Abstract
The human vision system appears to divide into two streams: a ventral stream from V1 to the inferior temporal cortex processing ‘vision for perception’, and a dorsal stream from V1 to the posterior parietal cortex processing ‘vision for action’. Among other characteristics, it has been suggested that dorsal processing is effortless, unconscious, and not bearing on central cognitive resources implicated in ventral processing. The present study shows that a typical dorsal task (i.e., grasping an object) is subject to a classical indicator of capacity limitations in dual-task situations, the psychological refractory period (PRP) effect. In particular, response times to task 2 (the grasping task) increased the more the two tasks overlapped in time, i.e., the shorter the time interval between the stimuli of the two tasks was. As is also common in PRP experiments, response times to task 1 were largely unaffected by this variation. The PRP effect was obtained despite careful control of strategic response deferment, and peripheral overlap of response modalities that may have artificially created performance costs in previous studies. Altogether, the present results show that dorsal processing is subject to the same capacity limitations that can almost universally be found with simple cognitive tasks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The existence of two cortical systems for processing visual information in the human brain has received a vast amount of attention throughout the last decades. With the division starting only after V1, it is by now widely accepted that a ‘ventral stream’ terminates in the inferior temporal cortex, and a ‘dorsal stream’ terminates in the posterior parietal cortex (Ungerleider and Mishkin 1982; for a review, see also Milner and Goodale 2006). However, the original interpretation advanced by Ungerleider and Mishkin (1982) of a ventral ‘What?’ and a dorsal ‘Where?’ pathway has been altered by Goodale and Milner (1992; Milner and Goodale 2006) with a lasting effect, now known as the action-perception model. This model assumes that both systems essentially process the same input but for different purposes. While the ventral pathway is held responsible for the identification and recognition of objects (= ‘vision for perception’), the dorsal pathway is the online-control mechanism for programming and guiding visually controlled motor actions (= ‘vision for action’). The general evidence for this distinction has recently been reviewed by Milner and Goodale (2006; Goodale 2008). In short, the main evidence is derived from (1) the neuropsychological double dissociation of visual agnosia (James et al. 2003) and optic ataxia (Perenin and Vighetto 1988; but see Pisella et al. 2006), and (2) the selective influence of visual illusions on the ventral (perception) system, but not on the dorsal (action) system (e.g., Aglioti et al. 1995; but see, e.g., Franz and Gegenfurtner 2008). Apart from their different neuroanatomical features, both pathways are assumed to differ in several characteristics and two of them are of special interest to the present study (for a more detailed comparison, see Norman 2002):
First, the ventral pathway is seen to process input in a holistic manner, whereas the dorsal pathway processes the input analytically and is more efficient at ignoring variations of task-irrelevant stimulus dimensions. This has nicely been demonstrated by Ganel and Goodale (2003) using a variant of Garner’s speeded classification task (Garner 1974, 1978; or more precisely, a filtering task; Posner 1964). In such a Garner task, participants have to classify stimuli according to one relevant dimension, while ignoring another task-irrelevant dimension. Critically, this is done under two experimental conditions: (1) in the baseline condition, the second, irrelevant dimension remains constant, whereas (2) in the filtering condition this irrelevant dimension varies. If the irrelevant dimension can effectively be ignored (i.e., this dimension is filtered out and the stimulus can be perceived analytically), baseline and filtering conditions should produce the same response times. However, if the irrelevant dimension cannot be ignored (i.e., the stimulus is perceived holistically), response times are longer in the filtering than in the baseline condition (= ‘Garner interference’). In Ganel and Goodale’s (2003) study, the participants either had to judge the width of rectangular objects (ventral perceptual judgment task) or to grasp the same objects across their width (dorsal grasping task). Note that the objects’ width was always the relevant dimension, and the objects’ length was the irrelevant dimension. Indeed, a Garner interference (i.e., longer response times in the filtering than in the baseline condition) was only found for the perceptual judgment task, but not for the grasping task. In other word: varying a task-irrelevant stimulus dimension affects response times in a ventral but not in a dorsal task. Thus, the dorsal pathway can effectively ignore these variations.
Secondly, processing in the ventral pathway is assumed to be far more resource-demanding and conscious than is processing in the dorsal pathway, which is generally assumed to work fast, automatic, and unaffected by any capacity limitations (e.g., Jeannerod and Jacob 2005; Liu et al. 2008; Norman 2002). Given that typical dorsal tasks, such as grasping an object, are highly practiced and skilled, this appears intuitive at first glance. However, as intuitive the claim might be, empirical results do not provide an unambiguous picture. For example, Singhal et al. (2007) combined in two experiments visually guided grasping and delayed (thus memory-driven) grasping with two different secondary tasks in a dual-task study. Although the degree of interference with the secondary task was larger for delayed grasping, there was also considerable dual-task interference for visually guided grasping. This should not be the case if dorsal processing does not require (central) processing resources. On the other hand, Liu et al. (2008) combined a (dorsal) pointing task with a rapid serial visual presentation (RSVP) task and concluded from their results that dorsal processing does not share resources with ventral processing. Recently, these results have been replicated for young adults, not though for old adults (Lee and Hsieh 2009). Another widely used methodological tool to investigate dual-task interference is the PRP paradigm. Here, two stimuli S1 and S2 are presented in rapid succession, each requiring its own response R1 and R2. The time interval between S1 and S2 onsets is referred to as the ‘stimulus onset asynchrony’ (SOA). The typical result obtained with PRP experiments is as follows: whereas RT1 (the time from S1 to R1) is relatively unaffected by the SOA manipulation, RT2 (the time from S2 to R2) increases with shorter SOAs (the PRP effect). To explain this effect, the widely accepted response selection bottleneck model (Pashler 1994; Welford 1952) divides each processing stream into three stages (cf. Fig. 1): a pre-central (A; e.g., perceptual processes), a central (B; e.g., response selection), and a post-central (C; e.g., motor execution) stage. The crucial assumption is that stages A and C can be performed in parallel to other concurrently processed stages, whereas at any given time, only one central stage B can be handled by the cognitive system. Thus, if the pre-central stage of task 2 (A2) is completed before the bottleneck has been released from the central stage of task 1 (B1; a situation most likely occurring with short SOAs), central processing of task 2 (B2) needs to be deferred until after completion of B1. This in turn yields the longer RT2s with shorter SOAs. In other words, the PRP effect arises only because both tasks require access to a common processing bottleneck. Note also that any task 2 manipulation prolonging its central (or post-central) stage should have the same effect on RT2 across all SOA values (i.e., SOA and the investigated manipulation combine additively). Kunde et al. (2007) used the perceptual judgment and the grasping task employed by Ganel and Goodale (2003) as task 2 in a PRP experiment (as most common, task 1 was a binary tone classification task). Participants were asked to either perceptually judge the width of objects or to grasp the objects across their width, while ignoring irrelevant variations of the objects’ length. Two notable results were obtained. First, variations of the objects’ length did affect the performance in the (ventral) perceptual judgment task but left unaffected the performance in the (dorsal) grasping task. Thus, the presence of a Garner interference in the ventral perceptual judgment task and the lack thereof in the dorsal grasping task were replicated, assuring that the general processing of both tasks was not altered by the dual-task setting. Interestingly, the Garner interference turned out to be additive to the SOA, suggesting that the Garner interference is resolved at a central (or probably post-central) rather than the pre-central (perceptual) stage (for a convenient introduction to the predictions derived from the central bottleneck model, see Pashler 1994). Second and most important, both tasks exhibited a PRP effect of comparable size. Hence, according to this study, dorsal processing, though seemingly fast and effortless, is subject to capacity limitations and therefore cannot be construed as automatic.
An illustration of a PRP logic based on the assumption of a response selection bottleneck: Stages A (pre-central perceptual stage) and C (post-central motor stage) can be processed in parallel to other stages, while the central stage B is assumed to constitute a structural bottleneck. Thus, only one stage B can be processed at any time. (SOA stimulus onset asynchrony, i.e., the time between the onset of stimulus 1 and 2)
However, on closer inspection, the findings of Kunde et al. (2007) may portray an incorrect picture. In particular, the PRP effect may also have arisen (1) from strategically withholding the grasping movement in order to comply with the instructions or (2) from peripheral interference due to an overlap in response modality. These arguments will be outlined in more detail later. Given the ambiguous conclusions from recent studies, we here pursue the PRP paradigm and present three experiments intended to rule out these alternative accounts. To resolve this issue is important for three reasons. First, knowing in which respects processing in the ventral and dorsal pathways differ (and in which respects they do not) will generally help to elaborate the perception–action model. Second, this question is also important from the perspective of dual-task research. The PRP effect has been shown to be very robust across a variety of tasks, and only a few exceptions seem to exist (Lien et al. 2006). One potential exception made use of eye movements as responses—a response mode perhaps controlled by specific neural circuits (Pashler et al. 1993). In light of these results, dorsally mediated grasping movements are indeed a good candidate for being another exception, and thus their sensitivity to dual-task interference deserves thorough experimental investigation. Third, knowing the capacity limitations of a typical dorsal task may also have implications for Human Factors research. After all, object-oriented tasks such as grasping or pointing (either directly or mediated by a tool) are part of many working environments. It is important to know whether other concurrent tasks (such as verbal communication) do interfere with, or are affected themselves, by apparently simple dorsal motor tasks.
Experiment 1
In a previous study that combined a binary choice reaction task as task 1 with a dorsal grasping task as task 2 in a PRP paradigm, the mean response time to task 1 (RT1) was roughly 400 ms. Critically, the mean response time in the grasping task (RT2) with an SOA of 1000 ms—hence without considerable task overlap—was only about 300 ms (see Fig. 2 in Kunde et al. 2007). Thus, in principle, grasping could have been performed faster than task 1 at the shortest SOA of 50 ms, at least in the majority of the trials. Yet, having been instructed to respond in task order and to focus on task 1 performance, the participants were obliged to strategically withhold grasping until after the response to task 1 was given in order to not commit an error. This in turn could have produced the observed PRP effect. Experiment 1 was modelled closely after the study by Kunde et al. (2007), but importantly the instructions placed equal emphasis on both tasks and grasping ahead of the task 1 response was not counted as erroneous (for a similar approach see Ruthruff et al. 1995, Experiment 1). We expected to replicate the presence of a Garner interference with perceptual judgment and the lack thereof with the grasping task. The most important question was whether the abandonment of a certain task order would remove (or at least reduce) the PRP effect for the dorsal grasping task when compared with a ventral judgement task.
Method
Participants
Sixteen undergraduate students from Dortmund University of Technology (3 male, mean age = 22;8 years) participated in return of course credit.
Design, apparatus, and stimuli
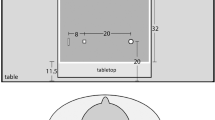
Each participant performed in two sessions of a PRP experiment. In both sessions, task 1 was a binary tone classification task with a left hand key press as the response. Stimuli were 300 and 900 Hz tones (50 ms) presented via headphones. Task 2 was either a grasping or a perceptual judgment task, varying across both sessions. Both tasks were modelled after Ganel and Goodale (2003): in the grasping task, participants naturally grasped a concrete stimulus across its width with their right hand using a precision grip; in the perceptual judgment task, they indicated the same stimuli’s width with a right hand key press. Stimuli were four wooden objects constructed according to a factorial combination of their width (30 and 35.7 mm) and length (63 and 75 mm). Participants wore computer-controlled PLATO shutter glasses (Translucent Technologies) during the experiment, and the stimuli were presented on a small custom-made table where they depressed a hidden micro switch. RT1 was measured from tone presentation until the left hand response, RT2 from the shutter glasses’ opening until the right hand’s response (perceptual judgment) or until the participants’ left index finger left a home button (grasping). For the grasping task movement time was additionally calculated from leaving the home button until lifting the stimulus object.
Procedure
Each participant performed in four experimental blocks of 72 trials each. Two blocks were ‘baseline’ blocks in which only the two task 2 stimulus objects of the same length were used, the remaining ‘filtering’ blocks used all four task 2 stimuli. Before each block, participants were shown those objects used in the upcoming block. Four block orders were applied resulting from counterbalancing the two baseline and the two filtering blocks with the order of the two baseline blocks also counterbalanced. The experimental blocks were preceded by an unanalyzed practice block of 24 trials. Each trial began with a short warning click after which the tone of task 1 was presented. Following a varying SOA of 50, 500, or 1000 ms, the shutter glasses opened and provided view of the stimulus object (see Fig. 2 for an illustration of a trial’s time-course). All task 1 errors and task 2 perceptual judgment errors were detected automatically, task 2 grasping accuracy was judged by the experimenter: a grasp was only judged correct if a precision grip was used. The experimenter gave feedback after each trial. Then, the shutter glasses became opaque again and the experimenter initiated the next trial. Instructions placed equal emphasis on both tasks allowing the participants to respond in whatever order they want, as long as they responded as fast and accurate as possible.
The order in which grasping and perceptual judgment were applied as task 2 (2), order of experimental blocks (4), and the stimulus–response mapping of task 1 (2) were counterbalanced across participants. Each baseline block comprised 2 (task 1 stimuli) × 2 (task 2 stimuli) × 3 (SOA) × 6 (repetitions) trials in a random order; filtering blocks comprised 2 (task 1 stimuli) × 4 (task 2 stimuli) × 3 (SOA) × 3 (repetitions) trials in a random order.
Data treatment and analyses
Analyses were mainly done by means of analysis of variance (ANOVA) with the factors condition (baseline vs. filtering) and SOA (50 vs. 500 vs. 1000 ms) as repeated measures. Mauchley’s test was used to assess violations of the sphericity assumption, and where necessary, Greenhouse-Geisser corrections were applied. (However, for easier communication, we report uncorrected degrees of freedom.) Additional analyses will be introduced where necessary in the results section.
Trials with general errors (e.g., too slow responses, response 2 given before glasses were opened, …) were excluded right out, and error analyses are based on the remaining trials with task 1 and task 2 accuracy as the dependent measure. For RT analyses, only those trials were further considered where both task 1 and 2 responses were correct. Additionally, RTs less than 150 ms or exceeding the individual’s mean by more than 2.5 individual standard deviations (calculated separately for each participant and analyzed condition) were excluded. An alpha level of .05 was adopted throughout this paper, and sample effect sizes are reported as partial η².
Results
RT and MT analyses
Perceptual judgment as task 2
Mean RTs are summarized in Table 1. On a descriptive level, mean RTs in task 1 were slightly longer in the filtering condition than in the baseline condition, but were roughly on the same level across all SOA conditions. Neither the two main effects were significant, SOA: F(2,30) = 1.04, P = .34, partial η² = .07, ε = .68, condition: F(1,15) = 0.84, P = .38, partial η² = .05, nor was the interaction, F(2,30) = 0.91, P = .36, partial η² = .06, ε = 56.
Mean RTs in task 2 were longer in the filtering condition than in the baseline condition and exhibited a large decrease with an increasing SOA. Accordingly, the main effects were significant, SOA: F(2,30) = 119.57, partial η² = .89, ε = .56, condition: F(1,15) = 5.51, partial η² = .27. The interaction was not reliable, F(2,30) = 1.63, P = .22, partial η² = .10, ε = 73. Repeated contrasts on the factor SOA showed a significant decrease in RT2 from SOA = 50 ms to SOA = 500 ms, F(1,15) = 161.88, partial η² = .92, as well as from SOA = 500 to SOA = 1000 ms, F(1,15) = 39.08, partial η² = .72.
Grasping as task 2
Mean RTs and MTs are summarized in Table 1 and visualized in Fig. 3. Mean RTs in task 1 were slightly longer in the filtering condition compared to the baseline condition and seemed to increase with increasing SOA. This was confirmed by a significant main effect SOA, F(2,30) = 8.38, partial η² = .36, ε = .65. No other effect was significant, condition: F(1,15) = 2.05, P = .17, partial η² = .12, SOA × condition: F(2,30) = 0.71, P = .50, partial η² = .12.
Mean RTs in task 2 were again slightly longer in the filtering condition than in the baseline condition, and largely decreased with an increasing SOA. While the latter aspect is supported by a significant effect of SOA, F(2,30) = 206.10, partial η² = .93, ε = .62, the difference between conditions was not reliable, F(1,15) = 1.20, P = .29, partial η² = .07, as was the interaction, too, F(2,30) = 0.26, P = .77, partial η² = .02. Repeated contrasts on the factor SOA showed a significant decrease in RT2 from SOA = 50 ms to SOA = 500 ms, F(1,15) = 231.59, partial η² = .94, as well as from SOA = 500 to SOA = 1000 ms, F(1,15) = 87.39, partial η² = .85. There were no reliable effects on MTs (all Ps > .2).
Error analyses
Mean percentages of errors are summarized in Table 2. With perceptual judgment as task 2, there were two significant effects. First, error rates in task 1 decreased with an increasing SOA, F(2,30) = 3.82, partial η² = .20. Secondly, in task 2, error rates decreased with an increasing SOA in the baseline condition, but the opposite was true for the filtering condition. Accordingly, the interaction of SOA and condition was significant, F(2,30) = 4.33, partial η² = .22. With grasping as task 2, the analyses uncovered only one marginally significant effect suggesting decreasing error rates in task 1 with an increasing SOA, F(2,30) = 3.20, P = .06, partial η² = .18. No other effects were reliable (all Ps ≥ .1).
Discussion
In Experiment 1, participants took part in a PRP experiment with either grasping (conceived as a dorsal task) or a perceptual judgment (conceived as a ventral task; see also Ganel and Goodale 2003) as task 2. The goal of Experiment 1 was to test whether the PRP effect on grasping observed by Kunde et al. (2007) was due to the instructions focusing on task 1 performance, rather than the existence of a central processing stage in grasping. Our approach was to re-run the original study with altered instructions placing equal emphasis on both tasks and allowing a freely chosen response order (see also Ruthruff et al. 1995).
Despite the altered instructions and some minor differences of mostly technical nature, there is a remarkable amount of commonalities with the results observed by Kunde et al. (2007). With the perceptual judgment as task 2, there was clear evidence of a PRP effect for task 2, and additionally the Garner interference, i.e., the fact that the filtering condition caused longer RTs than the baseline condition did (Ganel and Goodale 2003), was additive with the SOA. Of more importance are the results related to grasping as task 2. The non-significant Garner interference reassures a dorsal processing (Ganel and Goodale 2003), but despite the altered instructions, we observed a large PRP effect. In addition, the results showed a significant RT2 decrease from SOA = 500 ms to SOA = 1000 ms. At the 500 ms SOA, however, no need arises to strategically defer task 2 responding and the observed decrease adds evidence against the alternative account advanced in the introduction to Experiment 1. Somewhat unexpected is the increase of RT1 with an increasing SOA. This is neither predicted by all-or-none bottleneck accounts (Pashler 1994; predicting no effect on RT1 at all) nor by a capacity sharing model (Tombu and Jolicoeur 2003; predicting decreasing RT1 with an increasing SOA). We see two reasons for this: first, with the altered instructions, participants may have tended to group their responses (i.e., to respond more or less simultaneously). However, the effect of SOA on RT1 remains significant even after excluding trials with an inter-response interval of less than 50 ms (Miller and Ulrich 2008), F(2,30) = 4.00, partial η² = .21. Secondly, unlike RT1, the mean error percentages in task 1 decreased with an increasing SOA. This specific feature of the results might thus be due to a speed-accuracy trade-off. Yet, because the effects of theoretical interest occur in task 2, and are probably unaffected by this task 1 outcome, we won’t go into detail on this issue here.
Finally, an important and crucial question is: Did the participants accept the invitation to reverse their response orders, i.e., were the altered instructions successful? The data show that participants mostly did not. In fact, except for one, all participants almost always responded to task 1 before leaving the home button in task 2. The one exceptional participant is quite informative nevertheless, because she showed response reversals in 67, 36, and 12% of the trials (for the SOA conditions of 50, 500, and 1000 ms, respectively). Thus, in principle response reversals were possible under these experimental conditions. Moreover, treating the trials from this participant as independent observations revealed a significant PRP effect: RT2 significantly dropped from 726 to 347 ms over the full SOA range, F(2,274) = 38.23, partial η² = .24. Unfortunately, it is impossible to tell from our data whether the other participants were unable to reverse their response order or simply chose not to do so. Experiment 2 was run to more convincingly demonstrate response reversals but a persisting PRP effect.
Experiment 2
The large PRP effect in dorsal tasks (Kunde et al. 2007) can potentially be attributed to strategic task 2 deferments. To rule out this explanation, in Experiment 1, the participants were free to choose any response order they want to—a method that has been used earlier in PRP research (Ruthruff et al. 1995). Despite this altered instructions, we replicated the large PRP effect. Admittedly, however, only very rarely did the participants reverse their response order, leaving open whether or not the instructions were powerful enough to successfully rule out the earlier mentioned explanation. In Experiment 2, we thus attempt to further stimulate parallel processing and to provide converging evidence for a remaining PRP effect under these conditions.
One largely uncontroversial exception from the PRP effect appears to be saccadic eye movements as the task 2 response (Pashler et al. 1993; see Lien et al. 2006, for an overview). In this study, on some trials, a negative SOA was used, i.e., S2 appeared before (rather than after) S1. This manipulation was introduced to prevent participants from adopting an obvious response order (e.g., always respond first to task 1, then to task 2). In addition, parallel processing is induced when short SOAs occur more frequently than long SOAs (Miller et al. 2009). To maximize efforts, we combined both methods in Experiment 2: (1) we used negative SOAs (i.e., S2 appears before S1) and (2) the shortest SOAs (−50 and 50 ms) were thrice as frequent as the other SOAs were. Finding both response reversals and the PRP effect in the dorsal grasping task would be a strong empirical argument against the strategic deferment hypothesis and the remaining reservations concerning Experiment 1.
Method
Participants
Eight new undergraduate students from Dortmund University of Technology (1 male, mean age = 23;0 years) participated in return of course credit.
Design, apparatus, stimuli, and procedure
For the most parts, this experiment resembles Experiment 1 with three differences. First, participants took only part in one single session of a PRP experiment with the grasping task as task 2. Second, we did not distinguish between baseline and filtering blocks. Rather all four possible S2s were used throughout this experiment. The third change concerns the SOA manipulation. In the present experiment, we used SOAs of −150, −50, 50, 150, 250, and 500 ms. A negative SOA means that S2 was presented before (rather than after) S1 (Pashler et al. 1993). In addition, the frequency of the two shortest SOAs (−50 and 50 ms) was thrice higher (24 times each) than the other SOAs were (eight times each) to induce even more parallel processing (Miller et al. 2009). The experiment was divided into four experimental blocks of 80 trials each. The experimental blocks were preceded by an unanalyzed practice block of 22 trials. The instructions were similar to those in the Pashler et al. (1993) study and focused on speed while not giving priority to one task over the other or suggesting a preferred response order. Also similar to this study, no feedback was given to the participants.
The stimulus–response mapping of task 1 was counterbalanced across participants, and the eight possible combinations of S1 (low vs. high tone) and S2 (four wooden blocks) occurred equally often at each SOA level in a random order.
Data treatment and analyses
Analyses were done by means of ANOVA with the factor SOA (-150 vs. −50 vs. 50 vs. 150 vs. 250 vs. 500 ms) as a repeated measure. Mauchley’s test was used to assess violations of the sphericity assumption, and where necessary, Greenhouse-Geisser corrections were applied. (However, for easier communication we report uncorrected degrees of freedom.)
Results
In a first step, we assessed whether participants did indeed vary their response order. Across the six SOA conditions, participants left the home button before they responded to the tone classification task in 77.0, 74.4, 73.2, 70.8, 65.3, and 36.8% of the trials. Clearly, the manipulations introduced in this experiment were successful, and we went on to analyze the RT/MT and error data in a second step.
RT and MT analyses
Mean RTs and MTs are illustrated in Fig. 4. RT1 showed an increase of 66 ms across the six SOA conditions. At the same time, RT2 showed a large decrease of 266 ms. SOA had a significant effect on the RTs of both tasks, task 1: F(5,35) = 4.85, partial η² = .41, and task 2: F(5,35) = 23.10, partial η² = .77, ε = .29. MTs remained almost constant (varying between 586 and 595 ms), and the effect of SOA was not significant, F(5,35) = 0.33, P = .70, partial η² = .05, ε = .36.
Error analyses
Mean task 1 error percentage tended to decrease slightly across the SOA conditions (11.1, 7.5, 6.8, 3.9, 2.7, and 4.5), and the effect of SOA was significant, F(5,35) = 4.07, partial η² = .37, ε = .36. On the descriptive level, the same pattern was found for mean task 2 error percentage (2.8, 2.5, 1.7, 1.2, 2.0, and 0.8), but the effect of SOA was not significant, F(5,35) = 1.02, P = .42, partial η² = .1.3.
Discussion
In Experiment 2, we maximized efforts to induce parallel processing of the tone classification task and the grasping task in a PRP experiment. This was done to unambiguously rule out strategic task 2 deferment as the source of the PRP effect found by Kunde et al. (2007) and also in our Experiment 1. To this end, we modified the standard PRP paradigm by adding two manipulations, namely, negative SOAs where S2 occurred prior to S1 (Pashler et al. 1993), and a larger likelihood of short SOAs, what also induces a shift from serial to parallel processing (Miller et al. 2009).
The results of this Experiment 2 are straightforward: although participants reversed their response order on the majority of the trials, the results are qualitatively similar to those of Experiment 1. In particular, RT1 showed a slight increase (we again suspect this in parts being due to a speed-accuracy trade-off). Contrary to that, RT2 exhibited the typical PRP effect, as indicated by a sharp decrease of 266 ms—more than twice as big as the one reported by Pashler et al. (1993). Note that even the RT2 decrease from the SOA of 50 ms to the SOA of 500 ms is 159 ms, which is more than thrice as much as the comparable effect in the Pashler et al. (1993) study.
In sum, Experiments 1 and 2 suggest that a typical dorsal grasping task is subject to massive interference from a concurrent choice reaction task, even when participants can perform the two tasks in any order they want to. This renders it unlikely that previous observations of PRP effects in dorsal tasks are due to a strategic task 2 deferment required to maintain a specific task order. Experiment 3 aims at ruling out a second alternative account.
Experiment 3
PRP effects can ensue for other reasons than central capacity limitations. One such reason is peripheral output interference. For example, shaving and tooth brushing are two tasks that essentially cannot be performed simultaneously since they (at least in the majority of cases) require the same effector (most often the right hand). A similar though more moderate argument may apply to earlier PRP studies with grasping as the secondary task as well. The responses in both tasks were manual: a key press in the tone classification task and a grasp reach in the grasping task (or another key press in the perceptual judgment task). One might argue that the observed interference was simply due to the identical response modality. It is well known that producing responses with both hands at the same time affects performance adversely (e.g., Heuer 1995), what is known as bimanual interference. A related, but more general, objection can be derived from multiple resource theories (e.g., Navon 1984; Wickens 1980, 1984) suggesting that the amount of interference relates somehow to the joint competition for specific resource pools. In fact, PRP experiments with two manual responses appear to produce larger dual-task costs than those with differing response modalities (Pashler 1990; Ruthruff et al. 2001). To circumvent such response modality-related accounts, we ran the grasping condition from Experiment 1 again but replaced the manual key press response in the tone classification task with a vocal response. The resulting task pairing (auditory–vocal/visual–manual) is also on par with what Hazeltine et al. (2006) have termed a standard pairing, in turn favoring parallel performance of two tasks.
Method
Participants
Sixteen new undergraduate students from Dortmund University of Technology (3 male, mean age = 23;8 years) participated in return of course credit.
Design, apparatus, stimuli, and procedure
In most parts, Experiment 3 resembles Experiment 1 with the exception that participants took part in only one single session where the grasping task was task 2 in a PRP experiment. Two other exceptions apply: first, the participants responded to the tone stimuli in the tone classification task with uttering either “tip” or “top”. RTs in this task were recorded using a voice key, and the accuracy of the responses was recorded by the experimenter. Secondly, the instructions focused on task 1 emphasizing speed and accuracy. In all other respects, Experiment 3 was similar to the grasping condition of Experiment 1.
Data treatment and analyses
The data were treated in the same way as described in Experiment 1. In addition, cross-experiment analyses are reported here and include ‘experiment’ as a between-subjects factor.
Results
RT and MT analyses
Mean RTs and MTs are summarized in Table 3 and visualized in Fig. 5. Similar to Experiment 1, mean RTs in task 1 were slightly longer in the filtering condition compared to the baseline condition and increased with increasing SOA. The latter finding was confirmed by a significant main effect of SOA, F(2,30) = 15.61, partial η² = .51. No other effect was significant, condition: F(1,15) = 1.63, P = .22, partial η² = .10, SOA × condition: F(2,30) = 2.02, P = .15, partial η² = .12.
Of main interest here is that mean RT2 showed, again, a large decrease with an increasing SOA, supported by a significant effect of SOA, F(2,30) = 284.24, partial η² = .95, ε = .60. Neither the difference between conditions was reliable, F(1,15) = 2.04, P = 17, partial η² = .12, nor was the interaction, F(2,30) = 2.74, P = .08, partial η² = .15. Repeated contrasts on the factor SOA support a significant decrease in RT2 from SOA = 50 ms to SOA = 500 ms, F(1,15) = 635.34, partial η² = .98, as well as from SOA = 500 to SOA = 1000 ms, F(1,15) = 82.93, partial η² = .85. Unexpectedly, the mean MTs also increased slightly with an increasing SOA, F(2,30) = 25.41, partial η² = .63. No other effect was significant, condition: F(1,15) = 0.64, P = .44, partial η² = .04, SOA × condition: F(2,30) = 2.60, P = .09, partial η² = .15.
Error analyses
Mean percentages of errors are summarized in Table 3. There were two significant effects on error rates. First, and similar to Experiment 1, error rates in task 1 decreased with an increasing SOA, F(2,30) = 3.98, partial η² = .21, ε = .72. Secondly, in task 2, error rates decreased with an increasing SOA in the baseline condition, and slightly increased (at least from SOA = 50 ms to SOA = 1000 ms) in the filtering condition. This resulted in a significant interaction, F(2,30) = 4.47, partial η² = .23. No other effects were reliable (all Ps ≥ .24).
Analyses across experiments
One might ask whether parts of the interference observed in Experiment 1 and by Kunde et al. (2007) diminished with the standard pairing used in Experiment 3 and thus can be attributed to response modality overlap. To this end, we compared the numerical size of the PRP effect (i.e., the difference in RT2 between SOA = 50 ms and SOA = 1000 ms) across both experiments. In Experiment 1, the PRP effect amounts to 447 ms, and with 568 ms, it was even larger in Experiment 3. Assessed with an independent-groups t-test, this difference was significant, |t|(30) = 2.77. On the other hand, the task 1 pairing used in Experiment 3 appears to be more difficult than that used in Experiment 1. RT1 was significantly larger in Experiment 3 (759 ms) than in Experiment 1 (491 ms), |t|(30) = 5.27. Similarly, mean error percentages were significantly higher in Experiment 3 (3.99%) than in Experiment 1 (1.44%), |t|(30) = 2.23.
Discussion
In Experiment 3, participants took part in a PRP experiment with a grasping task as task 2. Critically and different from Experiment 1, responses to task 1 (tone classification) were vocal utterances instead of manual key presses. This was done to ensure minimal effects of bimanual interference (e.g., Heuer 1995) and/or competition for a specific resource pool (e.g., Navon 1984; Wickens 1980, 1984) related to response modality overlap. This new design also resulted in standard pairings (Hazeltine et al. 2006) that have been shown to favor parallel processing without considerable dual-task costs (at least after extensive practice). Admittedly, planning processes for vocal utterances can potentially interfere with the planning of the hand movement. Still the amount of overlap is conceivably smaller in the case of one vocal and one manual response compared to two manual responses. Also, the combination of an auditory–vocal and a visual–manual task is a common feature of many though not all PRP studies in the literature as an attempt to minimize input and output interference. It has been shown to be the optimal pairing to reduce these types of interference in non-PRP studies (Shaffer 1975).
Despite this modification, the central and most critical finding remained stable: RTs in the grasping task were longer with shorter SOAs than with longer SOAs, the well-known PRP effect. Post hoc analyses identified the PRP effect in Experiment 3 as even larger than in Experiment 1. This finding, however, must be taken with caution. First, both estimates for the PRP effects were taken from different groups of participants. Second, the auditory–vocal pairing used in Experiment 3 seemed to have impeded task 1 performance compared to the auditory–manual paring of Experiment 1, evidenced both by prolonged RTs and larger error rates. This in turn might have enlarged the PRP effect, although the fact that the response selection, a prime candidate for central processing in the PRP paradigm, was not altered (it was a 2 on 2 mapping in both experiments), renders this speculation somewhat unconvincing. Note also that Hazeltine et al. (2006) reported the opposite observation, i.e., a more difficult auditory–manual pairing compared to an auditory–vocal pairing. The reasons for this discrepance are unclear thus far, but differences in the response selection (3 on 3) and general experimental design features may account for these diverging findings.
Similar to Experiment 1 and again not compatible with either all-or-none bottleneck models (Pashler 1994) or a capacity sharing model (Tombu and Jolicoeur 2003) is the increase of RT1 with an increasing SOA. However, since at the same time error rates decreased, this fact might again reflect a speed-accuracy trade-off in task 1. The reasons for the slight, but significant, increase in MTs are thus far unknown to us.
General discussion
According to the action-perception model (Goodale and Milner 1992; Milner and Goodale 2006), the same visual input is processed by two different cortical systems for different purposes. A ventral pathway (roughly from V1 to the inferior temporal cortex) processes visual input to create a conscious representation of a percept, while a dorsal pathway (roughly from V1 to the posterior parietal cortex) processes the same visual input in order to program and guide visually controlled actions. It has been suggested that one core feature differing between both pathways is their consciousness and need for central processing capacity: in contrast to ventral processing, dorsal processing is described as effortless, fast, and automated, thus not needing central processing capacity (e.g., Jeannerod and Jacob 2005; Liu et al. 2008; Norman 2002). A recent study using the PRP paradigm seriously questioned this assertion (Kunde et al. 2007). These authors demonstrated that a dorsal grasping task exhibited a PRP effect of the same size as a ventral perceptual judgment task (both tasks were modeled after Ganel and Goodale 2003). Apparently, dorsal processing required some central capacity, and this contrasts the action-perception model. Unfortunately, this finding could have been due to (at least) two alternative interpretations: (1) the participants strategically withheld the grasping initiation to comply with the instruction to respond in stimulus order. Or (2), since both tasks required a manual response, the observed interference resulted from overlap in the response modality (Heuer 1995; Navon 1984; Wickens 1980, 1984).
The primary goal of the present research was to rule out these alternative accounts for the Kunde et al. (2007) results. To this end, we conducted three PRP experiments in which task 1 was always a binary tone classification. In Experiment 1, we used a modified instruction allowing a freely chosen response order (Ruthruff et al. 1995), and in Experiment 2, we took another step towards inducing parallel processing of both tasks by introducing negative SOAs (Pashler et al. 1993) and a higher likelihood of short SOAs (Miller et al. 2009). Finally, in Experiment 3, we replaced the manual task 1 response with a vocal response. This minimized the overlap in response modality and such a standard pairing favors parallel processing (Hazeltine et al. 2006). The main outcome of all three experiments can be summarized quickly: we successfully replicated the PRP effect in the grasping task, thus re-assuring the idea that—from the viewpoint of the PRP paradigm—dorsal processing calls for central resources and cannot be construed as automatic. Corroborating this conclusion, considerable dual-task interference has also been observed in a study by Singhal et al. (2007). In contrast, Liu et al. (2008) (see also Lee and Hsieh 2009) came to different conclusions. In their experiment, an RSVP task was combined with a pointing movement to a second peripheral target (either a to-be-identified letter or a simple disc) appearing after a variable delay following the first target (their Experiment 1). The main result was that (successful) identification of the first target interfered with the initiation time of the pointing movement, but not with the movement time. Referring to Goodale and Milner (2004) the authors predicted these results, since “action planning and action execution are controlled by the ventral and dorsal streams, respectively” (Liu et al. 2008, p. 710). Yet, the term ‘planning’ is used in a vague and loose way here. Planning in a broad sense such as “deciding upon one course of action rather than another” is indeed attributed to the ventral stream (Goodale 2008, p. 904). In contrast, planning in the sense of programming the initial movement parameters is nevertheless dependent on dorsal processing (Goodale and Milner 2004, p. 38). Conceivably, decisions like action-selection, the goal of the action, and so on are inherent in the instructions and thus have already been established in the studies by Liu et al. (2008) and Lee and Hsieh (2009). What is left is the final programming of the movement, and this is—according to Goodale and Milner (2004)—attributed to the dorsal pathway. As such, the initiation time appears to measure dorsal rather than ventral processing and the results are well in line with the conclusion we have based on our own experiments. That movement times were largely unaffected by the SOA manipulation in our experiments indicates that all relevant programming by the dorsal pathway has been finished before the movement itself is initiated.
We used the Garner interference (Ganel and Goodale 2003) as an indicator whether a task is processed ventrally or dorsally. A noteworthy side-aspect of our data is that the Garner interference (in the perceptual judgment task of Experiment 1) combined additively with the SOA. This suggests that the Garner interference is resolved only at a central processing stage (e.g., Pashler 1994). Hence, although somewhat counterintuitive, resolving the Garner interference appears to be an example of perceptual operations requiring central processing resources (see Pashler and Johnston 1998).
In sum, the present results support the conclusion that dorsal processing is not automatic but rather interferes with concurrent tasks. For the PRP and attention researcher, this might come to some disappointment since dorsal processing was indeed a likely candidate for being an exception from the robust PRP effect. Whether these limitations arise through the need to share a limited central capacity (Tombu and Jolicoeur 2003) or through bottleneck mechanisms requiring the serial processing of two tasks at a central processing stage (e.g., Pashler 1994) has to be clarified by future research.
References
Aglioti S, DeSouza JFX, Goodale MA (1995) Size-contrast illusions deceive the eye but not the hand. Curr Biol 5:679–685
Franz VH, Gegenfurtner KR (2008) Grasping visual illusions: consistent data and no dissociation. Cogn Neuropsychol 25:920–950
Ganel T, Goodale MA (2003) Visual control of action but not perception requires analytical processing of object shape. Nature 426:664–667
Garner WR (1974) The processing of information and structure. Erlbaum, Potomac
Garner WR (1978) Selective attention to attributes and to stimuli. J Exp Psychol Gen 107:287–308
Goodale MA (2008) Action without perception in human vision. Cogn Neuropsychol 25:891–919
Goodale MA, Milner AD (1992) Separate pathways for perception and action. Trends Neurosci 15:20–25
Goodale MA, Milner AD (2004) Plans for action. Behav Brain Sci 27:37–40
Hazeltine E, Ruthruff E, Remington RW (2006) The role of input and output modality pairings in dual-task performance: evidence for content-dependent central interference. Cogn Psychol 52:291–345
Heuer H (1995) Models for response-response compatibility: the effects of the relation between responses in a choice task. Acta Psychol 90:315–332
James TW, Culham J, Humphrey GK, Milner AD, Goodale MA (2003) Ventral occipital lesions impair object recognition but not object-directed grasping: a fMRI study. Brain 126:2463–2475
Jeannerod M, Jacob P (2005) Visual cognition: a new look at the two-visual systems model. Neuropsychologia 43:301–312
Kunde W, Landgraf F, Paelecke M, Kiesel A (2007) Dorsal and ventral processing under dual-task conditions. Psychol Sci 18:100–104
Lee T-Y, Hsieh S (2009) The limits of attention for visual perception and action in aging. Aging Neuropsychol Cogn 16:311–329
Lien M-C, Ruthruff E, Johnston JC (2006) Attentional limitations in doing two tasks at once. The search for exceptions. Curr Dir Psychol Sci 15:89–93
Liu G, Chua R, Enns JT (2008) Attention for perception and action: task interference for action planning, but not for online control. Exp Brain Res 185:709–717
Miller J, Ulrich R (2008) Bimanual response grouping in dual-task paradigms. Q J Exp Psychol 61:999–1019
Miller J, Ulrich R, Rolke B (2009) On the optimality of serial and parallel processing in the psychological refractory period paradigm: effects of the distribution of stimulus onset asychronies. Cogn Psychol 58:273–310
Milner AD, Goodale MA (2006) The visual brain in action, 2nd edn. University Press, Oxford
Navon D (1984) Resources—a theoretical soup stone? Psychol Rev 91:216–234
Norman J (2002) Two visual systems and two theories of perception: an attempt to reconcile the constructivist and ecological approaches. Behav Brain Sci 25:73–144
Pashler H (1990) Do response modality effects support multiprocessor models of divided attention? J Exp Psychol Hum Percept Perform 16:826–842
Pashler H (1994) Dual-task interference in simple tasks: data and theory. Psychol Bull 116:220–244
Pashler H, Johnston JC (1998) Attentional limitations in dual-task performance. In: Pashler H (ed) Attention. Psychology Press, Hove, pp 155–189
Pashler H, Carrier M, Hoffman J (1993) Saccadic eye movements and dual-task interference. Q J Exp Psychol 46:51–82
Perenin MT, Vighetto A (1988) Optic ataxia: a specific disruption in visuomotor mechanisms. I. Different aspects of the deficit in reaching for objects. Brain 111:643–674
Pisella L, Binkofski F, Lasek K, Toni I, Rossetti Y (2006) No double-dissociation between optic ataxia and visual agnosia: multiple sub-stream for multiple visuo-manual integrations. Neuropsychologia 44:2734–2748
Posner M (1964) Information reduction in the analysis of sequential tasks. Psychol Rev 71:491–504
Ruthruff E, Miller J, Lachmann T (1995) Does mental rotation require central mechanisms? J Exp Psychol Hum Percept Perform 21:552–570
Ruthruff E, Johnston JC, van Selst M (2001) Why practice reduces dual-task interference. J Exp Psychol Hum Percept Perform 27:3–21
Shaffer LH (1975) Multiple attention in continuous verbal tasks. In: Rabbitt PMA, Dornic S (eds) Attention and performance V. Academic Press, San Diego, pp 157–167
Singhal A, Culham JC, Chinellato E, Goodale MA (2007) Dual-task interference is greater in delayed grasping than in visually guided grasping. J Vision 7:1–12
Tombu M, Jolicoeur P (2003) A central capacity sharing model of dual-task performance. J Exp Psychol Hum Percept Perform 29:3–18
Ungerleider LG, Mishkin M (1982) Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW (eds) Analysis of visual behavior. MIT Press, Cambridge, pp 549–586
Welford AT (1952) The ‘psychological refractory period’ and the timing of high-speed performance—a review and a theory. Br J Psychol 43:2–19
Wickens CD (1980) The structure of attentional resources. In: Nickerson R (ed) Attention and performance, vol 8. Erlbaum, Hillsdale, pp 239–257
Wickens CD (1984) Processing resources in attention. In: Parasuraman R, Davies DR (eds) Varieties of attention. Academic Press, Orlando, pp 63–102
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Janczyk, M., Kunde, W. Does dorsal processing require central capacity? More evidence from the PRP paradigm. Exp Brain Res 203, 89–100 (2010). https://doi.org/10.1007/s00221-010-2211-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-010-2211-9