Abstract
This study tested whether air-conducted sound and bone-conducted vibration activated primary vestibular afferent neurons and whether, at low levels, such stimuli are specific to particular vestibular sense organs. In response to 500 Hz bone-conducted vibration or 500 Hz air-conducted sound, primary vestibular afferent neurons in the guinea pig fall into one of two categories––some neurons show no measurable change in firing up to 2 g peak-to-peak or 140 dB SPL. These are semicircular canal neurons (regular or irregular) and regular otolith neurons. In sharp contrast, otolith irregular neurons show high sensitivity: a steep increase in firing as stimulus intensity is increased. These sensitive neurons typically, but not invariably, were activated by both bone-conducted vibration and air-conducted sound, they originate from both the utricular and saccular maculae, and their sensitivity underpins new clinical tests of otolith function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Some primary vestibular afferent neurons respond to stimuli that are normally thought to be auditory rather than vestibular: air-conducted sound (ACS) and bone-conducted vibration (BCV) (Young et al. 1977; Murofushi and Curthoys 1997; McCue and Guinan 1997; Curthoys et al. 2006; Curthoys 2010; Curthoys and Vulovic, unpublished observations). On the basis of such evidence, new clinical tests of vestibular function have been developed using ACS and BCV, since it is easier and safer to present these stimuli in a clinic rather than using whole-head or whole-body accelerations (see Curthoys 2010 for a review). In the light of this clinical application, it is important to clarify how vestibular neurons respond to ACS and BCV, and this review primarily covers the published data from the Curthoys laboratory on recordings of primary afferent neurons in the guinea pig (Curthoys et al. 2006), together with new, as yet unpublished research, which has confirmed and extended the earlier work (Curthoys and Vulovic 2010, unpublished observations).
Studies on isolated vestibular receptor cells have shown that they respond to any stimulus that causes the stereocilia to be deflected (e.g. Hudspeth and Corey 1977). Usually, the stereocilia are deflected by angular and linear accelerations of the head that cause displacement of the cupulae or maculae and so results in a deflection of the stereocilia. BCV generates pressure waves that travel through and around the head, and measurements by linear accelerometers on the mastoids show that these waves result in linear acceleration at the mastoids (von Békésy 1960; Iwasaki et al. 2008). So BCV can activate vestibular afferents because it generates linear acceleration. Very intense ACS can cause primary vestibular afferents to be activated (Murofushi and Curthoys 1997; McCue and Guinan 1997), probably because the stapes movement generates a pressure wave in the fluid of the inner ear that deflects the stereocilia of adjacent vestibular receptors and so activates primary vestibular afferent neurons. In mammals, the saccular macula and the utricular macula are closely adjacent to the stapes (Uzun-Coruhlu et al. 2007; Mukherjee et al. 2010). BCV and ACS appear to have very different transduction mechanisms, so a major question is whether the one neuron can be activated by both BCV and ACS.
The definition of activation
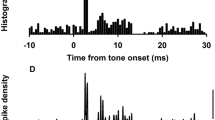
A central issue is defining “activation”. Young et al. (1977) defined activation as phase-locking of the primary neuronal response to the stimulus and reported that in the squirrel monkey, ACS and BCV stimuli caused phase-locking of neurons from all vestibular sensory regions at stimulus levels well below the levels necessary to elicit a detectable increase in neural firing. It is doubtful that phase-locking, without an increase in firing rate, could result in a measurable vestibulo-ocular or vestibulo-spinal response. Young et al. held the same view: “Most likely, phase-locking at audio frequencies would not, by itself, be recognized as an activation of the vestibular apparatus” (1977, p. 358).
In the study of guinea pig primary afferents to BCV, Curthoys et al. (2006) used a definition of activation that is more relevant for understanding vestibulo-ocular and vestibulo-spinal responses generated by ACS or BCV––that of an audibly detectable, stimulus-locked increase in firing rate. In that 2006 study, the stimulus-locked increase in firing was determined subjectively by listening to the unit firing in response to repeated presentations of the stimulus. More recently, the change in firing has been quantified objectively by measuring, at each stimulus level, the percentage increase in firing rate in spikes/s re the firing rate at baseline. Using the older definition, Curthoys et al. 2006 reported that primary otolith irregular neurons are activated by BCV, whereas regular otolith neurons and semicircular canal neurons are rarely activated and then only at high intensities. It is now clear that such a definition is too loose because it can result in neurons being categorized as similar (“activated”) that have very different sensitivities. For example, a neuron that has a very small (but audibly detectable) increase in firing rate at the highest stimulus intensity would be classed as equivalent to a neuron that shows a strong increase in firing even at very lowest stimulus intensities. These two neurons are obviously totally different in the sensitivity of their responses. It seems that the sensitivity of the neural response (as measured by per cent increase in firing per unit increase in stimulus level) is probably a better criterion for activation rather than a detectable increase in firing.
Curthoys et al. (2006) found that even the loose “detectable increase in firing rate” definition very clearly distinguished between (a) neurons that showed no detectable increase in firing rate up to the highest stimulus levels of BCV or ACS used (canal neurons and otolith regular neurons) and (b) neurons that showed a clear response at very low stimulus levels and a highly sensitive response to BCV (otolith irregular neurons).
The methods used in these studies comprise extracellular recording by glass microelectrodes in Scarpa’s ganglion visualized in the internal auditory meatus in acute ketamine-anaesthetized guinea pigs, after removal of the flocculus. The BCV was delivered by a Radioear B-71 bone oscillator cemented to the skull and the ACS delivered via a TDH 49 headphone coupled to a speculum in the ear canal. In the guinea pigs, the various branches of the VIII nerve are spatially separated at the internal auditory meatus, so it is possible to specify which branch of the vestibular nerve is being recorded from by visual observation. Additionally, the vestibular character of each neuron was verified by testing with tilts and rotations. A neuron was classed as a semicircular canal neuron if it showed an increase in firing rate during an angular acceleration, but returned to baseline firing at rest. A neuron was classed as otolithic if it showed a maintained change in firing rate during a maintained tilt position. In some otolithic neurons with a phasic response, the gain of the neuron to maintained tilts was small––Goldberg et al. (1990) had reported the low DC gain of some otolithic irregular neuron––so it was sometimes difficult to establish that the neuron was otolithic. However, these neurons typically responded at the onset of an abrupt tilt. In contrast, vertical canal neurons responded maximally at about peak angular velocity of sinusoidal tilts.
In these studies for both BCV and ACS, the stimulus was a 500 Hz sine wave lasting 0.5 s with square wave switching, presented at increasing stimulus levels with the stimulus being repeated three times at each stimulus level. Linear acceleration was measured by a sub-miniature triaxial linear accelerometer cemented to the skull (intensities up to 2g rms of 3 axes) and the ACS by a Brüel and Kjaer sound level meter (intensity up to 140 dB SPL). In addition, the auditory brainstem response (ABR) to sound and vibration was recorded by a silver ball electrode on the auditory cortex (Naito et al. 1999), and as a baseline for stimulus intensity, the ABR threshold of the wave I component of the ABR for various stimuli (clicks, single cycle and two cycle 500 Hz tones) for both ACS and BCV stimuli was measured to ensure that the stimuli used in this study were in a physiological range by being within 100 dB of ABR threshold. Each neuron was classified as regular or irregular on the basis of the normalized coefficient of variation (CV*) of interspike intervals of the resting discharge rate (CV* < 0.1 = regular; CV* > 0.1 = irregular) (see Curthoys et al. 2006). This simple approach was used since the prime concern was to show whether there was a very simple response pattern, not to establish a continuum of response as a function of CV*.
Otolith irregular neurons are selectively activated by sound and vibration
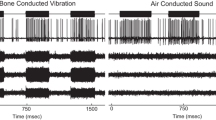
Tracks were made in the dorsal superior vestibular nerve and the inferior vestibular nerve, and cells activated by both ACS and BCV were found in both divisions of the nerve. Most neurons show no detectable change in firing up to the maximum intensities we used, and Fig. 1 shows an example: the response of a regular canal neuron to high-intensity BCV and ACS stimulation, and there is no detectable stimulus-locked change in firing. In contrast, a small number of neurons (perhaps only 10–15% of all afferents) responded at low threshold and with high sensitivity––a steep increase in firing as intensity was increased, and Fig. 2 shows an example: the response of an otolith irregular neuron in the inferior vestibular nerve, and so saccular, to low-intensity BCV and ACS stimulation. The stimulus-locked change in firing to both BCV and ACS is clear.
Time series of a regular semicircular-canal neuron during stimulation by BCV and ACS. The top trace (a) shows the command voltage indicating when the stimulus is on. The second trace shows the extracellular recording. The three bottom traces (x, y, z) show the triaxial accelerometer recording of the stimulus. The left panel is an example of BCV stimulation and the right of ACS stimulation of the same neuron. Note the scale of stimulus intensity in g at the left margin between traces x and y
Time series of an irregular otolith neuron during stimulation by BCV and ACS. The top trace (a) shows the command voltage indicating when the stimulus is on. The second trace shows the extracellular recording. The three bottom traces (x, y, z) show the triaxial accelerometer recording of the stimulus. The left panel is an example of BCV stimulation and the right of ACS stimulation of the same neuron. Note the scale of stimulus intensity in g at the left margin between traces x and y
Figure 3 shows that with increasing BCV stimulus peak intensity up to 2 g, some neurons showed no change in their spontaneous firing rate. These are the near horizontal lines in Fig. 3, and they were the typical response functions for semicircular canal neurons (regular or irregular) and otolith regular neurons. In sharp contrast, other neurons showed a very large increase in firing with only a very small increase in stimulus intensity. These were the near-vertical lines in Fig. 3, and they were the typical response functions of otolith irregular neurons. Their average sensitivity was of the order of 2000%/g (see Fig. 4). When activated, they show the phase-locking reported by Young et al. (1977). We quantified sensitivity by measuring the percentage increase in firing compared to baseline firing rate, as intensity was increased, and Fig. 4 shows the average sensitivity of different classes of neurons.
Examples of sensitivity plots of neurons to BCV (a) and ACS (b). Each point shows the increase in firing rate as the percentage of baseline firing rate during a single stimulus presentation. The stimulus intensity is calculated in g, root mean square of three axes as recorded by the accelerometer, for BCV and dB SPL for ACS. Each line is the best fit calculation of the responses for one neuron. The red X marks show the mean ABR thresholds with 95% confidence intervals for click, 1 and 2 cycle tone stimuli
Average sensitivities for neurons to BCV. The gradients of lines in Fig. 3 are averaged for each class of neuron, with 95% confidence intervals. The unit of sensitivity is per cent increase in firing rate per g above the resting discharge rate. The high sensitivity of otolith irregular neurons is clear
We then asked the question: can these same irregular otolithic neurons be activated by ACS? and the simple answer is yes; many, but not all, of the units activated by BCV can be activated by ACS (Fig. 2). For most neurons, the intensity of the ACS stimulus at threshold activation had to be about 60 dB above ABR threshold (i.e. about 115 dB SPL) before any increase in firing was detectable (Fig. 3), showing that BCV is a much more efficient vestibular stimulus than ACS.
Are these activated neurons really vestibular?
The direct visual electrode placement was into Scarpa’s ganglion of the vestibular nerve at the border of the temporal bone, so these are not cells of the interstitial nucleus of the vestibular nerve or the cochlear nucleus. Nor are they vestibular efferents because of their very short latencies (usually around 1 ms to single BCV click stimulation) (Curthoys et al. 2006). Irregular otolithic afferents are large diameter fibres originating from the calyx endings enveloping the type I receptors at the striola (Goldberg et al. 1990). These afferents have been shown to have a strong response to changes in linear acceleration (jerk) of the whole head rather than to maintained or low-frequency linear accelerations (Goldberg et al. 1990). The 500 Hz BCV stimulus is a powerful drive for them, because there are 500 steep changes in linear acceleration per second. Why is there such a clear dichotomy? Possibly, the choice of 500 Hz was optimal for showing the dichotomy since it was below the high frequency cut-off of the jerk-sensitive otolith irregular afferents, whilst being above the high frequency cut-off of regular otolith neurons and canal neurons.
Where were these neurons located? Some of these high-sensitivity neurons lie in the dorsal superior vestibular nerve and so are most probably utricular, whereas others lie in the inferior vestibular nerve and so are saccular (de Burlet 1924). It is important to stress that both utricular and saccular afferents can be stimulated by 500 Hz ACS and 500 Hz BCV, because there are some claims in the clinical literature that utricular neurons respond only to BCV and that saccular neurons respond only to ACS. These results show that both of those statements are incorrect––in fact, both utricular and saccular afferents respond to both BCV and ACS.
Why should otolith irregular neurons show such great sensitivity to vibration? In the frog gravity, sensing afferents from the saccule and lagena have been found to have remarkable sensitivity to vibration with a threshold of a few milli-gs (Narins and Lewis 1984; Koyama et al. 1982). This may relate to the need in these species to detect mating calls transmitted by ground-borne vibrations. It may be that the receptor mechanism allowing such great vibration sensitivity has been preserved by otolithic striola receptors, potentially giving these receptors a dual function––of detecting changes in linear acceleration and also detecting sound and vibration.
Comparison of guinea pig and squirrel monkey results
Young et al. (1977) reported that “responses to sound and vibration were seen in units from all 5 sense-organs” (p. 354), and “The squirrel monkey sacculus is, if anything, less sensitive to vibrational stimuli than are semicircular canals” (p. 358). In the guinea pig, using low-intensity 500 Hz stimuli, we found the opposite––that only a small subgroup of vestibular afferents could be activated by 500 Hz BCV and ACS (otolith irregular neurons) and that subgroup was not “from all 5 sense organs” but was specifically otolithic. Can these contradictory results be reconciled? There are many differences between the studies as Table 1 shows.
Young et al. (1977) undertook parametric investigation of a small group of neurons, using a range of frequencies, whereas the approach in the Curthoys laboratory has been to seek an overall population response pattern from many neurons using only 500 Hz stimuli. Activation was defined quite differently. There were major species and technical differences. We used large guinea pigs weighing around 800–1,200 g, and the thick skulls of our guinea pigs were rigidly held by a bolt cemented to the skull and bolted onto the stereotaxic frame. Young et al. (1977) used squirrel monkeys that appear to have more flexible skulls than the skulls of the large guinea pigs we used. The squirrel monkey skull was held by ear bars, whereas the guinea pig skull was held by a skull bolt. In young guinea pigs with small skulls, we could sometimes drive the B-71 oscillator sufficiently to activate a few semicircular canal neurons weakly by BCV at the highest intensities used (Curthoys et al. 2006, Table 1). This was simply not possible with large guinea pigs (over 1,000 g) with thick skulls. Possibly, the small skull of young animals allowed some flexion at the skull joints and so an angular rotation that would be sufficient to stimulate a semicircular canal neuron. But this was a completely different activation to the kind shown in Fig. 2.
The direction of the vibration stimulus for these two studies was different––predominantly rostro-caudal for Young et al. (1977) and predominantly dorso-ventral for Curthoys et al. (2006). Because of the host of differences, it is not possible to identify any particular factor as the cause of the disagreement. In the guinea pig, at 500 Hz, we continue to confirm that at low stimulus levels, semicircular canal neurons show no response (see Fig. 1), whereas otolith irregular neurons do (see Fig. 2). This differential response provides the foundation for clinical vestibular tests of otolith function using BCV and ACS.
Conclusion
In guinea pigs with normally enclosed labyrinths, sound and vibration can result in an increase in firing rate of some primary vestibular afferent neurons. In the guinea pig, otolithic irregular neurons, most probably from the striolae of the maculae, are activated at low stimulus levels by BCV and ACS. These neurons show a very sensitive response with a steep increase in firing with increases in intensity. At these stimulus levels, semicircular canal neurons do not respond. This dichotomous response has been used to underpin the interpretation of the human extraocular EMG response to low levels of BCV as an otolithic response, and to lay the groundwork for the clinical application of 500 Hz skull vibration of human patients as a specific test of otolith function.
Abbreviations
- ACS:
-
Air-conducted sound
- ABR:
-
Auditory brainstem response
- BCV:
-
Bone-conducted vibration
- B-71:
-
The standard clinical bone conduction oscillator (Radioear B71)
- CV*:
-
Normalized coefficient of variation
References
Curthoys IS (2010) A critical review of the neurophysiological evidence underlying clinical vestibular testing using sound, vibration and galvanic stimuli. Clin Neurophysiol 121:132–146
Curthoys IS, Kim J, McPhedran SK, Camp AJ (2006) Bone conducted vibration selectively activates irregular primary otolithic vestibular neurons in the guinea pig. Exp Brain Res 175:256–267
de Burlet HM (1924) Zur Innervation der Macula sacculi bei Säugetieren. Anat Anzeig 58:26–32
Goldberg JM, Desmadryl G, Baird RA, Fernandez C (1990) The vestibular nerve of the chinchilla IV. Discharge properties of utricular afferents. J Neurophysiol 63:781–790
Hudspeth AJ, Corey DP (1977) Sensitivity, polarity, and conductance change in the response of vertebrate hair cells to controlled mechanical stimuli. Proc Natl Acad Sci USA 74:2407–2411
Iwasaki S, Smulders YE, Burgess AM, McGarvie LA, MacDougall HG, Halmagyi GM, Curthoys IS (2008) Ocular vestibular evoked myogenic potentials to bone conducted vibration of the midline forehead at Fz in healthy subjects. Clin Neurophysiol 119:2135–2147
Koyama H, Lewis ER, Leverenz EL, Baird RA (1982) Acute seismic sensitivity in the bullfrog ear. Brain Res 250:168–172
McCue MP, Guinan JJ (1997) Sound-evoked activity in primary afferent neurons of a mammalian vestibular system. Am J Otol 18:355–360
Mukherjee P, Uzun H, Curthoys IS, Jones AS, Bradshaw AP, Pohl D (2010) Three dimensional analysis of the vestibular endorgans in relation to the stapes footplate for stapes piston placement. Otol Neurotol (in press)
Murofushi T, Curthoys IS (1997) Physiological and anatomical study of click-sensitive primary vestibular afferents in the guinea pig. Acta Otolaryngol 117:66–72
Naito R, Murofushi T, Mizutani M, Kaga K (1999) Auditory brainstem responses, electrocochleograms and cochlear microphonics in the myelin deficient hamster ‘bt’. Hear Res 136:44–48
Narins PM, Lewis ER (1984) The vertebrate ear as an exquisite seismic sensor. J Acoust Soc Am 76:1384–1387
Uzun-Coruhlu H, Curthoys IS, Jones AS (2007) Attachment of the utricular and saccular maculae to the temporal bone. Hear Res 233(1–2):77–85
von Békésy G (1960) Experiments in hearing. McGraw-Hill, New York
Young ED, Fernandez C, Goldberg JM (1977) Responses of squirrel monkey vestibular neurons to audio-frequency sound and head vibration. Acta Otolaryngol 84:352–360
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia and the Garnett Passe and Rodney Williams Memorial Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Curthoys, I.S., Vulovic, V. Vestibular primary afferent responses to sound and vibration in the guinea pig. Exp Brain Res 210, 347–352 (2011). https://doi.org/10.1007/s00221-010-2499-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-010-2499-5