Abstract
Proper movement timing is essential to the successful execution of many motor tasks and may be adversely affected by muscle fatigue. This study quantified how muscle fatigue affected task performance during a repetitive upper extremity task. A total of 14 healthy young adults pushed a low load back and forth along a low-friction horizontal track in time with a metronome until volitional exhaustion. Kinematic, force, and electromyography (EMG) data were measured continuously throughout the task. The first and last 3.5 min were analyzed to represent “early” and “late” fatigue. Means and standard deviations of movement distance, speed, and timing errors were computed. We also decomposed variations in movement distance and speed into deviations that directly affected achieving the task goal and those that did not, by identifying the goal equivalent manifold (GEM) of all valid solutions to this task. Detrended fluctuation analysis was used to quantify the temporal persistence in each time series. Principle components analysis provided a direct measure of alignment with the GEM. Median power frequencies of the EMG significantly decreased in six of the nine muscles tested indicating that subjects did fatigue. However, there were no differences in the means or variability of movement distance, speed, or timing errors. Thus, subjects maintained overall performance despite fatigue. Subjects applied slightly higher peak handle forces when they were fatigued (P = 0.032). Muscle fatigue caused significant reductions in the temporal persistence of movement speed (P = 0.037) and timing errors (P = 0.046), indicating that subjects corrected errors more quickly when fatigued. Mean deviations and variability perpendicular to the GEM were much smaller than variability along the GEM (P < 0.001). Deviations perpendicular to the GEM were also corrected much more rapidly than those along the GEM (P < 0.001). Subjects aligned themselves very closely (<±7°), but not exactly (P < 0.001), with the GEM. These measures were not significantly affected by muscle fatigue. Overall, these results indicated that subjects altered their biomechanical movement patterns in response to muscle fatigue, but did so in a way that specifically preserved the goal relevant features of task performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rhythmic movements performed during daily activities are often triggered and sustained by external signals (e.g., auditory, visual, etc.) (Bove et al. 2007). Timing is often critical to these repetitive movement tasks. During coordinated movements, specific muscles must be activated and/or inactivated in both the correct sequence and at appropriate times (O’Boyle et al. 1996). Muscle fatigue can alter muscle timing (Wilder et al. 1996; Strange and Berg 2007) and muscle coordination (Corcos et al. 2002; Goerlick et al. 2003; Billaut et al. 2005), thus impeding task performance. However, exactly how muscle fatigue affects the control of timing during repetitive tasks has not been established.
Muscle fatigue is defined as a decrease in the force generating capacity of a muscle or muscle group after activity (Bigland-Ritchie and Woods 1984; DeLuca 1984; Gandevia 2001). Fatigue is a combination of both central and peripheral processes (Gandevia 2001). At the peripheral level, there is a loss of force generating capacity of individual motor units (Selen et al. 2007). To maintain force, the central nervous system can increase its drive to the muscles. This causes already active motor units to fire more frequently and causes larger motor units to be recruited. This leads to an increased sense of effort (Gandevia 2001). As fatigue progresses, the number of active motor units decreases, muscle fiber conduction velocity decreases (Farina et al. 2002), motor units fire more slowly (Bigland-Ritchie and Woods 1984), and the motor units become more synchronized (Arihara and Sakamoto 1999). This leads to decreased mean or median frequencies of the electromyogram (EMG) signal (Bigland-Ritchie and Woods 1984) and eventually to task failure (Hunter et al. 2004).
Muscle fatigue may impair a person’s ability to properly execute a task. Reaction time during a choice reaction time task increased with muscle fatigue (Lorist et al. 2002). fMRI studies showed increased activity in the prefrontal areas of the brain after muscle fatigue, which may explain this increase in processing time (van Duinen et al. 2007). At the muscle level, fatigue causes an increase in muscle response time or “electromechanical delay” (Wilder et al. 1996), possibly due to the decrease in muscle fiber conduction velocity. Muscle fatigue may also affect the body’s ability to successfully reproduce a movement. In a study of rapid elbow flexion/extension, fatigue of the extensor muscles caused an undershoot of the final position during extension but had no affect on flexion (Jaric et al. 1999). However, other studies showed no affect of fatigue on the end-point trajectories in multi-joint tasks (Lucidi and Lehman 1992; Côté et al. 2002; Heuer et al. 2002; Selen et al. 2007). In each of these studies, it was presumed that subjects changed their neural control (muscle activation) or coordination strategies to achieve the same overall task goal.
Little is known about how muscle fatigue affects movement control. To determine this, it is important to define what parameters humans actively try to control. Since movement variability may increase with muscle fatigue (Selen et al. 2007), there may be active higher level processes occurring to combat this variability in order to retain accuracy. Many multi-joint tasks exhibit an infinite number of possible movement solutions (i.e., equifinality), so determining an optimal control strategy is difficult. One way to quantify the system’s degree of control is to study how quickly subjects respond to deviations away from the task goal. Cusumano and Cesari (2006) introduced the idea of a “goal equivalent manifold” (GEM), which provided a rigorous approach to quantifying motor redundancy in goal-directed movements. This method defines an explicit mapping between the variability of the body state variables (e.g., position, speed) and variability of the goal variables defined by the task. All possible solutions to the task lie along the GEM. Using this approach one can determine whether muscle fatigue affects the outcome (i.e., the “goal”), the body, or both.
One way to determine how quickly deviations are corrected is to quantify the temporal correlation structure of the variations in a time series (Hausdorff et al. 1995; Peng et al. 1995). When deviations in one direction are more likely to be followed by deviations in the opposite direction, the time series exhibits “anti-persistent” correlations. This indicates a highly controlled process. When deviations in one direction are more likely to be followed by deviations in the same direction (i.e., the deviations get bigger in magnitude) the time series exhibits “persistent” correlations. To date, only one group has examined the affect of fatigue on such temporal correlations. Localized muscle fatigue of the ankle plantarflexors caused the center of pressure trajectories during quiet standing to become more anti-persistent (Corbeil et al. 2003). These results suggested that the actions taken by the postural control system to maintain balance were more frequent post-fatigue (Corbeil et al. 2003).
The goal of this project was to determine how muscle fatigue affected the control of repetitive goal-directed upper extremity movements. Subjects performed a repetitive sawing-like task in time with a metronome until volitional exhaustion. The increased force variability and delayed reaction times associated with muscle fatigue (Bigland-Ritchie and Woods 1984; Farina et al. 2002; Lorist et al. 2002; van Duinen et al. 2007) suggest that task performance should deteriorate with fatigue: i.e., that timing errors would increase in magnitude. Alternatively, subjects could alter their movement patterns to maintain task performance: i.e., they would continue to achieve the task goal (Côté et al. 2002; Selen et al. 2007). We hypothesized that subjects would adopt a control strategy that aligned their movements with the GEM for this task, i.e., that deviations perpendicular to the GEM would be much smaller in magnitude and would be corrected more rapidly than deviations along the GEM. We further hypothesized that while subjects would alter their movement patterns to combat the effects of muscle fatigue, those features of motor performance that were specifically “goal relevant” would not change.
Methods
Subjects
A total of 14 healthy right-handed subjects (nine male, five female) participated. Their mean ± SD age, body mass, and height were 27 ± 2.7 year, 72.5 ± 16.9 kg and 1.72 ± 0.10 m, respectively. All participants signed institutionally approved informed consent forms and were screened to ensure that no subject had a history of medications, surgeries, injuries, or illnesses that might have affected their upper extremity joint movements. To determine handedness, subjects completed a modified version of the Edinburgh Inventory (Oldfield 1971). This inventory indicates the level of dominance of one hand over another. A score of 0/10 indicates a complete left-handed preference, while a score of 10/10 indicates a complete right-handed preference. All subjects scored at least 9/10 on the Edinburgh Inventory, indicating a strong right-handed dominance.
Experimental protocol
To monitor timing parameters during fatigue, we built a device to simulate a repetitive work task similar to sawing (Fig. 1). Subjects made bi-directional horizontal movements in the anterior–posterior direction with their right arm while holding a handle mounted to a carriage riding on a low friction track attached to a support frame. Inertial resistance was supplied by an adjustable set of weights mounted on the carriage. Therefore, the resisting load was always opposed to the direction of motion so the arm extensors were the primary agonists during the pushing stroke, while the flexors were the primary agonists on the pulling stroke.
The experimental setup. Subjects were seated in a high-back chair and restrained by belts across the waist and shoulders. A handle with an adjustable weight stack was able to slide with low friction across a horizontal track. This track was adjusted to the level of the subject’s sternum. A single marker on top of the handle quantified the anterior–posterior motions of the handle (X H). EMG electrodes (not shown) recorded the electrical activity of nine major arm and torso muscles used in the task
The device was adjusted so the subject’s legs were at a 90° angle with the ground. The height of the bar was adjusted so the midpoint between the third and fourth finger was in line with the xiphoid process. The front/back position of the chair was adjusted to be comfortable for the subject and allow for a full range of motion. This was defined as a maximum point almost to full extension (no hyperextension) and a minimum point at the level of the sternum.
To ensure the task resistance was comparable across subjects, we first measured each subject’s maximum pushing/pulling force using a second custom handle attached to a Baseline® dynamometer that was rigidly mounted on a table. Subjects alternately pushed and then pulled on this rigidly fixed handle as hard as they could for 5 s each, three times, with 60 s of rest in between each attempt. The average of these six peak forces applied during each maximal effort defined that subject’s maximum isometric pushing/pulling strength.
Subjects were instructed to move in time with a metronome. To ensure the task was dynamically equivalent across subjects, the frequency of the metronome was set to twice the average of the predicted resonant frequencies of the upper arm and forearm segments of each subject (two beats per cycle). The natural frequency, f n, of a rigid body pendulum is
where m is the mass of the limb segment, I o is the moment of inertia of the limb segment about the axis of rotation, and r is the distance from the axis of rotation to the center of mass of the limb segment. Given each subject’s height and weight, values for m, I o, and r were estimated from standard anthropometric tables (Winter 2005). The average natural frequency was 1.07 ± 0.03 Hz.
Subjects performed the sawing task with the weight mounted on the carriage (Fig. 1) set to 15% of this maximum pushing/pulling force. The actual forces experienced by each subject were a function of this external load, their hand acceleration (through F = m × a), and to some extent friction. Because movement distance and frequency were also both scaled to each subject, so were these external forces. Therefore, the forces applied to the handle by each subject were monitored throughout the trial by a six-axis load cell (JR3 Inc., Woodland, CA, USA) mounted at the base of the handle.
Our goal was to quantify the effects of muscle fatigue on movement timing and coordination. However, our findings could have been potentially seriously confounded if subjects simultaneously exhibited changes due to learning of the task. Humans can adjust grip force to accommodate simple inertial loads imposed by typical rigid objects within as little as 135 ms during a single movement (Bock 1993). When subjects lift objects of unusually high densities, they adapt their responses within fewer than five movements (Gordon et al. 1993). Even for more complex modifications of the arm’s inertial properties, adaptation is typically completed within 40–50 movements (Sainburg et al. 1999). Thus, we expected subjects would “learn” to manipulate the simple inertial load used in the present experiment vary quickly. Pilot testing confirmed that subjects did indeed learn this task (i.e., their mean errors approached zero) within just a few (<10) movements. Thus, to ensure that our results were not influenced by learning effects, subjects were asked to perform a warm up trial, moving in time with the metronome, for a minimum of 30 s (∼30 cycles) or until they felt completely comfortable with the task. Subjects then rested for 1 min to minimize any fatigue effects that may have occurred during this practice period.
Subjects then performed the fatigue task by sawing until they reached voluntary exhaustion. Once the fatigue trials began, data collection did not begin until subjects visually reached steady-state (an additional ∼20 cycles). Subjects were given strong verbal encouragement to continue and were told to “focus on the metronome and keep time with the beat” when they exhibited any difficulty maintaining timing. They were similarly instructed to maintain full range of motion if they began making smaller movements than specified.
A single reflective marker was placed on the top of the handle (Fig. 1) to define the beginning and end of each cycle. The 3D position of this marker was recorded continuously during each fatigue trial at 60 Hz using an 8–camera Vicon-612 motion analysis system (Oxford Metrics, Oxford, UK). Nine preamplified EMG surface electrodes (Delsys Inc., Boston, MA, USA) were attached to the dominant arm and torso to record activity in the middle trapezius, pectoralis major, deltoids (anterior, lateral and posterior), triceps (lateral head), biceps, flexor carpi radialis, and extensor carpi radialis longus. Electrodes were positioned over each muscle according to accepted recommendations (Konrad 2005). EMG and metronome data were recorded continuously at 1,080 Hz during all trials. Additionally, ratings of perceived exertion (RPE) were recorded once every 3 min during each trial using the modified Borg scale (Borg 1974, 1982), on which subjects subjectively rated their level of fatigue on a scale from 0 (none at all) to 10 (maximal exertion).
Data analyses
Raw EMG data were band-pass filtered from 20 to 400 Hz. Time points defining the beginning, middle, and end of each cycle, as determined from the marker data were used to split each EMG signal into the push stroke and pull stroke. Median power frequencies (MdPF) of the EMG signals were used to indicate muscle fatigue (DeLuca 1984). The MdPF for each stroke (either push or pull) was computed from the power spectrum of the signal using Welch’s method (MATLAB, Mathworks, Natick, MA, USA). The MdPF for each complete movement cycle (push plus pull) was calculated as the average of the MdPFs for the push and pull strokes (MacIsaac et al. 2001). For each muscle, the average MdPF for the first 224 cycles and the last 224 cycles (approximately 3.5 min) were compared using paired t-tests. This range was chosen because the other analyses used (see below) required relatively long time series. The need for longer time series was balanced against the need to capture only the earliest stages of fatigue. Data from two subjects for the middle deltoid and one subject for the wrist flexor were omitted due to technical problems during data collection.
The kinematic data from the handle marker (Fig. 1) were filtered using a fifth order low-pass Butterworth filter with a cutoff frequency of 6 Hz. These marker data were then resampled to 1,080 Hz using a piecewise cubic interpolant in Matlab (Mathworks, Natick, MA, USA) to match the sampling frequency of the metronome. The beginning and end of each movement stroke (i.e., either push or pull) were defined as the minimum and maximum excursions of the marker in the anterior–posterior direction. These minima/maxima were found by first differentiating the marker trajectory data and then locating the zero crossings of the velocity. For each movement cycle (i.e., push followed by pull), i, movement distance, d(i), was defined as the maximum minus the minimum anterior-posterior marker excursions for the push phase. Movement speeds, s(i), were defined as the movement distance, d(i), divided by the elapsed time for each movement cycle. Timing errors, e(j) were calculated separately for each stroke, j, by subtracting the time the handle marker reached a maximum or minimum from the time of the nearest metronome signal (Fig. 2; Chen et al. 1997; Ding et al. 2002). Thus, negative timing errors indicated that the subject lagged behind the metronome, while positive value indicated that they were ahead of it.
a An example segment of the handle marker trajectory (X H) is shown with points of minimum and maximum extension highlighted (×). Errors were defined as the elapsed time between these points and the metronome signal (.). b Example time series of timing errors for the same representative subject: S12. This subject consistently lagged behind the metronome. Points where the errors switch sign indicate where the subject was so far behind that they became more aligned with the subsequent metronome signal
To appropriately non-dimensionalize these variables (Hof 1996), movement speed was rescaled by a factor 1/(H × f m ), where H was the subject’s height and f m was the frequency of the metronome. Movement distance was rescaled by 1/H. The non-dimensional distance, D(i), was equal to the non-dimensional speed, S(i), at every location where the goal, f m , was reached. Because the same characteristic length (H) was used to non-dimensionalize both movement distance and speed, this choice did not affect the GEM analyses. Time series of non-dimensionalized E(j), D(i), and S(i) values were analyzed to quantify task performance and the overall movement patterns subjects used to achieve that performance.
In addition to these measures, we also used a performance analysis based on the idea of body-goal variability mapping (Cusumano and Cesari 2006). The primary goal of this task was to maintain movement time, T, with the metronome on each movement. However, there are an infinite number of combinations of movement distance, D, and speed, S, that will achieve this goal, so long as D/S = T. These [D, S] combinations define the goal equivalent manifold (GEM) for this task (Fig. 3). It should be noted that this GEM is defined explicitly by the goal of the task itself. It exists independent of our choice of analyses and independent of whether and how (or even if) subjects choose to control their movements in relation to the GEM. Given the existence of this GEM, we can then decompose the variability in the body movement parameters, D(i) and S(i), into components that directly affect task performance (i.e., achieving the task goal) and those that have no effect on achieving the task goal (Fig. 3; Cusumano and Cesari 2006). Variability in D(i) and S(i) were thus decomposed into variability tangent to and perpendicular to the GEM:
where \( \overset{\lower0.5em\hbox{$\smash{\scriptscriptstyle\rightharpoonup}$}} {\delta } {\left( i \right)} \) was the vector-valued error in movement time for movement i, δ T (i) was the corresponding magnitude of the deviation tangent to the GEM, δ P (i) was the corresponding magnitude of the deviation perpendicular to the GEM, and \( \hat{e}_{T} \) and \( \hat{e}_{P} \)were unit vectors defining directions tangent to and perpendicular to the GEM (Fig. 3). Any scalar deviations \( \delta _{T} {\left( i \right)} = \overset{\lower0.5em\hbox{$\smash{\scriptscriptstyle\rightharpoonup}$}} {\delta } {\left( i \right)} \cdot \hat{e}_{T} \) do not contribute to errors in movement time, while the deviations \( \delta _{P} {\left( i \right)} = \overset{\lower0.5em\hbox{$\smash{\scriptscriptstyle\rightharpoonup}$}} {\delta } {\left( i \right)} \cdot \hat{e}_{P} \) do. Thus, changes in the magnitude, variability, and/or cycle-to-cycle dynamics of these δ P (i) deviations would indicate changes in motor performance that were specifically “goal relevant.”
a Example of the goal equivalent manifold (GEM) analysis. The non-dimensional movement speed (S) is plotted versus non-dimensional movement distance (D). All combinations where S is equal to D achieve the “goal” of matching the metronome frequency, and thus define the GEM (diagonal line). Deviations perpendicular to and tangent to the GEM are denoted δ P and δ T , respectively. b Example time series of δ P and δ T fluctuations for a representative subject: S03. Fluctuations along the manifold were larger than those perpendicular to the manifold
Because the GEM is defined strictly by the task itself, this does not mean that subjects will take advantage of the GEM in regulating their movements. Computing the relative magnitudes of the variability of deviations in the δ P and δ T directions provides some insight as to how people regulate their movements. However, the ratio of these variances alone does not directly test the issue of alignment itself. To directly quantify how well each subject aligned their movements with the GEM, we also performed a principle components analysis (Daffertshofer et al. 2004) on the data obtained from each subject during each trial. We computed the eigenvector associated with the largest eigenvalue of the covariance matrix. This vector is known as the first principle component (PC1) because it defines the direction in which the greatest variance occurs. We then computed the angle (θ) between this PC1 vector and the GEM using the dot product. Values of θ very close to zero would indicate that subjects were indeed aligning their movements with the GEM.
Detrended fluctuation analysis (DFA) was used to determine the degree to which each time series exhibited persistent or anti-persistent temporal correlations across successive movements. This method has been used extensively in the analysis of experimental time series because it reduces noise effects and removes local trends making it less likely to be affected by nonstationarities (Hausdorff et al. 1995). Complete details of the methodology are published elsewhere (Peng et al. 1993, 1994, 1995; Hausdorff et al. 1995). In brief, the data sequence of length N was first integrated and then divided into equal, non-overlapping segments of length n. In each segment, the series was detrended by subtracting a least squares linear fit to that segment. The squares of the integrated, detrended data points (i.e, residuals) were then averaged over the entire data set and the square root of the mean residual, F(n), was calculated. This process was repeated for different values of segment lengths, n, ranging from 4 to N/4.
Typically, F(n) increases with n and a graph of log[F(n)] versus log(n) will often exhibit an approximately power-law relationship indicating the presence of scaling, such that F(n) ≈ n α (Hausdorff et al. 1995; Peng et al. 1995). These log[F(n)] versus log(n) plots were fitted with a linear function using least squares regression. The slope of this line defined the scaling exponent α (Fig. 4). A value of α = 0.5 indicates the time series is completely uncorrelated (i.e., random white noise). When, α < 0.5, the time series contains anti-persistent temporal correlations. This indicates a highly controlled process where deviations in one direction are more likely to be followed by deviations (i.e., corrections) in the opposite direction. Persistent temporal correlations are present when 0.5 < α ≤ 1.0 (Hausdorff et al. 1995). In this case, deviations in one direction are likely to be followed by deviations in the same direction (i.e., the deviations are not immediately corrected).
a Examples of two generic simulated stochastic time series, x(i), in arbitrary units (a.u.) with equal variance (both data sets were normalized to unit variance), but very different temporal correlation structures. The top graph clearly shows temporal persistence, whereas the bottom graph fluctuates much more rapidly. b DFA analyses of the two corresponding time series shown in a. The different temporal correlation patterns are clearly reflected in the different scaling exponent values obtained (α = 1.17 vs. α = 0.47). Thus, the α exponent obtained from DFA quantifies temporal correlations (persistence vs. anti-persistence) in a time series, independent of the magnitude of the variance
DFA was performed on the series of timing errors, E(j), movement distances, D(i) and movement speeds, S(i), as well as the deviations perpendicular to, δ P (i), and along, δ T (i), the GEM. The first and last i∈[1,..., 224] movement cycles (i.e., j ∈ [1,..., 448] timing errors) from each experiment were analyzed (approximately 3.5 min) to obtain “early” and “late” fatigue measures. Early/Late comparisons for means, standard deviations, and α of each of these time series were made using paired t-tests (Minitab 14, Minitab Inc, State College, PA, USA). Comparisons between directions (tangent vs. perpendicular to the GEM) were also made using paired t tests.
Results
Subjects performed the task for 23.93 ± 10.44 min (range 8.64–41.20 min). At the end of the first three minutes, subjects’ rates of perceived exertion (RPE) ranged from 2 to 6 (mean = 3.8), while at the beginning of the last segment all subjects had an RPE of 9 or higher. All subjects exhibited localized muscle fatigue as measured by decreased MdPF of the EMG signals (Fig. 5). These decreases were statistically significant for six of the nine muscles tested (P < 0.039) and nearly significant (P = 0.052 and P = 0.088) for two others.
Median power frequencies (MdPF) of the EMG signals decreased for all nine muscles tested. These decreases were statistically significant for the posterior deltoid (PD, P = 0.006), middle deltoid (MD, P = 0.039), anterior deltoid (AD, P = 0.002), triceps (TR, P < 0.001), wrist flexors (WF, P = 0.006), and wrist extensors (WE, P < 0.001). Decreases in MdPF were nearly statistically significant for the trapezius (TP, P = 0.052) and biceps (BI, P = 0.088), but were not significant for the pectoralis (PC, P = 0.760). Error bars indicate ±1 between-subject standard deviations about the mean
To verify that subjects had sufficiently “learned” the sawing task prior to data collection, the data from the first 240 cycles were divided into 24 non-overlapping bins of 10 movements each. Mean timing errors (E) and perpendicular distances from the GEM (δ P ) were computed within each bin to quantify performance accuracy. These data were compared statistically using a single-factor ANOVA to test for differences across the 24 bins. Neither timing errors nor δ P deviations changed over this period (P = 0.336 and 0.770, respectively; Fig. 6). Indeed, the δ P deviations tended to actually increase slightly, indicating that subjects were slightly less able to maintain proper timing. Thus, there was no evidence that any subject exhibited any further learning during the early fatigue phases of these experiments.
a Mean timing errors and b Perpendicular deviations (δ P ) from the GEM across the first 240 cycles of each fatigue trial. For each subject, average values for each measure were computed within 24 non-overlapping bins of ten movements each. Symbols (*) represent the mean values across all subjects. Error bars represent between-subject standard deviations. Both measures were very close to zero from the very beginning of the fatigue trials. Timing errors did not change over the first 240 cycles. Mean δ P deviations grew slightly larger over time. These data demonstrate that subjects had fully learned the task prior to data collection
Mean values of the non-dimensional movement distance (D), speed (S), and timing errors (E) were not affected by muscle fatigue (P = 0.958, 0.245, and 0.404, respectively; Fig. 7a). Timing errors were typically slightly negative, indicating that subjects were responding to the metronome signal rather than anticipating it. The magnitudes of the variability (Fig. 7b) for the task parameters D and S were unaffected by muscle fatigue (P = 0.695 and 0.538, respectively). The variability of timing errors exhibited a slight decrease with muscle fatigue that did not reach statistical significance (P = 0.192). However, α did decrease significantly with muscle fatigue (Fig. 7c) for both movement speed (P = 0.046) and timing errors (P = 0.037), but not for movement distance (P = 0.472). On average, all of these variables exhibited persistent temporal correlations (0.5 < α < 1.0), suggesting that they were not tightly controlled.
a The mean values of the movement distance (D), speed (S), and timing errors (E) were not affected by muscle fatigue (P = 0.958, 0.245, and 0.407, respectively). b Magnitudes of the variability (standard deviation) for these metrics were also not significantly affected by muscle fatigue (P = 0.695, 0.538, 0.192, respectively). c There was a significant decrease in α(with muscle fatigue for movement speed (S; P = 0.037) and timing errors (E; 0.046), but not distance (D; P = 0.472). On average, all variables exhibited persistent cycle-to-cycle correlations (0.5 < α < 1.0). Error bars indicate ±1 between-subject standard deviations about the mean
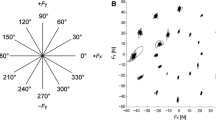
The overall patterns of the forces subjects applied to the handle appeared quite similar during both early and late fatigue (Fig. 8a). However, the average magnitudes of the peak forces each subject applied did increase significantly (P = 0.032) with muscle fatigue from 28.5 ± 6.0% to 30.7 ± 7.5% of each subjects maximum pushing/pulling force. These increases, although small, were generally consistent across subjects (Fig. 8b).
a Average handle force profiles for early and late fatigue movements, normalized to percent MVC and percent cycle time. Dotted lines represent ±1 between-subject standard deviation bands for each block of movements (Early and Late). b Average peak handle forces (F Peak) for each subject for both Early and Late fatigue. Each symbol and line type represents the average value for one subject. While these changes were relatively small, they were consistent across subjects to be statistically significant (P = 0.032)
The initial GEM decomposition revealed a strong tendency for subjects to align themselves with the GEM. Deviations perpendicular to the GEM were much smaller than those tangent to the GEM (Figs. 9, 10). There were differences between subjects in their responses to fatigue, however (Fig. 9). Some subjects increased both movement amplitude and speed post-fatigue (Fig. 9a), while others showed a decrease (Fig. 9d). A few subjects became less variable after fatigue (Fig. 9b), while others were consistently unable to keep time with the metronome post-fatigue (Fig. 9c).
GEM decomposition for four subjects representing the different behaviors exhibited. Points represent the combination of non-dimensional distance (D) and speed (S) for each movement. Symbols “×” and “.” represent the first and last 300 movements, respectively. The solid diagonal line is the goal equivalent manifold (GEM). Subjects tended to align with the GEM, such that there were greater deviations along the GEM than perpendicular to it. Two subjects showed an increase in movement amplitude and speed post-fatigue (e.g., a), while four others showed a decrease (e.g., d). Five subjects showed a decrease in variability post-fatigue (e.g., b). Three subjects showed an inability to keep time with the metronome post-fatigue (e.g., c)
a The mean magnitudes of the deviations perpendicular to (δ P ) to the GEM were significantly smaller than the deviations tangent to (δ T ) the GEM (P < 0.001). Perpendicular deviations tended to get slightly larger (i.e., more negative) with muscle fatigue (δ P : P = 0.087). Deviations along the GEM were not affected by muscle fatigue (δ T : P = 0.573). b The variability of deviations perpendicular to the GEM was significantly smaller than that of deviations tangent to the GEM (P < 0.001). Variability was not affected by muscle fatigue (δ P : P = 0.593, δ T : P = 0.837). c Deviations perpendicular to the GEM exhibited significantly less cycle-to-cycle temporal persistence (i.e., smaller α) than deviations tangent to the GEM (P < 0.001). There was a slight tendency for α to decrease with fatigue for deviations tangent to the GEM (δ T : P = 0.122). Fatigue did not affect the persistence of deviations perpendicular to the GEM (δ P : P = 0.497). Error bars indicate ±1 between-subject standard deviations about the mean
The mean magnitudes of the deviations perpendicular to (δ P ) and tangent to (δ T ) the GEM were significantly different (P < 0.001; Fig. 10a). Fatigue did not affect the mean deviations tangent to the GEM (P = 0.573). Deviations perpendicular to the GEM tended to increase slightly post-fatigue (P = 0.087). The magnitude of the variability (Fig. 10b) was unaffected by muscle fatigue (δ P : P = 0.593, δ T : P = 0.837). The magnitude of variability perpendicular to the GEM was significantly less than that tangent to the GEM (P < 0.001). Deviations perpendicular to the GEM were also significantly less persistent (Fig. 10c) than deviations along the GEM (αP < αT; P < 0.001). This indicated that the δ P deviations were corrected more quickly than δ T deviations. There was also a slight tendency for α(to decrease in late fatigue for δ T deviations (P = 0.122), whereas temporal correlations in δ P deviations were unaffected by fatigue (P = 0.497).
The principle components analyses (Fig. 11) revealed a slightly different view of how well subjects aligned their movements with the GEM. In most cases, the differences in alignment (θ) between the GEM and the first principle component (PC1) of the subjects’ data were less than ±10° (Early: 5.9° ± 7.8°, Late: 6.1° ± 6.8°). Across subjects, however, θ was significantly positive (P < 0.001), as determined from a single sample t-test with the null hypothesis that μ = 0. There were no significant changes in alignment of the data with the GEM between early and late fatigue (P = 0.93). Thus, even though subjects did exhibit positive ratios of δ T to δ P variability (Figs. 9, 10b), their movements were not aligned exactly along the GEM itself (Fig. 11b).
a Plots of movement distance (D) versus speed (S) for one example subject showing the orientation of the GEM as defined by the sawing task and the orientation of the first principle component (PC1) as determined from the subject’s data. The angle θ defines the orientation of PC1 relative to the GEM. For this subject, θ = −1.4° during early fatigue and θ = +19.0° during late fatigue. b Values of the angle θ for all subjects during both early and late fatigue. On average, these angles were slightly positive (P < 0.001) for most subjects, indicating that subjects did not align their movements exactly with the GEM. Across subjects, these angles did not change significantly from early to late fatigue (P = 0.93)
Discussion
Muscle fatigue can alter movement timing (Lorist et al. 2002; van Duinen et al. 2007). However, little is known about how muscle fatigue affects performance and control in repetitive tasks. This study was conducted to determine how muscle fatigue affected task performance during a repetitive sawing-like task. We analyzed the magnitudes, variations and temporal correlations of movement-to-movement timing errors, E(j), and cycle-to-cycle distances, D(i), and speeds, S(i). We also analyzed performance by decomposing variations in the task variables (D and S) into those that directly affected achieving the task goal (δ P ) and those that did not (δ T ), by identifying the goal equivalent manifold (GEM) of all valid solutions to this task. Using these analyses, we could determine if muscle fatigue affected overall task performance at the “goal” level, the biomechanical movement patterns subjects used to achieve this performance at the “body” level, or both.
The increased rates of perceived exertion (RPE) and decreased EMG median frequencies (Fig. 5) demonstrate that the sawing task did induce significant muscle fatigue in these subjects. The primary task goal was to perform the reaching task in time with the metronome. The lack of significant changes in average movement distance, speed, and timing errors (Fig. 7a) demonstrate that overall task performance did not change with fatigue. Similar results were reported for other upper extremity repetitive tasks (Lucidi and Lehman 1992; Côté et al. 2002; Heuer et al. 2002; Selen et al. 2007). The trend for mean deviations perpendicular to the GEM (δ P ) to increase slightly (but not quite significantly) with fatigue (Fig. 10a) suggests that there was some (albeit slight) deterioration in task performance. However, the lack of any significant changes (P > 0.49) in either the variability (Fig. 10b) or the temporal correlation structure (Fig. 10c) of δ P deviations or in the alignment of subjects’ movements with the GEM (Fig. 11) shows that subjects also maintained the same goal relevant performance with respect to the GEM.
Conversely, the cycle-to-cycle temporal persistence (α) of movement speed (S) and timing errors (E) both significantly decreased with fatigue (Fig. 7c). This indicates that subjects corrected timing errors more quickly when their muscles were fatigued. These decreases in α are supported by similar decreases found with fatigue during standing (Corbeil et al. 2003). Subjects also exhibited small, but fairly consistent increases in the peak forces they applied to the handle (P = 0.032; Fig. 8b). Likewise, 6 of the 14 subjects shifted their operating point along the GEM by making either longer faster movements (Fig. 9a) or shorter slower movements (Fig. 9d) with fatigue. The remaining subjects exhibited other qualitative changes in their movement patterns relative to the GEM (Fig. 9b, c). Together, these findings indicate that subjects significantly altered their biomechanical movement patterns in response to fatigue. However, these changes were made in such a way that those features of motor performance that were specifically “goal relevant” (Figs. 10, 11) did not change.
The goal equivalent manifold (GEM) approach adopted here is similar in some respects to the uncontrolled manifold (UCM) approach described in recent literature (Scholz and Schöner 1999; Latash et al. 2002; Schöner and Scholz 2007). Both approaches identify a sub-set of body-level variables, assumed to have the structure of a manifold, that define a full set of motor solutions that equally achieve a given task goal (Cusumano and Cesari 2006). The UCM approach then attempts to tie the resulting geometrical structure of this manifold directly to control by assuming control will be exerted only orthogonal to this manifold and not along it. Based on this assumption, putative control variables are identified by quantifying the ratio of the variance components parallel and perpendicular to the manifold (Latash et al. 2002). Conversely, the GEM approach makes no a priori assumptions about which variables are being “controlled.” This is because the GEM exists even for purely passive systems where no control is applied at all. Also, there is no particular reason to necessarily associate “control” with only one of the variance components (Cusumano and Cesari 2006). If (but only if) the body variables being analyzed do turn out to be the variables that are controlled, then the GEM would also be a UCM. However, this is not necessary and the ratio of the parallel and perpendicular variance components alone does not guarantee this. The GEM approach is thus both more general and more precise than the UCM approach.
Additionally, there were several technical differences between the analyses presented here and those associated with the UCM approach. First, the UCM approach assumes that stability can be equated with variability (Latash et al. 2002; Schöner and Scholz 2007). However, standard deviations only quantify the average magnitudes of the variations that occur across many cycles and do not directly quantify how a system responds to perturbations from one cycle to the next (Dingwell and Cusumano 2000; Dingwell and Kang 2007). The DFA analyses presented here (Figs. 4, 7c, 10c) provide an additional measure of cycle-to-cycle dynamics that is independent of variability (Peng et al. 1994; Hausdorff et al. 1995). Furthermore, variance ratios alone (Latash et al. 2002) do not directly quantify how closely each subject’s movements are aligned with the GEM. To assess this, we also conducted principle components analyses (PCA; Fig. 11) on our data. While previous authors have commented on the relationship between UCM and PCA (Schöner and Scholz 2007), the present analyses are the first we know of to simultaneously apply both approaches to directly assess alignment with the GEM. Even though subjects exhibited greater variability along the GEM than perpendicular to it (Fig. 10b), their performance was not aligned exactly with the GEM (Fig. 11). This suggests, contrary to the UCM interpretation, that there was indeed at least some coupling between performance (and likely also control), both perpendicular to and along the GEM.
The results presented here provide some new insights into the general nature of movement timing control and how this control is affected by muscle fatigue. For example, it is clear that subjects altered their biomechanical movement patterns in response to fatigue only in such a way that they specifically preserved the goal relevant features of their motor performance. However, these findings do not reveal which specific variables were being “controlled” or whether the changes that were observed were caused by physical changes in the plant (i.e., the musculoskeletal system being controlled), by changes in the underlying control policy (i.e., the specific instructions that define how to produce the desired output) each subject adopted, or possibly both. This issue is particularly complicated in the context of fatigue since the physical properties of the end effectors (i.e., the muscles) change due to fatigue. Therefore, it is possible that even the same governing control policy could induce different behaviors of these effectors. Therefore, contrary to the typical UCM interpretation (Schöner and Scholz 2007), we make no claims that subjects actively controlled their movements perpendicular to the GEM but not along it (Cusumano and Cesari 2006), nor that this control was preserved in the face of fatigue. What we can say is that the lack of fatigue-related changes in the goal relevant (i.e., δ P ) performance measures (Figs. 10, 11) suggests that any peripheral changes that might have directly affected these variables were compensated for by concurrent changes in the control policies subjects adopted.
As subjects tried to maintain time with the metronome, they oscillated between leading it and lagging behind it (e.g., Fig. 2b). The mean errors were negative (Fig. 7a), indicating that most subjects reacted to the stimulus rather than anticipating it. This result differs from previous findings on finger tapping (Aschersleben 2002). One reason for this is likely the larger inertial load involved in this task. This was substantiated by the DFA results (Figs. 7c, 10c), which showed that all five time series measures quantified in this study exhibited statistical persistence (i.e., α > 0.5). To exhibit anti-persistence (i.e., α < 0.5), subjects would have to be able to make immediate adjustments to each movement based on the error from the previous movement (e.g., Fig. 4; Bottom row). The significant inertial load involved in this task likely did not allow for such rapid corrections to be possible. Nevertheless, the finding that the δ P deviations perpendicular to the GEM were significantly less persistent than the δ T deviations along the GEM (i.e.,(αP < αT; Fig. 10c) strongly suggests that subjects adopted a general control strategy that corrected deviations in δ P more rapidly than deviations in δ T .
As mentioned, all five time series measures quantified in this study exhibited statistical persistence (i.e., α > 0.5). It has previously been proposed that findings of α > 0.5 from the DFA algorithm used here indicate the presence of “long-range correlations” such that the underlying time series can be characterized as having an infinite decorrelation time (Peng et al. 1993, 1994, 1995; Hausdorff et al. 1995). However, this algorithm was recently shown to yield “false positives” for many processes with finite correlation times (so-called “short-range correlations”). For example, many linear auto-regressive models can also result in findings of α > 0.5 (Maraun et al. 2004; Gates et al. 2007). Thus, here we make no claims that these α values represent true “long-range correlations” (Maraun et al. 2004). For our purposes, however, α still remains a valid measure of how rapidly the time series is fluctuating and thus provides a valid indication of how rapidly subjects were correcting deviations from each movement to the next.
We expected that the variability of the performance measures might increase with muscle fatigue (Selen et al. 2007). Instead, the variability of the timing errors actually decreased while the distance, speed, and deviations perpendicular and tangent to the GEM remained relatively constant (Figs. 7, 9). Although kinematic variability and force variability have been shown to increase in static tasks such as target tracking (Selen et al. 2007), these changes have not been documented in goal-directed movement tasks that require varying forces to achieve the goal.
Despite making the task as dynamically equivalent as possible, differences between subjects remained, particularly for time to exhaustion. One reason for this is that since this task was inherently redundant, there were numerous alternative modalities subjects could use to compensate for those that were altered by fatigue. Thus, it is easily possible different subjects compensated for fatigue in different ways. For example, each subject showed a unique pattern as to which muscles were most affected by fatigue during the task. By using different muscles, they were still able to perform the task accurately with no increase in movement variability. So while overall muscle force variability may increase (Selen et al. 2007), this effect could be counteracted by changing coordination strategies to use less fatigued muscles. The between-subject variability observed in this study was similar to that observed in previous studies of fatigue in complex multi-joint tasks (Nussbaum et al. 2001; von Tscharner 2002; Madigan and Pidcoe 2003; Voge and Dingwell 2003).
Another possible explanation is that the subjects fatigued to different degrees. Subjects could stop the experiment as soon as they felt they could no longer continue the task. This “threshold” could be different for the different subjects, depending on their motivation level and previous experience pushing themselves past the early stages of fatigue. To test this possibility, we correlated the difference between “early” and “late” fatigue for each dependent measure to each subject’s time to exhaustion. Early-late differences in variability of movement distances (D) were positively correlated with time to exhaustion (r 2 = 37.8%; P = 0.019). Subjects who performed longer showed greater decreases in movement distance variability, while subjects who stopped sooner showed greater increases. Early-late differences in α of timing errors (E) were negatively correlated with time to exhaustion (r 2 = 29.8%; P = 0.044). Subjects who performed longer showed smaller decreases to slight increases in α of timing errors. None of the other 13 comparisons were statistically significant (0.4% < r 2 < 23.2%; 0.82 > P > 0.08). Thus, while time to exhaustion had some impact on some of our results, the overall effects were not particularly strong.
It is possible that subjects experienced mental fatigue as well as muscle fatigue, since trials lasted up to 41 min and were fairly tedious. Cognitive factors can affect these processes during finger tapping where increasing the mental challenge of the task can lead to more persistent (i.e., larger α) temporal correlations (Ding et al. 2002). Previous work using fMRI has shown that after motor fatigue, activity in the prefrontal areas of the brain increases during reaction time task performance (van Duinen et al. 2007). This results in increased reaction times during auditory choice reaction time tasks (Lorist et al. 2002). Therefore, if the task became more mentally challenging, it is possible that this could offset the effect of muscle fatigue in some subjects. This may explain why the affect in α(is smaller in subjects who performed the task for a longer duration.
In summary, subjects significantly altered their biomechanical movement patterns in response to muscle fatigue. Muscle fatigue caused the deviations in movement speed and timing errors to become more anti-persistent. This suggests that subjects made more frequent corrections when their muscles were fatigued. Subjects also increased the peak forces they applied to the handle and exhibited qualitative changes in their behavior relative to the GEM when they were fatigued. However, the lack of significant changes in either the variability or temporal dynamics of the δ P deviations perpendicular to the GEM demonstrate that these changes were made only in such a way that they specifically preserved the goal relevant features of task performance.
References
Arihara M, Sakamoto K (1999) Contribution of motor unit activity enhanced by acute fatigue to physiological tremor of finger. Electromyogr Clin Neurophysiol 39:235–247
Aschersleben G (2002) Temporal control of movements in sensorimotor synchronization. Brain Cogn 48:66–79
Bigland-Ritchie B, Woods JJ (1984) Changes in muscle contractile properties and neural control during human muscular fatigue. Muscle Nerve 7:691–699
Billaut F, Basset FA, Falgairette G (2005) Muscle coordination changes during intermittent cycling sprints. Neurosci Lett 380:265–269
Bock O (1993) Early stages of load compensation in human aimed arm movements. Behav Brain Res 55:61–68
Borg GA (1974) Perceived exertion. Exerc Sport Sci Rev 2:131–153
Borg GA (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14:377–381
Bove M, Tacchino A, Novellino A, Trompetto C, Abbruzzese G, Ghilardi MF (2007) The effects of rate and sequence complexity on repetitive finger movements. Brain Res 1153:84–91
Chen Y, Ding M, Kelso JAS (1997) Long memory processes (1/f α Type) in human coordination. Phys Rev Let 79:4501–4504
Corbeil P, Blouin J, Begin F, Nougier V, Teasdale N (2003) Perturbation of the postural control system induced by muscular fatigue. Gait Posture 18:92–100
Corcos DM, Jiang HY, Wilding J, Gottlieb GL (2002) Fatigue induced changes in phasic muscle activation patterns for fast elbow flexion movements. Exp Brain Res 142:1–12
Côté JN, Mathieu PA, Levin MF, Feldman AG (2002) Movement reorganization to compensate for fatigue during sawing. Exp Brain Res 146:394–398
Cusumano JP, Cesari P (2006) Body-goal variability mapping in an aiming task. Biol Cybern 94:367–379
Daffertshofer A, Lamoth CJC, Meijer OG, Beek PJ (2004) PCA in studying coordination and variability: a tutorial. Clin Biomech 19:415–428
DeLuca CJ (1984) Myoelectrical manifestations of localized muscular fatigue in humans. Crit Rev Biomed Eng 11:251–279
Ding M, Chen Y, Kelso JAS (2002) Statistical analysis of timing errors. Brain Cogn 48:98–106
Dingwell JB, Cusumano JP (2000) Nonlinear time series analysis of normal and pathological human walking. Chaos 10:848–863
Dingwell JB, Kang HG (2007) Differences between local and orbital dynamic stability during human walking. J Biomech Eng 129:586–593
Farina D, Fattorini L, Felici F, Filligoi G (2002) Nonlinear surface EMG analysis to detect changes of motor unit conduction velocity and synchronization. J Appl Physiol 93:1753–1763
Gandevia SC (2001) Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81:1725–1789
Gates DH, Su JL, Dingwell JB (2007) Possible biomechanical origins of the long-range correlations in stride intervals of walking. Phys A Stas Med Appl 380:259–270
Goerlick M, Brown JMM, Groeller H (2003) Short-duration fatigue alters neuromuscular coordination of trunk musculature: implications for injury. Appl Ergon 34:317–325
Gordon AM, Westling G, Cole JK, Johansson RS (1993) Memory representations underlying motor commands used during manipulation of common and novel objects. J Neurophysiol 69:1789–1796
Hausdorff JM, Peng CK, Ladin Z, Wei JY, Goldberger AL (1995) Is walking a random walk? Evidence for long-range correlations in stride interval of human gait. J Appl Physiol 78:349–358
Heuer H, Schulna R, Luttmann A (2002) The effects of muscle fatigue on rapid finger oscillations. Exp Brain Res 147:124–134
Hof AL (1996) Scaling gait data to body size. Gait Posture 4:222–223
Hunter SK, Duchateau J, Enoka RM (2004) Muscle fatigue and the mechanisms of task failure. Exerc Sport Sci Rev 32:44–49
Jaric S, Blesic S, Milanovic S, Radovanovic S, Ljubisavljevic M, Anastasijevic R (1999) Changes in movement final position associated with agonist and antagonist muscle fatigue. Eur J Appl Physiol 80:467–471
Konrad P (2005) The ABC of EMG: a practical introduction to kinesiological electromyography, Noraxon, Inc
Latash ML, Scholz JP, Schöner G (2002) Motor control strategies revealed in the structure of motor variability. Exerc Sport Sci Rev 30:26–31
Lorist MM, Kernell D, Meijman TF, Zijdewind I (2002) Motor fatigue and cognitive task performance in humans. J Physiol 545:313–319
Lucidi CA, Lehman SL (1992) Adaptation to fatigue of long duration in human wrist movements. J Appl Physiol 73:2596–2603
MacIsaac D, Parker PA, Scott RN (2001) The short-time Fourier transform and muscle fatigue assessment in dynamic contractions. J Electromyogr Kinesiol 11:439–449
Madigan ML, Pidcoe PE (2003) Changes in landing biomechanics during a fatiguing landing activity. J Electromyogr Kinesiol 13:491–198
Maraun D, Rust HW, Timmer J (2004) Tempting long-memory—on the interpretation of DFA results. Nonlinear Processes Geophys 11:495–503
Nussbaum MA, Clark L, Kirst M, Rice K (2001) Fatigue and endurance limits during intermittent overhead work. AIHA J 62:446–456
O’Boyle DJ, Freeman JS, Cody FWJ (1996) The accuracy and precision of timing of self-paced, repetitive movements in subjects with Parkinson’s disease. Brain 119:51–70
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Peng CK, Buldyrev SV, Havlin S, Simons M, Stanley HE, Goldberger AL (1994) Mosaic organization of DNA nucleotides. Phys Rev E 49:1685–1689
Peng CK, Havlin S, Stanley HE, Goldberger AL (1995) Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos 5:82–87
Peng CK, Mietus J, Hausdorff JM, Havlin S, Stanley HE, Goldberger AL (1993) Long-range anticorrelations and non-Gaussian behavior of the heartbeat. Phys Rev Let 70:1343–1346
Sainburg RL, Ghez C, Kalakanis D (1999) Intersegmental dynamics are controlled by sequential anticipatory, error correction, and postural mechanisms. J Neurophysiol 81:1045–1056
Scholz JP, Schöner G (1999) The uncontrolled manifold concept: identifying control variables for a functional task. Exp Brain Res 126:289–306
Schöner G, Scholz JP (2007) Analyzing variance in multi-degree-of-freedom movements: uncovering structure versus extracting correlations. Motor Control 11:259–275
Selen LPJ, Beek PJ, van Dienn JH (2007) Fatigue-induced changes of impedance and performance in target tracking. Exp Brain Res 181:99–108
Strange AJ, Berg WP (2007) Fatigue-induced adaptive changes of anticipatory postural adjustments. Exp Brain Res 178:49–61
van Duinen H, Renken R, Maurits N, Zijdewind I (2007) Effects of motor fatigue on human brain activity, an fMRI study. NeuroImage 35:1438–1449
Voge KR, Dingwell JB (2003) Relative timing of changes in muscle fatigue and movement coordination during a repetitive one-hand lifting task. In: Proceedings of 25th international conference of IEEE EMBS, Cancun
von Tscharner V (2002) Time-frequency and principal-component methods for the analysis of EMGs recorded during a mildly fatiguing exercise on a cycle ergometer. J Electromyogr Kinesiol 12:479–492
Wilder DG, Aleksiev AR, Magnusson ML, Malcolm PH, Spratt KF, Goel VK (1996) Muscular response to sudden load: a tool to evaluate fatigue and rehabilitation. Spine 21:2628–2639
Winter DA (2005) Biomechanics and motor control of human movement, 3rd edn. Wiley, New York
Acknowledgments
Funding for this project was provided by grant #EB003425 from the National Institutes of Health. The authors thank Dr. Joseph P. Cusumano for many insightful discussions and comments on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gates, D.H., Dingwell, J.B. The effects of neuromuscular fatigue on task performance during repetitive goal-directed movements. Exp Brain Res 187, 573–585 (2008). https://doi.org/10.1007/s00221-008-1326-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-008-1326-8