Abstract
The main objective of this study was to determine whether bone-conducted vibration (BCV) is equally effective in activating both semicircular canal and otolith afferents in the guinea pig or whether there is preferential activation of one of these classes of vestibular afferents. To answer this question a large number (346) of single primary vestibular neurons were recorded extracellularly in anesthetized guinea pigs and were identified by their location in the vestibular nerve and classed as regular or irregular on the basis of the variability of their spontaneous discharge. If a neuron responded to angular acceleration it was classed as a semicircular canal neuron, if it responded to maintained roll or pitch tilts it was classified as an otolith neuron. Each neuron was then tested by BCV stimuli—either clicks, continuous pure tones (200–1,500 Hz) or short tone bursts (500 Hz lasting 7 ms)—delivered by a B-71 clinical bone-conduction oscillator cemented to the guinea pig's skull. All stimulus intensities were referred to that animal's own auditory brainstem response (ABR) threshold to BCV clicks, and the maximum intensity used was within the animal's physiological range and was usually around 70 dB above BCV threshold. In addition two sensitive single axis linear accelerometers cemented to the skull gave absolute values of the stimulus acceleration in the rostro-caudal direction. The criterion for a neuron being classed as activated was an audible, stimulus-locked increase in firing rate (a 10% change was easily detectable) in response to the BCV stimulus. At the stimulus levels used in this study, semicircular canal neurons, both regular and irregular, were insensitive to BCV stimuli and very few responded: only nine of 189 semicircular canal neurons tested (4.7%) showed a detectable increase in firing in response to BCV stimuli up to the maximum 2 V peak-to-peak level we delivered to the B-71 oscillator (which produced a peak-to-peak skull acceleration of around 6–8 g and was usually around 60–70 dB above the animal's own ABR threshold for BCV clicks). Regular otolithic afferents likewise had a poor response; only 14 of 99 tested (14.1%) showed any increase in firing rate up to the maximum BCV stimulus level. However, most irregular otolithic afferents (82.8%) showed a clear increase in firing rate in response to BCV stimuli: of the 58 irregular otolith neurons tested, 48 were activated, with some being activated at very low intensities (only about 10 dB above the animal's ABR threshold to BCV clicks). Most of the activated otolith afferents were in the superior division of the vestibular nerve and were probably utricular afferents. That was confirmed by evidence using juxtacellular injection of neurobiotin near BCV activated neurons to trace their site of origin to the utricular macula. We conclude there is a very clear preference for irregular otolith afferents to be activated selectively by BCV stimuli at low stimulus levels and that BCV stimuli activate some utricular irregular afferent neurons. The BCV generates compressional and shear waves, which travel through the skull and constitute head accelerations, which are sufficient to stimulate the most sensitive otolithic receptor cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mammalian primary vestibular neurons can, under the appropriate conditions, respond to auditory stimulation. Mikaelian (1964) used extracellular single neuron recordings of primary vestibular afferents in a strain of mutant mice without cochleas and found that vestibular neurons in these mice responded to air-conducted (AC) sound—after their bony labyrinth had been opened. The response of vestibular neurons to AC sound in animals with intact labyrinths has been confirmed and extended to other species (squirrel monkey—Young et al. 1977; cat—McCue and Guinan 1994a, b; guinea pig—Murofushi et al. 1995; chinchilla—Carey et al. 2004). Indeed this response has been used as the basis of a widespread clinical test of human vestibular function: intense AC clicks or short tone bursts activate vestibular receptors and result in a short latency vestibular inhibitory potential in neck muscles. This is called the vestibular evoked myogenic potential (VEMP) (Colebatch and Halmagyi 1992). Recordings of primary vestibular afferents from both cat and guinea pig identify the saccular macula as being the vestibular sensory region preferentially activated by such AC stimuli (McCue and Guinan 1994a, b, 1995, 1997; Murofushi et al. 1995; Murofushi and Curthoys 1997). Irregular saccular neurons, which probably originate predominantly from the striola of the saccular macula (Baird et al. 1988; Goldberg 2000) were preferentially activated by AC click stimulation at low stimulus levels. Although, many hundreds of semicircular canal neurons were tested in the Murofushi studies only a few responded at levels up to the maximum intensities delivered (Murofushi et al. 1995; Murofushi and Curthoys 1997). Murofushi et al. concluded the neurons activated by AC clicks were saccular because (1) they responded to maintained pitch tilts as opposed to angular accelerations, (2) they were located in the inferior division of the vestibular nerve, and (3) there was retrograde transport of biocytin from the site of the recorded neuron cell-bodies in Scarpa's ganglion to the central region of the saccular macula (Murofushi et al. 1995; Murofushi and Curthoys 1997). McCue and Guinan also independently concluded that irregular saccular neurons were activated by AC sound (1995, 1997) on the basis of cellular staining and tracing of single sound-activated irregular vestibular neurons to the saccular macula in the cat.
Recent clinical studies have shown that bone conducted skull vibrations—BCV stimuli consisting of 7 ms tone bursts (500 Hz) delivered by a small clinical bone conduction oscillator on the skin over the patient's mastoid may be an even more effective and clinically more acceptable stimulus than AC clicks for the VEMP test, and such a short tone burst BCV stimulus has been reported to be more effective at eliciting a response in comparison to a single click (Welgampola and Colebatch 2005; Rosengren et al. 2005; Jombik and Bahyl 2005). However, with the exception of the study by Young et al. (1977) there is scant evidence about which vestibular sensory regions are activated by such BCV stimuli in mammals. In that study, a total of 34 vestibular neurons in the squirrel monkey were classified as originating from various vestibular sensory regions on the basis of the neuron's location in the superior or inferior division of the vestibular nerve and by the neuron's response to natural vestibular stimulation—angular accelerations and linear accelerations. The BCV stimulus was delivered by an electrodynamic transducer (akin to a loudspeaker driver) coupled by a plexiglas rod to the animal's frontal bone. The stimulus magnitude was measured by “hand-held accelerometers” placed on the skull. It was reported that neurons from all vestibular sensory regions were activated by BCV stimuli and that canal neurons were as sensitive as otolith neurons.
In order to use BCV VEMPs validly in a clinical situation there needs to be direct physiological evidence demonstrating that the BCV stimuli used in the clinical test activate vestibular neurons and evidence about which classes of vestibular afferents are activated by BCV stimuli. In this study, we tested the effect of BCV clicks, tone bursts, and continuous tones on primary vestibular afferents. We sought to answer the question: are receptors across all vestibular sensory regions activated by BCV stimuli equally as Young et al. reported for the squirrel money or is there a differential preference by some vestibular regions for BCV stimuli? Of particular interest was the question of the uniformity of vestibular activation at low stimulus levels. In this study the sound source was a B-71 bone oscillator (Radioear, New Eagle, PA, USA) rigidly attached by dental cement to the animal's skull and the vibrations from this oscillator were conducted through the head. This is in contrast to our previous study where the sound source was a headphone and the vibrations from the diaphragm in that headphone were conducted to the eardrum by variations in air pressure and we refer to that stimulus as AC sound.
We used extracellular single neuron recording from a large number of primary vestibular neurons identified by their location and their response to natural stimulation and we tested each one with BCV stimuli. Neurons were simply classified into one of two broad classes—regular or irregular on the basis of the normalized coefficient of variation (CV*) of the interspike interval during spontaneous discharge (Goldberg et al. 1984): a neuron was classed as regular if the CV* was less than 0.1 and irregular if the CV* was greater than 0.1 (Young et al. 1977; Hullar et al. 2005). Natural rotational and positional stimulation of the en bloc animal in yaw, pitch and roll was used to identify the probable vestibular sensory region innervated by these neurons by determining whether the neuron responded to angular accelerations or static pitch or roll positions. In order to deliver a range of natural vestibular stimuli the animal was placed on a small platform, which could be yawed, pitched or rolled. Following Young et al. (1977) if the neuron responded only to angular accelerations it was classed as a semicircular canal neuron and the canal of origin was identified by relating the axis of angular rotation which facilitated the neuron, to the known orientation of the guinea pig semicircular canals (Curthoys et al. 1975). If the neuron showed a maintained change in firing at a static position it was classed as an otolith neuron.
One particular question was whether BCV activated some utricular afferents. Utricular afferents course in the superior vestibular nerve (de Burlet 1929) together with a small bundle of fibers from the anterior (“hook”) region of the saccular macula (Hardy 1934). Neurons in the superior vestibular nerve which respond to maintained tilts are thus more likely to be utricular rather than saccular afferents, however given the curvature of the guinea pig utricular and saccular macula and the broad range of preferred directions of primary otolithic afferents (Fernandez and Goldberg 1976b) the assignment is not definite. To test this question of utricular activation more conclusively we used retrograde transport of neurobiotin injected close to cells, which showed BCV sensitivity to trace the site of origin of these activated neurons.
Preliminary reports of some of these data have appeared; Society for Neuroscience, San Diego 2004, 2005; Barany Society meeting, Paris, July 2004, David Zee Festschrift, Siena 2004.
Methods
Animals
A total of 31 pigmented guinea pigs weighing between 520 and 1,300 g were used. The “Principles of Laboratory Animal Care” (NIH publication no. 86–23, revised 1985) were followed and all experimental procedures were approved by the Animal Ethics Committee of the University of Sydney (AEC approval number L29/1-2003/3/3684, approvals date 9.12.2002).
Surgical procedures
Animals were anesthetized with separate intramuscular injections of ketamine 100 mg/kg (Ketamil, Troy laboratories, NSW, Australia) and xylazine 4 mg/kg (Xylase, Parnell laboratories, NSW, Australia). They were given supplementary injections as required during the experiment (usually at 50% of the initial dose every 2 h). Heart rate and EMG activity were monitored continuously throughout the experiment to ensure the depth of anesthesia was adequate. Temperature was maintained by a heating pad. Animals breathed oxygen-enriched air spontaneously throughout the experiment (a tracheal cannula was not used).
The dorsal surface of the skull was exposed and a slot (4×8 mm) was drilled into the skull at the right fronto-parietal area to accommodate a stainless steel head holding machine screw (3.4 mm diameter) which was used to hold the animal's head. A nut was tightened on this screw, effectively locking the screw to the skull and the whole nut and screw assembly were cemented to the skull. This machine screw (and thus the skull) was bolted to one end of a sturdy brass supporting rod 10 cm long with the other end of this rod being attached to a ball and socket joint which allowed the head to be positioned with great flexibility but then rigidly locked into place. The standard head position was nose-down by around 40° to bring the horizontal semicircular canals into an earth horizontal plane (Curthoys et al. 1975) and close to the intersection of the three rotational axes of a turntable, which could be yawed, pitched and rolled (see below).
For the auditory brainstem response (ABR) electrode a hole 1 mm in diameter was drilled at the level of the posterior end of the zygomatic arch and 3–4 mm left of the sagittal suture to allow placement of a silver ball electrode on the dura close to the auditory cortex. The bone overlying the left cerebellum was removed and the flocculus and paraflocculus were removed by aspiration to expose Scarpa's ganglion for extracellular single neuron recording (Curthoys 1981a, b, 1982; Murofushi et al. 1995).
The BCV stimuli were delivered by a Radioear B-71 bone conduction oscillator and skull vibration was measured by a sensitive single axis linear accelerometer (Analog Devices, MA, USA, ADXL105) in both cases rigidly attached to the skull. The accelerometer was oriented with its sensitive axis in a rostro-caudal direction. As shown in Fig. 1a, the accelerometer was rolled around the animal's x-axis relative to vertical by 20–30° and was oriented to produce a predominantly dorsoventral force. Machine screws were used as “stand-offs” to allow the B-71 oscillator and ADXL105 surface mount linear accelerometer to be positioned about 10 mm from the skull surface whilst still in rigid contact with the skull by means of machine nuts cemented onto the skull (2 for 5 mm screws and 2 for 2 mm screws), which served as anchor points for the bolts. The target locations of these nuts are shown in Fig. 1a: the two nuts for supporting the B-71s were located at the level of the zygomatic arch and the two for holding the accelerometers were located further caudal.
A matching machine screw, M5 × 10 mm long, was cemented to the flat bakelite driving plate of each of two standard clinical BC oscillators—Radioear B-71 BC oscillator. A 4BA screw (2×5 mm) long was cemented to the linear accelerometers. The B-71 with bolt was then tightened into the M5 nut to form a firm, tight bond for transmitting the vibration to the skull. The same method was used for coupling the linear accelerometers to the skull. Usually, two B-71s and two ADXL105s were used (over the ipsilateral and contralateral forebrain regions) (see Fig. 1a). By screwing the machine screw into the nut cemented to the skull, the B-71 or ADXL105 accelerometer were coupled directly to the skull. The placement was symmetrical so we could examine the effect of ipsilateral or contralateral placements (see Fig. 1b).
The mechanism of action of the B-71 is that energizing the device causes linear movement of a small captive iron slug which strikes the bakelite driving plate of the B-71 in a direction which is perpendicular to the face of the driving plate so a shock wave is transmitted along the axis perpendicular to the driving plate. With the B-71 positioned as in this experiment the driving plate was parallel to the skull surface so the shock wave was largely perpendicular to the skull surface and the force was directed primarily in a dorso-ventral direction. In some animals a TDH 49 headphone (Telephonics, New York, USA) was used to deliver AC clicks to the left ear, using the speculum coupler we described previously (Murofushi et al. 1995) to allow comparison of the sensitivity to air conducted sound and BCV.
Recording
A glass microelectrode filled with 2 M NaCl with impedance of 1–2 MΩ was attached to a Kopf stereotaxic carriage and guided under visual control into the exposed Scarpa's ganglion with the aid of an operating microscope (Curthoys 1981a, 1982). All neurons were recorded on the left side. Action potentials of neurons were recorded extracellularly and since these were usually biphasic we concluded they were usually from cell-bodies of vestibular afferents in Scarpa's ganglion rather than fibers. The reference electrode was a silver plate implanted in the neck muscles. Standard acquisition and computer analysis techniques were used. The normalized CV* was determined from the neuron's resting activity by dividing the standard deviation of the interspike intervals by the mean interval and using the formula published by Hullar et al. (2005) for the chinchilla. Following Young et al. (1977) a neuron was classed as regular if CV* was less than 0.1 and irregular if CV* was greater than 0.1.
Natural vestibular stimulation
Animals were supported on a small turntable (30 cm diameter), which could be rotated in the animal's yaw plane for testing horizontal canal activation. The platform supporting this turntable was mounted on a swing, allowing static or dynamic pitch stimulation to be delivered. When the animal was locked in a yaw position with the axis of swing rotation along the interaural line, pitch static and dynamic stimulation was tested. Changing the yaw angular position of the animal by 90° converted the swing stimulation to roll and so allowed neurons to be tested for static or dynamic responses to roll. The angular extent was through ±30°, with a peak velocity of 50°/s.
In cases where the neural response was not clear the animal could be yawed so that either ipsilateral anterior or posterior canal could be aligned with the plane of the angular stimulation (Curthoys et al. 1977). All stimulation was done by hand, since it was important to identify very quickly the stimulus which activated the neuron and so infer the sensory region from which the neuron originated and then to move on to testing the response of the neuron to BC vibration.
The assumption we made was that neurons which showed a maintained increase in discharge rate to static pitch or roll tilts were otolithic. If a neuron showed an increase in firing only to an angular acceleration it was deemed to be from a semicircular canal (Curthoys and Markham 1971; Blanks and Precht 1976). Only neurons where we conclusively tested all angular and linear accelerations in yaw, pitch and roll are included here.
Neurobiotin labeling
Juxtacellular labeling of BCV activated neurons was done with electrodes containing 4% neurobiotin in 2 M NaCl, with the neurobiotin being injected iontophoretically 5 nA alternating once per second between anodal and cathodal pulses. Following perfusion with saline followed by 5% paraformaldehyde and 1% glutaraldehyde the whole macula was removed and neurobiotin labeling was demonstrated by treatment with an avidin-biotinylated horseradish peroxidase procedure (ABC kit Vector Laboratories, Burlingame, CA, USA) with diaminobenzidine as the chromogen, using the procedures of Lee et al. (2005). The whole-mount of the macula was treated and cleared in glycerol.
Measurement of stimulus magnitude
In order to keep all stimulus levels within a physiological range, all stimulus intensities were referred to the baseline value which in this case was the threshold for that animal's own ABR threshold to BCV clicks, and the maximum intensity delivered was around 70 dB above ABR threshold to BCV clicks. This ensured that the intensities in this study were in physiological levels and were analogous levels to the levels used for human clinical studies (Welgampola and Colebatch 2005).
Skull vibration was measured using miniature high sensitivity linear accelerometers rigidly attached to the skull. However, the threshold of the ABR to a BCV click stimulus (Wada and Starr 1983; Naito et al. 1999) was used as the reference level to which stimulus intensity was referred to ensure that the stimuli remained within physiological levels. The ABR threshold was determined by recording the cortical potential by a silver ball recording electrode on the dura close to the auditory cortex and averaging the evoked response to 128 presentations of the BCV click stimulus (Wada and Starr 1983; Naito et al. 1999). The positive electrode was a silver ball electrode close to the left dorso-lateral auditory cortex and the reference electrode was implanted in the neck muscles. To determine the threshold of the ABR, the responses to 128 clicks (at 5 Hz) at high stimulus levels were averaged. The intensity was then attenuated to determine the level required for threshold of the P3 and P4 components of the ABR. The threshold was determined by visual inspection (Cazals et al. 1982) and defined as the lowest stimulus level at which the P3 or P4 wave of the ABR could just be detected (see Fig. 2). That level was called 0 dB and all intensities for that animal were referred to this biological reference level. In this way, the stimulus levels presented to guinea pigs were referred to that animal's own threshold and, by analogy, we verified that the stimulus levels delivered to guinea pigs were comparable to the values delivered to human subjects and patients in a clinical setting (Welgampola and Colebatch 2005), in that for both guinea pigs and humans the stimuli were a comparable number of decibels above threshold.
In fact high intensity AC sound did produce (very small) skull acceleration as shown by the stimulus column in Fig. 2, most likely because of the tight coupling between the headphone casing and the rigid plastic speculum which caused, at very high AC sound levels, very small vibrations of the headphone casing to be transmitted to the skull via the speculum. These vibrations were not of sufficient amplitude to activate neurons most sensitive to BCV.
Although, it was considered unlikely that the laterality of stimulation affected neural responses, two B-71s were used as ipsilateral and contralateral BCV transducers and two linear accelerometers were used for measuring the skull vibration.
Unit responses
Once classified according to natural stimulation, each neuron's response to BCV stimuli was assessed. Usually, the stimulus was clicks at the maximum stimulus level (2 V p–p) which we delivered to the B-71 oscillator (which corresponded to a level of around 6 g and was usually around 50–70 dB above BCV threshold) and if this level activated the neuron the voltage was decreased using a decade attenuator to identify the threshold for that neuron. Activation was defined as a detectable stimulus-locked increase in firing rate and that was established by repeated presentations of the stimulus. Measures showed that our threshold for detection was around a 2–10% increase in firing. If no response could be elicited at the maximum level the neuron was categorized as “not responding.”
The response to clicks was usually tested first, followed by the response to continuous pure tones, the frequency of which was varied from 200 to 2,000 Hz. At the intensities we used, few neurons responded above about 1 kHz. The output from the linear accelerometers showed there was a marked skull resonance around 500–600 Hz. The tones were generated by a Trio AG-202A audio generator and amplified by a custom made amplifier (DC-100 kHz) and attenuated by a Tech TE-111 decade attenuator. In later experiments computer generated stimulus waveforms in LabView (National Instruments, Austin, TX, USA) were used to present either single clicks or 500 Hz short tone burst stimuli (7 ms long) identical to those used in human clinical studies (Welgampola et al. 2003).
Accelerometers
The single axis linear accelerometers which we used can only give an indication of the acceleration component in one plane whereas the head vibration is a very complex stimulus with components in all three planes. Given that suitable technology was not available for miniature triaxial accelerometers, which could fit on a guinea pig's head and measure the full stimulus pattern, we chose to use single axis accelerometers to monitor stimulus occurrence and magnitude and give an approximate measure of the relative magnitude of the vibration stimuli. We used two small surface mount device sensitive single axis linear accelerometers cemented to bolts attached to the skull. Although, these were small devices they were still large compared with the guinea pig skull (Fig. 1) and there was not enough room for them to be cemented directly to the skull, so they had to be mounted on two bolts as “standoffs.” This is not ideal for giving precise information about the magnitude of the linear acceleration since the vibration of the supporting standoff affects the measured skull vibration but these are adequate as an indicator of the stimulus delivery, relative magnitude and skull ringing without providing exact measures of the linear acceleration components which will require miniature triaxial accelerometers cemented directly to the skull. We oriented the accelerometer so the sensitive axis was rostro-caudal since that was the direction of the applied force in the Young et al. (1977) study.
Results
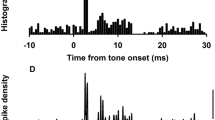
A total of 346 neurons were identified as being vestibular by their response to natural vestibular stimulation and their location in Scarpa's ganglion. They were tested for response to BC vibration and a total of 71 of those 346 neurons met the criterion for responding to BC stimuli, by showing a stimulus-locked increase in firing in response to a BC vibration. Examples of neural responses to brief BCV clicks (Fig. 3), short tone bursts (Fig. 4) and long duration tonal stimuli (Fig. 5) are shown and are discussed in detail below. Where a neuron was activated by a click it was invariably activated by sinusoidal BC vibrations. To single clicks, neurons typically showed a response with a short fixed latency around 1.1–2 ms (see Fig. 3).
Examples of three neurons activated by single click stimuli delivered by the B-71 bone oscillator to show activation at short fixed latency following single clicks. Each panel shows superimposed traces from four or five repetitions of the low intensity (threshold-straddling) stimulus so that on some presentations the neuron does not fire, allowing the baseline activity to be shown and the action potential to be identified clearly. The stimulus trace is from the skull-mounted linear accelerometer. Each neuron was an irregular otolithic neuron sensitive to static pitch tilts
Examples of the responses of two separate otolithic neurons (sensitive to tilt) to brief 500 Hz BCV tone bursts (a) and brief clicks (b) by the B-71 oscillator. In both cases the effects of decreasing intensity are shown. The stimulus traces are from the skull-mounted linear accelerometer. The neuron fires on the first four peaks of the tone burst stimulus, and five repetitions of the stimulus are superimposed. b response to three intensity levels (re ABR) of single 0.1 ms bone clicks, as recorded from a unit sensitive to left-ear down static head tilt
Activation of an irregular otolithic neuron to a 500 Hz continuous tone to show the synchronization of the action potential to the stimulus. a With a long time base and b with a short time base. The stimulus-locked sinusoidal deflection in the unit trace in b is the cochlear microphonic to the vibratory stimulus and shows the stimulus locking of this neuron clearly: the neuron fires close to the peak of the cochlear microphonic on each occasion. This stimulus locking confirms the observations of Young et al. (1977) for vestibular afferents in the squirrel monkey
The latencies of activation of these primary afferent neurons were very short—for AC stimuli the average latency was of the order of 0.9 ms (Murofushi et al. 1995) and the shortest latencies to BCV stimuli were close to that value. However, measuring latencies for BCV clicks is more problematic since the BCV stimulus is not an abrupt sharp onset square wave as it is for AC clicks. The linear accelerometer showed that a single click delivered to the B-71 oscillator caused a damped oscillation of the skull and some neurons fired on the second peak of this damped oscillation. So increasing intensity caused a “quantal” jump in latency up to the earlier acceleration peak, rather than a small decrement in the latency of the response. Many neurons did respond to BCV clicks with a latency of around 1–2 ms, and such a short latency excludes the possibility that these were efferent fibers.
Figure 4a shows that during a high intensity 7 ms 500 Hz BCV tone burst the otolithic responsive neurons generate a barrage of stimulus-locked action potentials on four or five of the stimulus cycles whereas with a single click there are just one or two action potentials (Fig. 4b also see Fig. 3). In terms of generating a motor response, it is very likely that temporal summation during the 7 ms 500 Hz tone burst would act to enhance the motor response to this otolithic stimulus.
We confirmed the observation of Young et al. of the tight synchronization of activation of vestibular neurons in response to BC vibration. For example in Fig. 5 the neuron is activated by the continuous tone but does not fire on each peak, however, when the neuron fires it is tightly linked to the peak of the stimulus acceleration as shown by the accelerometer record. Following the short tone burst there is a period where there are no action potentials generated for some 10–15 ms (Fig. 6) which appears to be an example of postexcitatory disfacilitation.
Irregular otolith neurons activated by BCV stimulation were sensitive, with thresholds for activation as low as 10 dB above ABR threshold. Across many otolith irregular neurons the median threshold for neural activation was 44 dB above ABR threshold (n = 38) (see Fig. 7).
Most of the otolith irregular neurons activated by BC vibration were in the superior division of the vestibular nerve and responded to either pitch or roll tilt, and we concluded that these neurons probably originated from the utricular macula because there is only a very small projection from the saccular macula in the superior vestibular nerve. Support for that conclusion was provided by evidence from juxtacellular labeling of responsive neurons. We made a number of attempts at using neurobiotin to stain neurons activated by BCV and some attempts were successful: all of which showed staining only in the utricular macula. The cristae of the horizontal, anterior, and posterior canals were prepared and examined in the same way as the utricular and saccular maculae but the only region which ever showed labeling by neurobiotin was the utricular macula. No labeling was encountered in the cristae, emphasizing the differential distribution of BCV activated neurons between the utricular macula and the cristae. Only one labeling attempt was made on each nerve to avoid ambiguity during afferent recovery.
In the example shown, we injected neurobiotin in close proximity to one pitch sensitive otolith neuron which was activated by BCV stimuli and following processing we were able to identify calyx endings filled with neurobiotin located in the region of the striola of the utricular macula using a whole mount of the utricular macula (see Fig. 8). There was no evidence of neurobiotin labeling in the saccular macula or cristae of the semicircular canals.
We specifically sought to compare the response of individual neurons to AC and BCV stimuli but none of the neurons activated by BCV stimuli could be activated by AC stimuli up to the maximum intensities we used.
We examined whether there was a relationship between CV* and threshold intensity for activation but probably because of the relatively small number of activated neurons and the variability of the data, no systematic relationship was detected—product moment correlation coefficient = 0.33, P = 0.30.
Figure 9 shows that the answer to the main question we addressed—was there preferential specificity for any one vestibular sensory region for BC sound?—is clearly yes: otolithic irregular neurons are selectively activated. Comparatively few primary semicircular canal neurons could be activated by BC vibration. None of the 133 regular semicircular canal neurons tested showed a detectable increase in firing to the BCV stimuli presented. A few irregular canal neurons (9/56 = 16%) were activated by BCV stimuli. For regular otolithic neurons, few could be activated by BCV: only 14 of 99 tested (14.1%). However, most irregular otolithic neurons were activated: 48/58 tested = 82.8%). Of those otolithic neurons which were activated, some were activated at very low intensities—only about 10 dB above the ABR threshold for BCV click stimuli. The preferential activation of irregular neurons was confirmed both across animals and within individual animals: in the one electrode track we frequently encountered regular canal or otolith neurons which could not be activated by BCV stimuli in close juxtaposition to irregular otolithic neurons which showed sensitive response to exactly the same BCV stimulus, showing that activation by BCV stimuli was not due to artifact such as the state of anesthesia, etc.
Laterality
As we had expected, BCV stimuli activated irregular primary otolithic afferent neurons on both sides of the head—with a small increase in latency for contralateral stimulation due to conduction time across the skull.
Discussion
This study has confirmed that primary vestibular neurons respond to BCV stimulation at low stimulus levels. The BCV generates compressional and shear waves, which travel through the skull and are sufficient to stimulate the most sensitive otolithic receptor cells.
When a guinea pig skull is struck by a vibrating stimulus such as the moving metal slug inside the B-71 bone conduction oscillator, waves are transmitted through the skull. Two important waves are compressional and shear waves propagated through the skull where the compression wave (the P-wave) produces displacement in the direction of the propagated wave and the shear wave (the S-wave) produces displacement perpendicular to the direction of wave propagation (Doyle 1995; Stein and Wysession 2003). In addition there are surface waves (Rayleigh and Love waves). These various waves will be propagated and reach and activate receptor hair cells in the inner ear, depending on many factors, including the magnitude and direction of the applied stimulus vibration, the location of the vibrator and the receptor hair cell preferred orientation. Understanding how the vibration affects the vestibular receptors will require systematic changes in the direction of the applied stimulus to relate this complex stimulus in a structure as complex as a skull.
The neurons activated are vestibular and not cochlear since (1) they were located in the vestibular division of the VIII nerve and (2) they responded to natural vestibular stimulation (angular or linear accelerations). Up to the maximum stimulus levels we used, there was not a uniform activation: regular otolithic neurons and neurons from semicircular canals (regular or irregular) rarely showed activation by BCV stimuli up to our maximum levels, whereas 82% of the irregular otolithic neurons tested showed a detectable increase in firing to BC stimulation. If more intense stimuli had been used then it is very likely more neurons would have responded, but such intense stimuli would be at high and possibly unphysiological levels, and the primary focus in this study was the response preferences at stimulus levels within physiological range and more especially those close to threshold.
This preferential activation of otolithic as opposed to semicircular canal neurons for BCV sound is similar to what we reported with AC sound (Murofushi et al. 1995) where none of 300 guinea pig horizontal semicircular canal neurons responded to AC clicks. In both studies there was also an almost complete absence of response by semicircular canal neurons to the low level stimuli used. Many neurons in this study, which were activated by BCV stimuli were tested with AC stimuli but could not be activated. One reason may be that most of the neurons in this study were in the superior division rather than in the inferior vestibular nerve. The thresholds would be different if there were different mechanisms of hair cell activation by AC and BCV stimuli and it is probable that is the case.
These responses to BCV stimuli are not injury discharges as shown by the smooth unitary waveform and the fact that some of these neurons could be held for hours. We can also exclude the possibility that they are from efferent fibers (either vestibular or cochlear) because of the very short latencies (around 1.5–2 ms) of neural activation to BCV click stimulation (Fig. 2).
Sampling
Because of the operational approach and the configuration of Scarpa's ganglion in the guinea pig (Curthoys 1981a, b) most of the tracks were in the superior division of the vestibular nerve. It was difficult to access neurons in the inferior vestibular nerve, and that is probably the reason these cells are under-represented in the total tested—for example there are very few cells from the posterior semicircular canal nerve but those neurons which were tested showed a similar lack of response to BCV stimuli as neurons from the other semicircular canals.
If a neuron was in the inferior vestibular nerve and responds to pitch then it is likely to be saccular. Neurons in the superior vestibular nerve responding to tilts may be utricular [since some utricular receptors and afferents prefer forces directed in the pitch plane (Fernandez and Goldberg 1976a)] but there is a small possibility that such neurons may be saccular since some saccular afferents course in the superior vestibular nerve (de Burlet 1929; Hardy 1934).
The assignment of neurons to be utricular or saccular cannot be definite because the anatomical configuration of the planes of the utricular and saccular maculae in the guinea pig (Curthoys et al. 1999) shows that the saccular macula has a projection into the frontal plane, so some saccular neurons could show responses to static roll tilts. Similarly, the utricular macula in the guinea pig is not a flat surface but has a distinct upturn at the rostral portion of the macula (Fig. 1 of Curthoys et al. 1999) so some utricular afferent neurons could show responses to static pitch tilt stimuli. Nevertheless, the evidence of labeled calyces in the utricular macula after juxtacellular neurobiotin injection adjacent to a BCV sensitive neuron shows that some utricular afferents do respond to BCV stimuli. Although juxtacellular labeling is not as definite as intracellular labeling, it provides strong evidence of the origin of the neurons close to the injection site (Pinault 1994). It is likely that saccular afferents are also activated by BCV but we cannot offer definitive evidence on that point from this study.
Latency
The latencies of activation of these primary afferent neurons were very short—for AC stimuli the average latency was of the order of 0.9 ms (Murofushi et al. 1995) and the shortest latencies to BCV stimuli were close to that value. However, measuring latencies for BCV clicks is more problematic: the stimulus is not an abrupt sharp onset square wave as it is for AC clicks. The linear accelerometer showed that a single click delivered to the B-71 oscillator caused a damped oscillation of the skull and some neurons fired on the second peak of this damped oscillation. So increasing intensity caused a “quantal” jump in latency up to the earlier acceleration peak, rather than a small decrement in the latency of the response. Many neurons did respond to BCV clicks with a latency of around 1–2 ms, and such a short latency excludes the possibility that these were efferent fibers.
With both AC and BCV stimuli it must be emphasized that there are factors contributing to latency, which are not present with electrical stimulation, specifically the transduction time and time to cross the receptor-afferent synapse. We used mechanical, not electrical stimuli—and the short latency we recorded to this mechanical stimulus is appropriate for primary afferents.
In some neurons which were activated by BCV stimuli the frequency of the BCV stimulus was varied but it is not possible to give definite information about the full frequency response of BCV activated neurons because of the transducer resonance and head resonance and the complex damped oscillation which was caused by the BCV stimulus. Most neurons could be activated up to 800 Hz and few responded to frequencies above 1,000 Hz. While high, these frequencies are lower than have been reported for horizontal semicircular canal neurons to high frequency mechanical indentations of the canal (Wit et al. 1984).
Auditory brainstem response
One clear next step is to compare directly the stimulus levels required for activation by AC versus BCV stimuli in the one neuron. That direct comparison could not be carried out in this study because most of the neurons were in the superior vestibular nerve and could not be activated by AC stimuli. That result is consistent with our previous result that AC clicks preferentially activate saccular neurons in the inferior vestibular nerve. That direct comparison of the effectiveness of AC and BCV stimuli in the one neuron must await recording from cells in the inferior vestibular nerve. Comparison of our data here with previously published data shows indirectly that the BCV stimuli are more effective at activating otolithic neurons than AC stimuli. The median threshold for otolith irregular neurons for BCV clicks here was 44 dB above ABR threshold (n = 38) whereas the median threshold of neurons to AC clicks in the Murofushi et al. (1995) study was 60 dB above ABR threshold (n = 37). That difference may be due to the different modes of receptor cell activation by these very different stimuli.
Comparison with the results of Young et al. (1977)
Young et al. (1977) reported that BCV stimuli uniformly activated neurons from all vestibular sensory regions, without any clear preference. Since the result of this study contrasts with that result it is necessary to consider the possible reasons for the disagreement.
Species differences
Young et al. used squirrel monkeys held by standard ear bars whereas the skulls of our guinea pigs were rigidly held by a bolt cemented to the skull and locked to the stereotaxic frame. The ear bars may have allowed a different pattern of skull vibration or skull movement during the BCV stimulus.
Transducers
Young et al. used an electrodynamic driver coupled to the frontal bone at the midline via a plexiglas rod cemented to the driver's diaphragm which would have been capable of delivering much larger stimuli than the small B-71 oscillator we used.
Criterion for activation
Apparently Young et al. mostly determined sensitivity by means of a phase-locking criterion. They found that some vestibular neurons show phase locking at stimulus levels well below (20 dB below) the levels required for an audibly detectable change in firing rate (see their Fig. 1, p 355). This very sensitive criterion is appropriate for auditory studies but has rarely been used in vestibular studies where most investigators seek changes in firing rate which could produce a measurable response (such as an eye movement or neck muscle activation). It is doubtful that a change in phase locking, without an increase in firing rate could result in a measurable movement. Interestingly, Young et al. held the same view: “most likely, phase-locking at audio frequencies would not, by itself, be recognized as an activation of the vestibular apparatus” (1977, p.358). In the present study we sought to measure neural changes which “would be recognized as an activation of the vestibular apparatus,” and so we used the more common but less sensitive criterion of an audibly detectable change in firing rate, which seems more relevant for accounting for the generation of short latency vestibulo-ocular and vestibulo-spinal responses.
What could be the mechanism for this response? In a number of species it has been shown that some otolithic afferents are sensitive to BCV stimuli. In the frog for example saccular afferents are extremely sensitive to BCV stimuli (Koyama et al. 1982) with thresholds of micro-gs: as low as 0.005 cms−2. Our results indicate that some irregular otolithic afferents in guinea pigs exhibit analogous sensitivity to BCV stimuli. Irregular otolith afferents originate predominantly from the central striolar region of the maculae where the calyx nerve endings of irregular primary neurons envelop Type I receptors (Baird et al. 1988; Fernandez et al. 1990; Goldberg et al. 1990; Goldberg 2000). Such irregular neurons show pronounced tuning for rapid accelerations—changes in linear acceleration (jerk) (Fernandez and Goldberg 1976c). Possibly this preferential tuning of the irregular otolithic afferents for dynamic stimuli may be responsible for the results we have obtained. BCV stimuli are unusual vestibular stimuli but they may have a particular advantage for investigating the very early physiological responses of vestibular receptors since BCV stimuli can be presented easily and repeatedly, unlike other vestibular stimuli such as calorics or rotations.
Abbreviations
- AC :
-
Air conducted
- BCV :
-
Bone conducted vibration
- ABR :
-
Auditory brainstem response
- VEMP :
-
Vestibular evoked myogenic potential
- CV :
-
Coefficient of variation
References
Baird RA, Desmadryl G, Fernandez C, Goldberg JM (1988) The vestibular nerve of the chinchilla. II. Relation between afferent response properties and peripheral innervation patterns in the semicircular canals. J Neurophysiol 60:182–203
Blanks RHI, Precht W (1976) Functional characterization of primary vestibular afferents in the frog. Exp Brain Res 25:369–370
de Burlet HM (1929) Zur vergleichenden Anatomie der Labyrinthinnervation. J Comp Neurol 47:155–169
Carey JP, Hirvonen TP, Hullar TE, Minor LB (2004) Acoustic responses of vestibular afferents in a model of superior canal dehiscence. Otol Neurotol 25:345–352
Cazals Y, Aran J-M, Erre J-P (1982) Frequency sensitivity and selectivity of acoustically evoked potentials after complete cochlear hair cell destruction. Brain Res 231:197–203
Colebatch JG, Halmagyi GM (1992) Vestibular evoked potentials in human neck muscles before and after unilateral vestibular deafferentation. Neurology 42:1635–1636
Curthoys IS (1981a) Scarpa's ganglion in the rat and guinea pig. Acta Otolaryngol 92:107–113
Curthoys IS (1981b) The organization of the horizontal semicircular duct, ampulla and utricle in the rat and guinea pig. Acta Otolaryngol 92:323–330
Curthoys IS (1982) The response of primary horizontal semicircular canal neurons in the rat and guinea pig to angular acceleration. Exp Brain Res 47:286–294
Curthoys IS, Markham CH (1971) Convergence of labyrinthine influences on units in the vestibular nuclei of the cat. I. Natural stimulation. Brain Res 35:469–490
Curthoys IS, Curthoys EJ, Blanks RHI, Markham CH (1975) The orientation of the semicircular canals in the guinea pig. Acta Otolaryngol 80:197–205
Curthoys IS, Markham CH, Blanks RHI (1977) Semicircular canal functional anatomy in cat, guinea pig and man. Acta Otolaryngol 83:258–265
Curthoys IS, Betts GA, Burgess AM, MacDougall HG, Cartwright AD, Halmagyi GM (1999) The planes of the utricular and saccular maculae of the guinea pig. Ann NY Acad Sci 871:27–34
Doyle H (1995) Seismology. Wiley, Chichester
Fernandez C, Goldberg JM (1976a) Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. I. Response to static tilts and to long-duration centrifugal force. J Neurophysiol 39:970–984
Fernandez C, Goldberg JM (1976b) Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. II. Directional selectivity and force-response relations. J Neurophysiol 39:985–995
Fernandez C, Goldberg JM (1976c) Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. III. Response dynamics. J Neurophysiol 39:996–1008
Fernandez C, Goldberg JM, Baird RA (1990) The vestibular nerve of the chinchilla. III. Peripheral innervation patterns in the utricular macula. J Neurophysiol 63:767–804
Goldberg JM (2000) Afferent diversity and the organization of central vestibular pathways. Exp Brain Res 130:277–297
Goldberg JM, Smith CE, Fernandez C (1984) Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of the squirrel monkey. J Neurophysiol 51:1236–1256
Goldberg JM, Desmadryl G, Baird RA, Fernandez C (1990) The vestibular nerve of the chinchilla. V. Relation between afferent discharge properties and peripheral innervation patterns in the utricular macula. J Neurophysiol 63:791–804
Hardy M (1934) Observations on the innervation of the macula sacculi in man. Anat Rec 59:403–418
Hullar TE, Della Santina CC, Hirvonen T, Lasker DM, Carey JP, Minor LB (2005) Responses of irregularly discharging chinchilla semicircular canal vestibular-nerve afferents during high-frequency head rotations. J Neurophysiol 93:2777–2786
Jombik P, Bahyl V (2005) Short latency responses in the averaged electro-oculogram elicited by vibrational impulse stimuli applied to the skull: could they reflect vestibulo-ocular reflex function. J Neurol Neurosurg Psychiatry 76:222–228
Koyama H, Lewis ER, Leverenz EL, Baird RA (1982) Acute seismic sensitivity in the bullfrog ear. Brain Res 250:168–172
Lee H-Y, Camp AJ, Callister RJ, Brichta AM (2005) Vestibular primary afferent activity in an in vitro preparation of the mouse inner ear. J Neurosci Methods 145:73–87
McCue MP, Guinan JJ (1994a) Acoustically responsive fibers in the vestibular nerve of the cat. J Neurosci 14:6058–6070
McCue MP, Guinan JJ (1994b) Influence of efferent stimulation on acoustically responsive vestibular afferents in the cat. J Neurosci 14:6071–6083
McCue MP, Guinan JJ (1995) Spontaneous activity and frequency selectivity of acoustically responsive vestibular afferents in the cat. J Neurophysiol 74:1563–1572
McCue MP, Guinan JJ (1997) Sound-evoked activity in primary afferent neurons of a mammalian vestibular system. Am J Otol 18:355–360
Mikaelian D (1964) Vestibular response to sound: single unit recording from the vestibular nerve in fenestrated deaf mice (Df/Df). Acta Otolaryngol 58:409–422
Murofushi T, Curthoys IS (1997) Physiological and anatomical study of click-sensitive primary vestibular afferents in the guinea pig. Acta Otolaryngol 117:66–72
Murofushi T, Curthoys IS, Topple AN, Colebatch JG, Halmagyi GM (1995) Responses of guinea pig primary vestibular neurons to clicks. Exp Brain Res 103:174–178
Naito R, Murofushi T, Mizutani M, Kaga K (1999) Auditory brainstem responses, electrocochleograms and cochlear microphonics in the myelin deficient hamster ‘bt’. Hear Res 136:44–48
Pinault D (1994) Golgi-like labeling of a single neuron recorded extracellularly. Neurosci Lett 170:255–260
Rosengren SM, Todd NPM, Colebatch JG (2005) Vestibular-evoked extra-ocular potentials produced by stimulation with bone-conducted sound. Clin Neurophysiol 116:1938–1948
Stein S, Wysession M (2003) An introduction to seismology, earthquakes and earth structure. Blackwell Publishing, Oxford
Wada S-I, Starr A (1983) Generation of the auditory brainstem responses (ABRs). I. Effects of injection of a local anesthetic (Procaine HCl) into the trapezoid body of guinea pigs and cat. Electroencephalogr Clin Neurophysiol 56:326–339
Welgampola MS, Colebatch JG (2005) Characteristics and clinical applications of vestibular-evoked myogenic potentials. Neurology 64:1682–1688
Welgampola MS, Rosengren SM, Halmagyi GM, Colebatch JG (2003) Vestibular activation by bone conducted sound. J Neurol Neurosurg Psychiatry 74:771–778
Wit HP, Bleeker JD, Mulder HH (1984) Responses of pigeon vestibular nerve fibers to sound and vibration with audiofrequencies. J Acoust Soc Am 75:202–208
Young ED, Fernandez C, Goldberg JM (1977) Responses of squirrel monkey vestibular neurons to audio-frequency sound and head vibration. Acta Otolaryngol 84:352–360
Acknowledgments
We thank Michael Halmagyi, Leigh McGarvie, Mike Todd, Warren Davies, Ann Burgess for their help. Darek Figa and his team for their care of the guinea pigs. This research was supported by a grant from the NH & MRC of Australia (253620).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Curthoys, I.S., Kim, J., McPhedran, S.K. et al. Bone conducted vibration selectively activates irregular primary otolithic vestibular neurons in the guinea pig. Exp Brain Res 175, 256–267 (2006). https://doi.org/10.1007/s00221-006-0544-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-006-0544-1