Timmermann L, Ploner M (2007) Gamma oscillations in human primary somatosensory cortex reflect pain perception. PLoS Biol 5(5):e133. https://doi.org/10.1371/journal.pbio.0050133
Harris JA, Arabzadeh E, Fairhall AL, Benito C, Diamond ME (2006) Factors affecting frequency discrimination of vibrotactile stimuli: implications for cortical encoding. PLoS ONE 1:e100. https://doi.org/10.1371/journal.pone.0000100
Hauck M, Domnick C, Lorenz J, Gerloff C, Engel AK (2015) Top-down and bottom-up modulation of pain-induced oscillations. Front Hum Neurosci 9:375. https://doi.org/10.3389/fnhum.2015.00375
Heid C, Mouraux A, Treede RD, Schuh-Hofer S, Rupp A, Baumgärtner U (2020) Early gamma-oscillations as correlate of localized nociceptive processing in primary sensorimotor cortex. J Neurophysiol 123(5):1711–1726. https://doi.org/10.1152/jn.00444.2019
Hoechstetter K, Rupp A, Stančák A, Meinck H-M, Stippich C, Berg P, Scherg M (2001) Interaction of tactile input in the human primary and secondary somatosensory cortex—a magnetoencephalographic study. Neuroimage 14(3):759–767. https://doi.org/10.1006/nimg.2001.0855
Iannetti GD, Hughes NP, Lee MC, Mouraux A (2008) Determinants of laser-evoked EEG responses: pain perception or stimulus saliency? J Neurophysiol 100(2):815–828. https://doi.org/10.1152/jn.00097.2008
Kakigi R, Jones SJ (1986) Influence of concurrent tactile stimulation on somatosensory evoked potentials following posterior tibial nerve stimulation in man. Electroencephalogr Clin Neurophysiol 65(2):118–129. https://doi.org/10.1016/0168-5597(86)90044-4
Kennett S, Taylor-Clarke M, Haggard P (2001) Noninformative vision improves the spatial resolution of touch in humans. Curr Biol 11(15):1188–1191. https://doi.org/10.1016/s0960-9822(01)00327-x
Kuroki S, Watanabe J, Nishida S (2017) Integration of vibrotactile frequency information beyond the mechanoreceptor channel and somatotopy. Sci Rep 7(1):2758. https://doi.org/10.1038/s41598-017-02922-7
Lautenbacher S, Prager M, Rollman GB (2007) Pain additivity, diffuse noxious inhibitory controls, and attention: a functional measurement analysis. Somatosens Mot Res 24(4):189–201. https://doi.org/10.1080/08990220701637638
Le Bars D, Dickenson AH, Besson JM (1979) Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain 6(3):283–304
Lee MC, Mouraux A, Iannetti GD (2009) Characterizing the cortical activity through which pain emerges from nociception. J Neurosci 29(24):7909–7916. https://doi.org/10.1523/jneurosci.0014-09.2009
Legrain V, Iannetti GD, Plaghki L, Mouraux A (2011) The pain matrix reloaded: a salience detection system for the body. Prog Neurobiol 93(1):111–124. https://doi.org/10.1016/j.pneurobio.2010.10.005
Liu Z, Zhang N, Chen W, He B (2009) Mapping the bilateral visual integration by EEG and fMRI. Neuroimage 46(4):989–997. https://doi.org/10.1016/j.neuroimage.2009.03.028
Madden VJ, Catley MJ, Grabherr L, Mazzola F, Shohag M, Moseley GL (2016) The effect of repeated laser stimuli to ink-marked skin on skin temperature—recommendations for a safe experimental protocol in humans. PeerJ. https://doi.org/10.7717/peerj.1577
Mejias JF, Murray JD, Kennedy H, Wang X-J (2016) Feedforward and feedback frequency-dependent interactions in a large-scale laminar network of the primate cortex. Sci Adv 2(11):e1601335. https://doi.org/10.1126/sciadv.1601335
Moayedi M, Liang M, Sim AL, Hu L, Haggard P, Iannetti GD (2015) Laser-evoked vertex potentials predict defensive motor actions. Cereb Cortex 25(12):4789–4798. https://doi.org/10.1093/cercor/bhv149
Moayedi M, Di Stefano G, Stubbs MT, Djeugam B, Liang M, Iannetti GD (2016) Nociceptive-evoked potentials are sensitive to behaviorally relevant stimulus displacements in egocentric coordinates. eNeuro. https://doi.org/10.1523/eneuro.0151-15.2016
Mouraux A, Iannetti GD (2008) Across-trial averaging of event-related EEG responses and beyond. Magn Reson Imaging 26(7):1041–1054. https://doi.org/10.1016/j.mri.2008.01.011
Nielsen J, Arendt-Nielsen L (1997) Spatial summation of heat induced pain within and between dermatomes. Somatosens Mot Res 14(2):119–125. https://doi.org/10.1080/08990229771123
Northon S, Rustamov N, Piche M (2019) Cortical integration of bilateral nociceptive signals: when more is less. Pain 160(3):724–733. https://doi.org/10.1097/j.pain.0000000000001451
Perchet C, Godinho F, Mazza S, Frot M, Legrain V, Magnin M, Garcia-Larrea L (2008) Evoked potentials to nociceptive stimuli delivered by CO2 or Nd:YAP lasers. Clin Neurophysiol 119(11):2615–2622. https://doi.org/10.1016/j.clinph.2008.06.021
Plaghki L, Mouraux A (2003) How do we selectively activate skin nociceptors with a high power infrared laser? Physiology and biophysics of laser stimulation. Neurophysiol Clin 33(6):269–277
Ploner M, Sorg C, Gross J (2017) Brain rhythms of pain. Trends Cogn Sci 21(2):100–110. https://doi.org/10.1016/j.tics.2016.12.001
Quevedo AS, Coghill RC (2007) Attentional modulation of spatial integration of pain: evidence for dynamic spatial tuning. J Neurosci 27(43):11635–11640. https://doi.org/10.1523/jneurosci.3356-07.2007
Ragert P, Nierhaus T, Cohen LG, Villringer A (2011) Interhemispheric interactions between the human primary somatosensory cortices. PLoS ONE. https://doi.org/10.1371/journal.pone.0016150
Ronga I, Valentini E, Mouraux A, Iannetti GD (2013) Novelty is not enough: laser-evoked potentials are determined by stimulus saliency, not absolute novelty. J Neurophysiol 109(3):692–701. https://doi.org/10.1152/jn.00464.2012
Rossiter HE, Worthen SF, Witton C, Hall SD, Furlong PL (2013) Gamma oscillatory amplitude encodes stimulus intensity in primary somatosensory cortex. Front Hum Neurosci 7:362. https://doi.org/10.3389/fnhum.2013.00362
Rustamov N, Northon S, Tessier J, Leblond H, Piche M (2019) Integration of bilateral nociceptive inputs tunes spinal and cerebral responses. Sci Rep 9(1):7143. https://doi.org/10.1038/s41598-019-43567-y
Saija JD, Başkent D, Andringa TC, Akyürek EG (2017) Visual and auditory temporal integration in healthy younger and older adults. Psychol Res. https://doi.org/10.1007/s00426-017-0912-4
Sambo CF, Forster B, Williams SC, Iannetti GD (2012) To blink or not to blink: fine cognitive tuning of the defensive peripersonal space. J Neurosci 32(37):12921–12927. https://doi.org/10.1523/jneurosci.0607-12.2012
Sandrini G, Serrao M, Rossi P, Romaniello A, Cruccu G, Willer JC (2005) The lower limb flexion reflex in humans. Prog Neurobiol 77(6):353–395. https://doi.org/10.1016/j.pneurobio.2005.11.003
Schnitzler A, Gross J (2005) Normal and pathological oscillatory communication in the brain. Nat Rev Neurosci 6(4):285–296. https://doi.org/10.1038/nrn1650
Schulz E, Tiemann L, Witkovsky V, Schmidt P, Ploner M (2012) gamma Oscillations are involved in the sensorimotor transformation of pain. J Neurophysiol 108(4):1025–1031. https://doi.org/10.1152/jn.00186.2012
Sherrington CS (1906) The integrative action of the nervous system. Yale University Press, New Haven
Sherrington CS (1910) Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J Physiol 40(1–2):28–121. https://doi.org/10.1113/jphysiol.1910.sp001362
Simões C, Alary F, Forss N, Hari R (2002) Left-hemisphere-dominant SII activation after bilateral median nerve stimulation. Neuroimage 15(3):686–690. https://doi.org/10.1006/nimg.2001.1007
Tabor A, Thacker MA, Moseley GL, Körding KP (2017) Pain: a statistical account. PLoS Comput Biol 13(1):e1005142. https://doi.org/10.1371/journal.pcbi.1005142
Tame L, Pavani F, Papadelis C, Farne A, Braun C (2015) Early integration of bilateral touch in the primary somatosensory cortex. Hum Brain Mapp 36(4):1506–1523. https://doi.org/10.1002/hbm.22719
Tan LL, Oswald MJ, Heinl C, Retana Romero OA, Kaushalya SK, Monyer H, Kuner R (2019) Gamma oscillations in somatosensory cortex recruit prefrontal and descending serotonergic pathways in aversion and nociception. Nat Commun 10(1):983. https://doi.org/10.1038/s41467-019-08873-z
Tiemann L, Schulz E, Gross J, Ploner M (2010) Gamma oscillations as a neuronal correlate of the attentional effects of pain. Pain 150(2):302–308. https://doi.org/10.1016/j.pain.2010.05.014
Tiemann L, May ES, Postorino M, Schulz E, Nickel MM, Bingel U, Ploner M (2015) Differential neurophysiological correlates of bottom-up and top-down modulations of pain. Pain 156(2):289–296. https://doi.org/10.1097/01.j.pain.0000460309.94442.44
Torta DM, Liang M, Valentini E, Mouraux A, Iannetti GD (2012) Dishabituation of laser-evoked EEG responses: dissecting the effect of certain and uncertain changes in stimulus spatial location. Exp Brain Res 218(3):361–372. https://doi.org/10.1007/s00221-012-3019-6
Torta DM, Legrain V, Mouraux A (2015) Looking at the hand modulates the brain responses to nociceptive and non-nociceptive somatosensory stimuli but does not necessarily modulate their perception. Psychophysiology 52(8):1010–1018. https://doi.org/10.1111/psyp.12439
Valentini E, Betti V, Hu L, Aglioti SM (2013) Hypnotic modulation of pain perception and of brain activity triggered by nociceptive laser stimuli. Cortex 49(2):446–462. https://doi.org/10.1016/j.cortex.2012.02.005
Willer JC (1977) Comparative study of perceived pain and nociceptive flexion reflex in man. Pain 3(1):69–80. https://doi.org/10.1016/0304-3959(77)90036-7
Woolf CJ, Ma Q (2007) Nociceptors—noxious stimulus detectors. Neuron 55(3):353–364. https://doi.org/10.1016/j.neuron.2007.07.016
Yarnitsky D (2010) Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol 23(5):611–615. https://doi.org/10.1097/ACO.0b013e32833c348b
Yue L, Iannetti GD, Hu L (2020) The neural origin of nociceptive-induced gamma-band oscillations. J Neurosci 40(17):3478–3490. https://doi.org/10.1523/jneurosci.0255-20.2020
Zhang ZG, Hu L, Hung YS, Mouraux A, Iannetti GD (2012) Gamma-band oscillations in the primary somatosensory cortex—a direct and obligatory correlate of subjective pain intensity. J Neurosci 32(22):7429–7438. https://doi.org/10.1523/jneurosci.5877-11.2012
Acknowledgements
This work was supported by a grant from the Natural Science and Engineering Research Council of Canada (#06659) and the Canadian Foundation for Innovation (#33731). The contribution of Stéphane Northon was supported by the Fonds de Recherche du Québec en Nature et Technologie. The contribution of Zoha Deldar was supported by the Department of Anatomy of the Université du Québec à Trois-Rivières and the Centre de recherche en Neuropsychologie et Cognition. The contribution of Mathieu Piché was supported by the Fonds de Recherche du Québec en Santé.
Funding
This work was supported by a grant from the Natural Science and Engineering Research Council of Canada (#06659) and the Canadian Foundation for Innovation (#33731). The contribution of Stéphane Northon was supported by the Fonds de Recherche du Québec en Nature et Technologie. The contribution of Zoha Deldar was supported by the Department of Anatomy of the Université du Québec à Trois-Rivières and the Centre de recherche en Neuropsychologie et Cognition. The contribution of Mathieu Piché was supported by the Fonds de Recherche du Québec en Santé.
Author information
Authors and Affiliations
Contributions
All authors contributed significantly to this study and has read the final version of the manuscript. SN contributed to data collection and analyses and wrote the first version of the manuscript. ZD contributed to data collection. MP contributed to study design, data collection, analyses and interpretation, wrote the final version of the manuscript and obtained funding.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Ethics approval
All experimental procedures conformed to the standards set by the latest revision of the Declaration of Helsinki and were approved by the Research Ethics Board of the Université du Québec à Trois-Rivières.
Consent to participate
All participants received written informed consent, acknowledged their right to withdraw from the experiment without prejudice, and received a compensation of $25 for their time.
Consent for publication
Not applicable.
Additional information
Communicated by Melvyn A. Goodale.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Northon, S., Deldar, Z. & Piché, M. Cortical interaction of bilateral inputs is similar for noxious and innocuous stimuli but leads to different perceptual effects. Exp Brain Res 239, 2803–2819 (2021). https://doi.org/10.1007/s00221-021-06175-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-021-06175-9