Abstract
Switching between two tasks results in switch costs, which are increased error rates and response times in comparison to repeating a task. Switch costs are attributed to a change in task set, which is the internalized rule of how to respond to a stimulus. However, it is not clear if this is because the instruction about which task to perform has changed, or because a programmed response has changed. We examined this question by changing the instruction about whether to perform a pro or an antisaccade to a stimulus, before or after the stimulus was presented. As a saccade response is specified by instruction plus stimulus position, changing the instruction after the stimulus was present resulted in a change in the specified response, whereas changing the instruction beforehand did not. Three experiments investigated; (i) if changing instruction alone or changing the specified response produced switch costs; (ii) if predictability of switching instruction influenced switch costs; and (iii) if predictability of stimulus position influenced switch costs. Regardless of instruction or stimulus predictability, switch costs for both pro and antisaccades consistently resulted if the specified response switched. This suggests that a pro or antisaccade motor program was automatically programmed based on a presented instruction and stimulus position. Therefore, the given physical information drove switch costs, even if subjects could predict a change in task. This study demonstrates that switch costs result if changing an instruction changes a programmed response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The choice to perform one task instead of another is a hallmark of human executive functioning. However, flexibly changing from one task to another results in impairments in performance. In the laboratory setting this is explored by monitoring performance when two or more tasks are interleaved. Namely, subjects are slower to respond if the task on the current trial is different from the task on the previous trial (Allport et al. 1994; Jersild 1927; Rogers and Monsell 1995; Spector and Biederman 1976). Subjects also make more errors when the task switches from one trial to the next. These increases in response time and error rates when switching task are referred to as ‘switch costs’.
Switch costs represent a basic but fundamental measure of the ease in which the brain can flexibly control behavior. Functional Magnetic Resonance Imaging (fMRI) studies suggest that flexibly switching task is mediated by the prefrontal cortex, the pre-supplementary motor area, basal ganglia and parietal cortex (e.g., Brass and von Cramon 2004, 2002; Monchi et al. 2001; Yeung et al. 2006). Lesions or degeneration in these areas can lead to task-switching impairments (Miller and Cohen 2001; Milner 1963; Monchi et al. 2004). However, the precise neural origins of switch costs are unclear.
There is substantial evidence that switching task requires executive processes to switch ‘task set.’ ‘Task set’ refers to the internalized rules about how to respond to a stimulus (e.g., Allport et al. 1994; Rogers and Monsell 1995; Schuch and Koch 2003). Evidence suggests that ‘task set’ can be subdivided into components that can be configured endogenously in advance of stimulus presentation, and into other components that are triggered upon presentation of the stimulus to act upon (Barton et al. 2006; De Jong 2000; Matthews et al. 2002; Monsell 2003; Rogers and Monsell 1995). Meiran (2000) proposed that task set can be divided into ‘stimulus set’ (prepared based on an instruction) and ‘response set’ (pertaining to the execution of a response to the stimulus). Therefore it is possible that task set prepared based on an instruction may simply be a rule about what to do; whereas task set triggered by the target stimulus may be a response program that is automatically prepared. The aim of the current study is to investigate how switching only an internalized rule differs from switching a programmed motor response.
Recent studies have investigated how switch costs are related to selecting and executing a response (e.g., Koch and Allport 2006; Meiran 2000; Schuch and Koch 2003; Wylie et al. 2004). These studies have used methods such as: priming the subject to execute one motor response over another (e.g., hit left key), interleaving no-go trials (where a response was not executed on the previous trial), and examining the interference from two responses that share a common response mapping (e.g., hitting left key as a valid response to either task). However, in each case the comparison (switch vs. repeat task) is made between the current trial that includes an instruction and a response, and the previous trial that also included an instruction and a response. Therefore, these studies cannot directly examine the difference between switching a response process versus switching only a rule. Comparing across trials mixes both processes, and can include other sources that affect responding, such as previous saccade direction and previous stimulus location (see Fecteau and Munoz 2003 for review).
In the present study, we used a mid-trial change of instruction that could occur before or after the target stimulus was presented. On each trial, a colored fixation point dictated to perform a prosaccade to a peripheral stimulus, or an antisaccade away from the stimulus. If this fixation point changed color before the stimulus was present, it did not constitute a change of response, only a change in the rule about what task to perform on that trial. However, if the instruction changed after the stimulus was present, the required response was also changed.
We used prosaccades and antisaccades because distinct responses (saccade left or saccade right) are dictated by an instruction plus stimulus position. A large body of literature exists on their neurophysiological processes, and we know that the presentation of a stimulus will automatically evoke response processes (Dorris and Munoz 1998; Everling and De Souza 2005; Everling and Munoz 2000; Munoz and Everling 2004). Secondly, studies have shown that the prefrontal cortex is involved in configuring to the appropriate saccade task using fMRI of humans (De Souza et al. 2003) and neurophysiology in monkeys (Everling and De Souza 2005). Furthermore, there are no issues of overlapping stimulus response mappings, as on each trial the leftward saccade response is exclusively associated with one task, and the rightward response is exclusively associated with the other task.
In Experiment 1, we investigated how manipulating when the instruction change occurred affected when switch costs occurred. Importantly, we could classify the instruction change as before or after a response could be programmed. In Experiment 2, we investigated the effect of prior information suggesting whether the instruction would change or remain the same, in order to determine if predicting instruction change would result in switch costs if a response could be voluntarily programmed in advance of stimulus presentation. Finally, in Experiment 3 we examined the possibility that subjects could prepare a saccade response in advance of the stimulus, if they could predict where the stimulus would appear.
Our results suggest that the appropriate saccade response was automatically prepared based on the instruction (fixation point color) and stimulus position. Switching the motor program (either prosaccade or antisaccade), and not switching the rule alone, was the critical element in switch cost production.
Experiment 1: Effect of instruction change
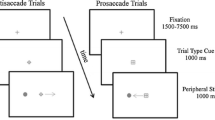
In Experiment 1 we established when a change of instruction during the trial produced switch costs. We varied the time at which the change in instruction could occur, henceforth known as ‘switch time,’ before stimulus onset, concurrently with stimulus onset, or after stimulus onset (Fig. 1). We also controlled for the fact that changes in fixation color or luminance alone could be responsible for switch costs, even if they did not convey instruction.
Parameters for experiment, showing timing of experimental events. Note that switch times are defined as when the second fixation point appeared relative to stimulus onset: a second fixation point appeared before stimulus onset (switch times of −800, −400, −200 ms); b second fixation point appeared at stimulus onset (switch time of 0 ms); c second fixation point appeared after stimulus onset (switch time of +200 ms) [red (dark gray circle) prosaccade, green (light gray circle) antisaccade condition]
Methods
Participants
All experimental procedures were reviewed and approved by the Queen’s University Human Research Ethics Board, and adhere to the Declaration of Helsinki. Twelve individuals with normal or corrected-to-normal vision were recruited from the Queen’s University population and provided their informed consent prior to participating. They were compensated for their participation ($10/h). Three of the participants were male, and the age range of the participants was 20–25.
Design and procedure
Horizontal eye position was monitored online with DC-electrooculography (EOG). To minimize DC drift the skin was cleaned with rubbing alcohol and the subjects wore the electrodes for approximately 5–10 min before the experiment began. Additional DC drift was corrected manually during the experiment. Stimulus presentation and monitoring of eye position were done using REX Version 5.4, sampling at 1,000 Hz (Hays et al. 1982). Prior to data collection the EOG signal was calibrated to 10° left, 10° right and 0°. Eye-movement and statistical analyses were conducted in MATLAB 7.0 (The Mathworks).
Subjects were seated 1 m from a tangent visual screen immediately behind an array of LED stimuli. A head rest was used to maintain head position. All of the experiments were conducted in the dark, however, the screen was diffusely illuminated for 600 ms between trials to prevent dark adaptation. The subjects performed a saccade task that required them to initiate a saccade to or away from a stimulus (red or green LED; dual red/green diode, red: 5.0 cd/m2, green: 15.0 cd/m2), that appeared 10° to the left or right of center. The basic parameters of the task remained the same across three separate days of the experiment, and are illustrated in Fig. 1. All 12 subjects participated in all 3 days, and performed either an interleaved pro-antisaccade task, or a blocked pro-antisaccade task depending on the day.
Interleaved pro-antisaccade task
Each trial began with the presentation of a fixation point at center (red or green LED, tri red/green/blue diode, red: 8.0 cd/m2, green: 3.0 cd/m2) (Fig. 1). Subjects were told that one fixation color dictated to perform a prosaccade (look towards) to the 10° stimulus, and the other color dictated to perform an antisaccade (look away) from the stimulus. Instruction color was counterbalanced between subjects, but for each subject the 10° stimulus was always the identical color as the prosaccade instruction color at fixation. The stimulus was presented pseudoramdomly to the left or right 1,300 ms after the subjects fixated the central fixation point. At five variable times relative to stimulus appearance (‘switch times’), the central fixation point was extinguished for 100 ms, and then reappeared as either the same or opposite color. About 50% of the trials incorporated this switching of fixation color. The onset of this second fixation point was −800, −400, −200, 0, 200 ms with respect to stimulus appearance, corresponding to the five different switch times in the experiment (recall that on only half the trials did the instruction actually switch). Early switch times were included to be sure we allowed sufficient time for a rule to be changed in advance of stimulus presentation (e.g., −800 ms). Note that on the 0 and +200 ms switch time trials the second fixation point appeared concurrently with (0) or after (+200) stimulus appearance. Therefore, subjects were told to obey the second instruction, and be sure to wait for its appearance before initiating the appropriate response. The 100 ms gap in fixation allowed us to compare trials in which the second fixation point was the same (‘nonswitch’) to those in which it switched (‘switch’), at each of the five switch times. The 100 ms gap also made sure that both switch and nonswitch trials had a change in stimulation at fixation.
Subjects were required to perform 200 correct trials per block, and completed 3 blocks in total. Before completing the 600 correct trials, subjects were given a practice block that consisted of nonswitch trials only of interleaved pro-antisaccades. Subjects were required to complete 100 correct practice trials.
Blocked pro-antisaccade task
The blocked pro-antisaccade task was conducted to control for the fact that switch costs were not driven only by a change in fixation color, but required a change in task instruction. Subjects performed blocks of prosaccades and antisaccades using the same parameters as in the interleaved pro-antisaccade task, but they did not have to use the instruction given by the fixation color, since they knew which task to perform for the entire block. The second fixation color always corresponded with a pro or antisaccade in each block, even though 50% of the trials were switch trials. Subjects were informed that the first fixation point may be a different color from the second, however, they were to always execute prosaccades or antisaccades depending on the block. Subjects performed 2 blocks of 150 pro trials, and 2 blocks of 150 anti trials. Pro or anti-block order was counterbalanced across subjects. Subjects were instructed to perform the desired saccade, making sure to wait until the second fixation light had appeared.
Order of tasks
All 12 subjects participated in the 3 sessions on 3 separate days, and performed the pro-antisaccade tasks in the following order: blocked pro-antisaccade task (Day 1), interleaved pro-antisaccade task (Day 2), blocked pro-antisaccade task (Day 3).
Analysis
Failure to fixate the first fixation point within 5 s, failure to maintain fixation, failure to initiate a saccade, and failure to fixate the saccade target for at least 160 ms were recorded as ‘rejection errors’ and removed from analysis. Responses were analyzed such that pro and antisaccade trials refer to the saccade dictated by the second fixation color. For example, prosaccade switch trials are those in which the first fixation color indicated an antisaccade, and the second fixation color indicated a prosaccade. Prosaccade nonswitch trials are those in which both the first and second fixation color indicated to make a prosaccade. Response time was defined as the time from when the stimulus appeared to when the first saccade away from fixation exceeded 30°/s. This meant that response times at the +200 ms switch time included a delay of 200 ms while the subjects waited to receive the second fixation point.
The errors of primary interest were those in which subjects executed the wrong saccade based on the second instruction (a prosaccade on anti instruction and vice versa), and errors in which the subject anticipated the response (executed a saccade on +200 ms trials before or within 70 ms of the onset of the second fixation instruction). These errors were labeled as ‘direction’ and ‘anticipatory errors’, respectively, and were analyzed separately. The percentage of direction and anticipatory errors were calculated by dividing the errors by the total number of valid trials (correct trials + direction error trials + anticipatory error trials).
Switch costs and switch benefits (for response time and direction errors) at each switch time were calculated by subtracting the mean of nonswitch trials from the mean of switch trials of the twelve subjects (see Supplementary Table 1). A positive value indicated a switch cost, and a negative value indicated a ‘switch benefit’. A priori, we wished to examine when switch costs were found across the switch times. T-tests were used for response time and Wilcoxon Signed Rank tests were used for direction errors. We also examined how switch costs depended on Day (blocked or interleaved tasks). Therefore, one way ANOVAs were performed at each switch time to compare how switch costs were affected by task (e.g. blocked or interleaved). For direction errors, we used the simple subtraction as an index of switch costs. For response time, however, we used a normalized index in the following way:
This index incorporates variability in response times, in addition to mean response times (Prince et al. 2002). We did not assume the variability in response time would be the same at each switch time or the same in the blocked and interleaved tasks. If the difference of the means is large and the variance is small, this index is close to ±1 depending on which mean is larger. However, if the variance is large, the switch cost indices are smaller.
A repeated-measures ANOVA across the switch times was not conducted, as the 200 ms delay would alter the statistical tests, and we were not interested in such interactions.
Results
Supplementary Table 1a–c shows when significant switch costs and switch benefits developed for Days 1, 2 and 3, respectively. Corresponding statistical tests are shown.
Response time
Large switch costs of 94 ms for prosaccades were found in the interleaved task (Day 2) at the +200 ms switch time (Fig. 2a, top-middle panel). Similarly, large switch costs of 88 ms for antisaccades were found at +200 ms. Switch costs were not found before stimulus onset in either the blocked or interleaved tasks (P > 0.14), however, switch benefits did occur at the −800 and −400 ms switch times (Fig. 2). There was an effect of whether subjects performed the blocked task or the interleaved task, such that switch costs were largest in the interleaved task at the +200 ms switch time (Fig. 2b). One-way ANOVAs demonstrated that these switch costs were significantly larger in the interleaved task than in the blocked task [prosaccades, F(1,2) = 6.00, P < 0.01; antisaccades, F(1,2) = 5.25, P < 0.05] (Fig. 2b). Paired t-tests revealed that switch costs did not differ between pro and antisaccades at any switch time in either the blocked or interleaved tasks (P > 0.07). To summarize, response time switch costs did not develop before stimulus onset, however, some significant switch benefits were found. Switch costs were significantly larger in the interleaved task (Day 2).
a Response times (mean ± SE) relative to stimulus onset for pro and antisaccade switch trials (solid traces) and for nonswitch trials (dotted traces) in Experiment 1. Left panels show response times across the five switch times for pro and antisaccades in the blocked pro-antisaccade task on Day 1. Center panels show response times in the interleaved pro-antisaccade task on Day 2. Right panels show response times in the blocked pro-antisaccade task on Day 3. b Comparison of response time switch cost indices (see “Methods”) between switch and nonswitch trials across Day 1, Day 2 and Day 3. Positive index values indicate switch costs
Direction and anticipatory errors
Direction error rates followed the same trends as response times. Switch costs occurred at the 0 ms and +200 ms switch times on Day 2 (Fig. 3a, b), and did not occur at pre-stimulus switch times. Switch costs were significantly larger in the interleaved task (Day 2) (P < 0.01) (Fig. 3b). Significant switch benefits were found at the −400 ms switch time, P < 0.01 (Fig. 3a, bottom-middle panel). This resulted in a marginally significant difference of switch benefits between pro and antisaccades at this switch time only (P < 0.05). No other switch costs or benefits were significant at any other switch time for either the blocked, P > 0.09, or interleaved task, P > 0.075.
a Percent direction errors (mean ± SE) for pro and antisaccade switch trials (solid traces) and for nonswitch trials (dotted traces) in Experiment 1. Left panels show direction errors across the five switch times for pro and antisaccades in the blocked pro-antisaccade task on Day 1. Center and right panels show direction errors for the interleaved task on Day 2, and blocked task on Day 3, respectively. b Comparison of direction error switch costs across the five switch times for Days 1, 2 and 3
Post-hoc analysis showed that subjects made significantly more errors altogether on prosaccade trials (switch + nonswitch) in the interleaved task than in the blocked task (t-test, P < 0.01). There was a significant difference in overall antisaccade direction errors in comparison to prosaccades at the +200 ms switch time in the interleaved task (P < 0.05). There was no significant differences at any other switch time (P > 0.07). There was not a significant difference between antisaccade direction errors across the 3 days, except at +200 ms (P < 0.01).
In the blocked tasks subjects made significantly more anticipatory errors, accounting for the low percentage of direction errors at the +200 ms switch time on Days 1 and 3. We subdivided these anticipatory errors into “anticipatory congruent” and “anticipatory incongruent” with reference to the first instruction (Fig. 4). Anticipatory congruent errors refer to those that were in the correct direction (towards or away from the stimulus) associated with the color of the first instruction. Incongruent refer to those errors that were in the direction against what was associated with the first instruction. For the blocked tasks, subjects consistently made significantly more anticipatory congruent errors on nonswitch trials than on switch trials (P < 0.05) (Fig. 4, left and right panels). In contrast, subjects made significantly more anticipatory incongruent errors on switch trials than on nonswitch trials (P < 0.05) (Fig. 4, left and right panels). In all cases, there was a significantly greater percentage of incongruent errors than congruent errors on switch trials (P < 0.05). In addition, there was a greater percentage of congruent errors than incongruent errors on nonswitch trials (P = 0.05). In the interleaved task, anticipatory errors were less than 12% (Fig. 4, middle panels). There was a significant difference of more anticipatory congruent errors for nonswitch prosaccades than switch prosaccades (P < 0.05). For the nonswitch prosaccades, anticipatory congruent errors were greater than incongruent (P < 0.05). To summarize, anticipatory errors were high in the blocked task, such that on switch trials subjects made mostly anticipatory incongruent errors, where as on nonswitch trials subjects made mostly anticipatory congruent errors with respect to the first instruction. This is consistent with the requirement to perform only pro or antisaccades depending on the block.
Percent anticipatory errors (mean ± SE) at the +200 ms switch time for pro and antisaccade switch trials (dark grey) and for nonswitch trials (light grey) in Experiment 1. Anticipatory congruent anticipatory errors are those that are associated with the response specified by the first fixation color and stimulus. Anticipatory incongruent anticipatory errors are those that are against the direction associated with the first fixation color and stimulus. Left panels show anticipatory errors in the blocked pro-antisaccade task on Day 1. Center and right panels show anticipatory errors for the interleaved task on Day 2, and blocked task on Day 3, respectively
Discussion
Switch benefits
Recent studies using interleaved pro and antisaccades have revealed response time switch benefits for antisaccades (Barton et al. 2006; Cherkasova et al. 2002; Fecteau et al. 2004; Manoach et al. 2002). Barton et al. (2006) suggested that the origin of these switch benefits results from persisting response-system inhibition from the previous antisaccade which required inhibiting a reflexive saccade to the stimulus. Antisaccades that follow prosaccades (switching task) do not have to overcome this inhibition (Barton et al. 2006). We observed switch costs and switch benefits regardless of pro or antisaccade (see Figs. 2, 3), suggesting that inhibition related to one task alone cannot explain our findings. We believe that in our task, subjects were volitionally controlling both pro and antisaccades, based on the difficulty of the interleaved task. We found that prosaccade response times were slower in comparison with those found in basic reflexive-prosaccade tasks (Fischer and Weber 1992, 1997; Munoz et al. 1998). Increased latency in both pro and antisaccades has also been found in other saccade studies of countermanding, dual-task performance and task-switching (Hunt and Klein 2002; Kristjansson et al. 2001; Matthews et al. 2002). As shown in Fig. 2, prosaccade response times were consistently faster in the blocked tasks than in the interleaved tasks (P < 0.05).
We cannot explain what is driving the switch benefits from this experiment. One possibility is that while we controlled for a temporal warning effect with the 100 ms gap, the saliency of a change of instruction prior to stimulus appearance may have had some additional effect on increasing the readiness to respond. This could lead to subjects executing the response faster on trials in which the instruction switched early in the trial.
Switch costs
We controlled for the fact that switch costs might be mediated by the changing color or luminance at fixation. There were small response time switch costs in the blocked tasks on Day 1 and 3, however, these costs were much smaller than on Day 2 and were not present in direction errors (Figs. 2, 3). Secondly, anticipatory errors suggest that subjects were not influenced by the first instruction in the blocked task (Fig. 4). If a high proportion of anticipatory congruent errors had resulted for either switch or nonswitch trials, this would suggest that the subjects had generated a response based on the color of the first fixation point, rather than simply failing to delay responding. This indicates that color change alone did not change task set.
The results from the interleaved task (Day 2) suggest that: (i) physical information is strong enough to drive switch costs, and (ii) switch costs do not result before the stimulus is present. Since the switch-trial probability was 50%, the first instruction carried no response-related information, so subjects had to rely on the instruction carried by the second fixation color to execute the correct response. Importantly, switch costs were still found for both prosaccades and antisaccades after stimulus onset. This suggests that the first instruction automatically induced a corresponding task set. The motivation of Experiment 2 was to examine the role of voluntarily changing task set. If switching of instruction could be predicted, switch costs might result at the negative switch times because subjects could use this information to change task set in advance of the stimulus. If this occurred, it would also demonstrate that the instruction induced a task set, before the stimulus was present.
Experiment 2: Effect of switch trial probability
In Experiment 1, switch costs resulted despite the subjects having no information of whether the instruction would change or remain the same. In Experiment 2 we manipulated the probability that the current trial would include a change in instruction. Previous studies have demonstrated that switch costs are reduced if one is able to predict that a switch will occur (Monsell 1996; Rogers and Monsell 1995). We predicted that switch costs might decrease, or reverse to switch benefits, if the subjects expected a switch of instruction, and therefore could switch task set. For example, switch benefits at +200 ms might imply that the subjects configured a task set against the first instruction, using the information that the instruction would likely switch.
Methods
Participants
Twelve different participants performed the interleaved pro-antisaccade task, in which the probability that the first fixation color would switch was varied. Two of the participants (IC and MW) were co-authors on the paper. Eight were male, and the age range was 20–28.
Design and procedure
Three sessions of the task were run, such that switch-trial probability in each session was either 25, 50 or 75%. Subjects performed the experiment across three separate days, and the order in which each subject received the three versions was counterbalanced. Subjects were not informed of the switching probability by the experimenter.
The experimental conditions were identical to those of Experiment 1, however, nine of the subjects performed the task in a different laboratory, where they were seated 80 cm from the screen. For these subjects, stimulus eccentricity was 15°. The performance of the nine subjects in the second laboratory was compared to the performance of three subjects in the first laboratory (1 m from screen, 10° targets) and did not show behavioral differences. To control for any differences between the laboratories, experimental conditions and subjects, we replicated the 50% interleaved pro-antisaccade task from Experiment 1 in addition to exploring the effects at 25 and 75% switch probability. Subjects were required to perform 200 correct trials per block, and completed 3 blocks in total as in Experiment 1. About 100 correct practice trials (interleaved pro-antisaccades of nonswitch trials only) were given beforehand.
Results
Supplementary Table 2 shows significant switch costs, switch benefits and statistical tests for Experiment 2.
Response time
As shown in Fig. 5a, b, increasing the switch probability from 25% to 50% and to 75% meant that switch costs decreased at the post-stimulus switch time. Large response time switch costs were always found at the +200 ms switch time (P < 0.01), however, as the switch probability increased to 75%, switch costs decreased, F(1,2) = 7.69, P < 0.01. For antisaccades, switch costs were also largest in the 25% condition at 0 ms, F(1,2) = 11.31, P < 0.001, and +200 ms, F(1,2) = 8.34, P < 0.01 (Fig. 5b). Switch costs were also consistently found at the 0 ms switch time in the 50 and 25% switch probability conditions, P < 0.01. However, switch costs at the 0 ms switch time were less than the switch costs found at the +200 ms switch time.
a Response times (mean ± SE) relative to stimulus onset for pro and antisaccade switch trials (solid traces) and for nonswitch trials (dotted traces) in Experiment 2. Left panels show response times across the five switch times for pro and antisaccades in the interleaved pro-antisaccade task where 25% of the trials were switch trials. Center panels show response times where 50% of the trials were switch trials. Right panels show response times where 75% were switch trials. b Comparison of response time switch costs indices across the three switch trial probabilities
In the 25% switch probability condition, small switch costs of 17 ms were found before stimulus onset at −200 ms for prosaccades (Fig. 5a, top-left panel). This was the only instance in which switch costs were found before stimulus onset.
Switch benefits were found at the −800 ms switch time for both prosaccades and antisaccades in all three probability conditions (P < 0.05). Switch benefits were also found at the −400 ms switch time in the 50% switch probability (Fig. 5a, middle panels), and at −200 ms for antisaccades at 75% switch probability (Fig. 5a, bottom-right panel). Response time switch benefits were not significantly modulated by switch probability (P > 0.08).
Switch costs were marginally higher for prosaccades in the 25% switch probability condition at the −200 ms switch time (t-test, P < 0.05). In the 50% switch probability condition, switch costs were significantly higher for prosaccades at the +200 ms switch time (P < 0.01). Similarly in the 75% condition, switch costs were significantly higher for prosaccades at 0 ms, P < 0.001, and at +200 ms, P < 0.01.
Direction errors
Figure 6 shows how the occurrence of direction errors changed systematically across the three switch trial probabilities. Significant switch costs were consistently found at +200 ms in all conditions (P < 0.05) (Fig. 6a), but reduced as the probability of switching instruction increased (Fig. 6). This resulted in switch costs being significantly largest in the 25% condition, and smallest in the 75% condition for antisaccades at +200 ms, F(1,2) = 3.58, P < 0.5 (Fig. 6b). Switch benefits for direction errors never occurred, P > 0.21.
a Percent direction errors (mean ± SE) for pro and antisaccade switch trials (solid traces) and for nonswitch trials (dotted traces) in Experiment 2. Left panels show direction errors across the five switch times for pro and antisaccades where 25% of the trials were switch trials. Center and right panels show direction errors for the 50 and 75% switch trial conditions, respectively. b Comparison of direction error switch costs across the three switch trial probabilities
Subjects made at most 3.8% anticipatory errors in Experiment 2, and thus will not be described further (t-test, P > 0.13). There was no significant difference in error rate switch costs between pro and antisaccades, however, there were marginally greater switch costs for antisaccades at the 0 ms switch time in the 25% condition (t-test, P < 0.05).
Discussion
Significant switch costs were consistently found at the +200 ms switch time regardless of switch trial probability. Information supporting switching could not eliminate switch costs. Therefore, while switch costs were affected by prior information, a component of task set appears to be triggered automatically by the color at fixation. We speculate that the first instruction biased the system to automatically program the corresponding response when the stimulus appeared. This occurred despite information suggesting the instruction would switch to the opposite task. Therefore, switch costs may result from the switching of a response program, automatically prepared at stimulus onset. Information related to switch probability modulated switch costs, suggesting that there is a second component of task set that was volitionally controlled. As there were small response time switch costs that appeared before stimulus onset (−200 ms, prosaccades, 25% switch probability) this suggests that the subjects were able to adopt a task set (internalized rule) based on the first instruction before stimulus onset.
Experiment 3: Effect of stimulus location probability
If switch costs result from the switching of a programmed motor response, switch costs also may result if the stimulus itself changes a motor program. Previous evidence has demonstrated that stimulus predictability can reduce pro and antisaccade latencies toward, or away from, the predicted stimulus location (Carpenter and Williams 1995; Dorris and Munoz 1998; Koval et al. 2004). Therefore, it is possible that a saccade response could be prepared to a predictable stimulus location. Switch costs might result before stimulus onset if the change in instruction changes a programmed response to this location. Alternatively, prior information relating to the stimulus location may not be able to change switch costs, if the instruction at fixation and stimulus have the strongest effect of triggering a response process.
Methods
Participants
Twelve different participants performed Experiment 3. Three were male and the age range was 19–28. The laboratory was identical to the one used in Experiment 1 for all subjects.
Design and procedure
Two sessions were run and the order was counterbalanced for the twelve subjects. In one session the stimulus appeared at the left and right location with equal probability. In the other session, the stimulus either appeared 75% on the left and 25% on the right for six subjects, or 25% on the left and 75% on the right for the other six subjects. Therefore, for each subject we had trials where the stimulus appeared at a low probability location (25%) and trials where the stimulus appeared at a high probability location (75%). These low and high probability locations were then compared to the second session of 50% stimulus probability, identical to Experiment 1 and Experiment 2. All other aspects of the analysis were identical to Experiment 1, and the probability that the instruction would switch was 50%. Subjects were not informed of the instruction switching or stimulus location probability.
The experimental conditions were identical to those of Experiments 1 and 2. Subjects were required to perform 200 correct trials per block, and completed 3 blocks in total. Practice trials were given as in Experiments 1 and 2.
Results
Supplementary Table 3 shows statistically significant switch costs and benefits for Experiment 3.
Response time
The manipulation of stimulus location probability did not significantly affect the magnitude of switch costs (Fig. 7b), F(1,2) < 2.28, P > 0.11, or when switch costs developed with respect to stimulus onset (Fig. 7a). As shown in Fig. 7a, response time switch costs and benefits followed similar trends to the 50% switch trial probability condition in Experiments 1 and 2. For instance, switch costs were consistently found at the +200 ms switch time (P < 0.01). Significant switch benefits occurred at the −800 and −400 ms switch times, but not at other switch times (Fig. 7a)
a Response times (mean ± SE) relative to stimulus onset for pro and antisaccade switch trials (solid traces) and for nonswitch trials (dotted traces) in Experiment 3. Left panels show response times for pro and antisaccades at the low stimulus probability location (25%). Center panels show response times when the stimulus location was 50% right and 50% left. Right panels show response times at the high stimulus probability location (75%). b Comparison of response time switch cost indices across the three stimulus probability locations
Switch costs at the +200 ms switch time for prosaccades were significantly greater than those for antisaccades at the high stimulus probability location (P < 0.05). The difference in switch costs at the 0 ms switch time between pro and antisaccades for the low stimulus probability location was also significant (P < 0.05).
Direction errors
As in response time analysis, there were no significant differences in switch costs or benefits between the three probability conditions F(1,2) < 1.62, P > 0.21 (Fig. 8b). Significant switch costs were observed at the +200 ms location in the 50% stimulus probability condition for antisaccades (Fig. 8a, bottom-middle panel), and this switch cost for antisaccades was significantly greater than for prosaccades (P < 0.01) at +200 ms. Significant switch costs were also observed at +200 ms in the 75% stimulus probability location for prosaccades and antisaccades (P < 0.05).
a Percent direction errors (mean ± SE) for pro and antisaccade switch trials (solid traces) and for nonswitch trials (dotted traces) in Experiment 3. Left panels show direction errors across the five switch times for pro and antisaccades where there was a low probability (25%) of the stimulus appearing at that location. Center panels and right panels show direction errors for the 50% and high (75%) stimulus probability locations. b Comparison of direction error switch costs across the three stimulus location probabilities
Subjects made altogether more errors in the 25% stimulus probability location for antisaccades than they did for prosaccades. These are errors in which the subjects looked to the stimulus (a prosaccade) which appeared in the low probability location. This was significant across all switch times except at +200 ms (P < 0.01). These errors were not significantly different across the probability locations, P > 0.09, other than at −800 ms, P < 0.01. Response times for these errors were greater than 210 ms in all instances.
Discussion
We predicted that if subjects had prior information suggesting that the stimulus will appear at one location, they may be able to use this information to program a saccade in advance of the stimulus. Therefore, switch costs might result before stimulus onset if the change in instruction changed a programmed saccade. The trend of antisaccade error rates across the probability locations suggests that subjects at least learned the location probability, and this affected their performance. However, the pattern of switch costs was identical to Experiments 1 and 2 (50% switch probability). This suggests that stimulus predictability was not enough to drive switch costs, and the main effect was from the change of instruction after the stimulus and first instruction specified a response.
General discussion
We identified in Experiment 1 that switching a response program driven by physical information is a fundamental property of task switching performance. Experiment 2 demonstrated that prior information about an instruction switching can influence switch costs, but switch costs still result when a specified response changes. Experiment 3 verified that switch costs are related to switching a physically specified response, and not due to response preparation based on probable stimulus location. We believe that a saccade response was automatically programmed based on a combination of physically available information of fixation point color and stimulus location. Switch costs result when the response is changed.
Accumulator model
A useful method to illustrate saccade response programming is with an ‘accumulator model.’ An accumulator model considers a response program as a neural signal that begins at a baseline of activity, rises toward a threshold level of activity, and triggers a response. The baseline level of activity and the rate of rise to threshold can influence when the response is executed, and whether this response can be inhibited by competing programs (Carpenter 1981; Carpenter and Williams 1995; Hanes and Carpenter 1999; Hanes and Schall 1996; Logan et al. 1984; Sinha et al. 2006). These principles have been validated in neurophysiological recordings of saccade-related neurons in the frontal eye-fields (FEF) and superior colliculus (SC) (Dorris and Munoz 1998; Hanes and Schall 1996; Munoz and Schall 2004; Paré and Hanes 2003) two critical areas for saccade generation (Schiller et al. 1980).
In the current experiment, response time switch costs can be modeled as saccade response signals that cross threshold later on switch trials than on nonswitch trials (Fig. 9a). We suggest that when the stimulus appears after the first instruction, a neural response signal (pro or antisaccade) is automatically initiated from baseline activity (following a delay in sensory to motor processing). On switch trials, the change in instruction requires the alternate saccade response signal to be initiated. Thus, switching the instruction after stimulus presentation requires reprogramming a new response and suppressing the old response. This is not required on nonswitch trials, resulting in nonswitch trial signals triggering a response earlier in time than switch trial signals. On a switch trial, if the saccade signal related to the original instruction is not inhibited and crosses the threshold, a direction error is triggered corresponding to an error rate switch cost.
Switch-cost model. a Instruction switch after stimulus onset (+200 ms switch time). A response is automatically programmed based on the 1st instruction and stimulus position. This signal begins at baseline and rises towards threshold (dotted line). On switch trials, the second instruction is different than the first instruction meaning that a new response sig nal must be initiated from baseline (solid line). This results in response signals on switch trials crossing threshold later in time, corresponding to switch costs. b Effect of probability. Information relating to the probability that the given instruction will switch can influence the rate of rise of activity on nonswitch trials. This results in nonswitch signals crossing threshold ahead of switch trial signals, but at different times depending on the level of activity upon presentation of the second instruction
On nonswitch trials, response signals that are initiated before the second instruction result in ‘correct’ trials provided the saccades are executed 70 ms after the second instruction (anticipatory if they cross before this time). If the saccade response is initiated to the first instruction, this would reduce the mean response time on correct nonswitch trials, but would reduce the mean response time of direction errors on switch trials. To examine this possibility behaviorally, we analyzed the response times of direction errors in the interleaved tasks at the +200 ms switch time. The mean response time for direction errors on antisaccade switch trials was significantly shorter than on antisaccade nonswitch trials in Experiments 1 (Day 2), Experiment 2, and Experiment 3 at the 50% stimulus location probability (P < 0.05). This trend was similar for prosaccades, however, it was only significant in Experiment 2. (Recall that the percentage of direction errors was consistently below 5% for nonswitch prosaccade trials, resulting in highly variable and sparse data for analysis). This supports the proposal that response signals initiated to the first instruction that cross threshold result in short latency correct nonswitch trials, but switch trial direction errors.
We propose that information related to the probability that the instruction will switch can affect the rate of rise of response signals initiated upon stimulus onset. This corresponds to a voluntary component of task set. Upon presentation of the second instruction there would be a difference in the level of activity depending on switch probability (Fig. 9b). It has been shown when the ‘go’ signal to execute a saccade is presented, the initial level of activity in the FEF and SC negatively correlates with saccade response times (Dorris et al. 1997; Dorris and Munoz 1998; Everling and Munoz 2000). It has also been shown that pre-stimulus activity in some FEF and SC saccade neurons can be biased by saccadic probability (Dorris and Munoz 1998). Therefore, when the probability of switching is low (25%), response related activity may increase to a higher level before the second instruction is delivered (Fig. 9b), resulting in increased switch costs. The small response time switch costs that occurred at -200 ms in the 25% probability condition suggest the possibility that at stimulus onset, another signal, related to the first rule, might be at a higher level than the signal related to the second rule. Therefore, if activity related to the representation of the first rule is still higher upon stimulus presentation, switch costs may result as the response signal on nonswitch would be initially closer to threshold. Indeed, Sinha et al. (2006) proposed a two-stage model of task-switching, such that recognizing an instruction to switch was a rise-to-threshold process that in turn dictated when the saccade response signal commences. We cannot discount other explanations, however, such as: reconfiguration to the new instruction takes longer than 200 ms and therefore is reconfigured after stimulus presentation on switch trials. This reconfiguration time might vary with switch probability, accounting for why this occurred only in the 25% switch condition (Monsell 1996; Rogers and Monsell 1995).
Switch benefits
Unlike switch costs, switch benefits were not influenced by instruction probability, and therefore do not reflect a change in task set. They may result from an altering effect that might alter the baseline activity or rate of rise to threshold, resulting in the response signals triggering a saccade earlier on switch trials.
Conclusions
We propose that switch costs result from the switching of a response program, defined by a combination of physically available information of stimulus position plus the presented instruction. Voluntary signals related to instruction probability can modulate switch costs; however, switch costs are still driven by the physical information. Switch costs do not result from switching an instruction alone. This suggests that we have the flexibility to change task set related to rule representation, however, we are impaired at switching task if a response processes is engaged.
References
Allport A, Styles EA, Hsieh S (1994) Shifting intentional set: exploring the dynamic control of tasks. In: Umilta C, Moscovitch M (eds) Attention and performance XV. MIT Press, Cambridge, pp 421–452
Barton JJ, Greenzang C, Hefter R, Edelman J, Manoach DS (2006) Switching, plasticity, and prediction in a saccadic task-switch paradigm. Exp Brain Res 68:76–87
Brass M, Von Cramon DY (2002) The role of the frontal cortex in task preparation. Cereb Cortex 12:908–914
Brass M, Von Cramon DY (2004) Decomposing components of task preparation with functional magnetic resonance imaging. J Cogn Neurosci 16:609–620
Carpenter RH (1981) Oculomotor procrastination. In: Fisher DF, Monty RA, Senders JW (eds) Eye movements: cognition and visual perception. Erlbaum, Hillsdale, pp 237–246
Carpenter RH, Williams ML (1995) Neural computation of log likelihood in control of saccadic eye movements. Nature 377:59–62
Cherkasova MV, Manoach DS, Intriligator JM, Barton JJ (2002) Antisaccades and task-switching: interactions in controlled processing. Exp Brain Res 144:528–537
De Jong R (2000) An intention-activation account of residual switch costs. In: Monsell S, Driver J (eds) Control of cognitive processes: attention and performance XVIII. MIT Press, Cambridge, pp 357–376
De Souza JF, Menon RS, Everling S (2003) Preparatory set associated with pro-saccades and anti-saccades in humans investigated with event-related FMRI. J Neurophysiol 89:1016–1023
Dorris MC, Munoz DP (1998) Saccadic probability influences motor preparation signals and time to saccadic initiation. J Neurosci 18:7015–7026
Dorris MC, Paré M, Munoz DP (1997) Neuronal activity in monkey superior colliculus related to the initiation of saccadic eye movements. J Neurosci 17:8566–8579
Everling S, Munoz DP (2000) Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. J Neurosci 20:387–400
Everling S, De Souza JF (2005) Rule-dependent activity for prosaccades and antisaccades in the primate prefrontal cortex. J Cogn Neurosci 17:1483–1496
Fecteau JH, Munoz DP (2003) Exploring the consequences of the previous trial. Nat Rev Neurosci 4:1–9
Fecteau JH, Au C, Armstrong IT, Munoz DP (2004) Sensory biases produce alternation advantage found in sequential saccadic eye movement tasks. Exp Brain Res 159:84–91
Fischer B, Weber H (1992) Characteristics of “anti” saccades in man. Exp Brain Res 89:415–424
Fischer B, Weber H (1997) Effects of stimulus conditions on the performance of antisaccades in man. Exp Brain Res 116:191–200
Hanes DP, Carpenter RH (1999) Countermanding saccades in humans. Vision Res 39:2777–2791
Hanes DP, Schall JD (1996) Neural control of voluntary movement initiation. Science 274:427–430
Hays AV, Richmond RJ, Optician LM (1982) A UNIX-based multiple process system for real-time data acquisition and control. WESCON Conf Proc 2:1–10
Hunt AR, Klein RM (2002) Eliminating the cost of task set reconfiguration. Mem Cognit 30:529–539
Jersild AT (1927) Mental set and shift. Archives of psychology, Whole No. 89
Koch I, Allport A (2006) Cue-based preparation and stimulus-based priming of tasks in task switching. Mem Cognit 34:433–444
Koval MJ, Ford KA, Everling S (2004) Effect of stimulus probability on anti-saccade error rates. Exp Brain Res 159:268–272
Kristjansson A, Chen Y, Nakayama K (2001) Less attention is more in the preparation of antisaccades, but not prosaccades. Nat Neurosci 4:1037–1042
Logan GD, Cowan WB, Davis KA (1984) On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform 10:276–291
Manoach DS, Lindgren KA, Cherkasova MV, Goff DC, Halpern EF, Intriligator J, Barton JJ (2002) Schizophrenic subjects show deficient inhibition but intact task switching on saccadic tasks. Biol Psychiatry 51:816–826
Matthews A, Flohr H, Everling S (2002) Cortical activation associated with midtrial change of instruction in a saccade task. Exp Brain Res 143:488–498
Meiran N (2000) Modeling cognitive control in task-switching. Psychol Res 63:234–249
Miller EK, Cohen JD (2001) An integrative theory of prefrontal cortex function. Ann Rev Neurosci 24:167–202
Milner B (1963) Effects of different brain lesions on card sorting. Arch. Neurol 9:90
Monchi O, Petrides M, Petre V, Worsley K, Dagher A (2001) Wisconsin card sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. J Neurosci 21:7733–7741
Monchi O, Petrides M, Doyon J, Postuma RB, Worsley K, Dagher A (2004) Neural bases of set-shifting deficits in Parkinson’s disease. J Neurosci 24:702–710
Monsell S (1996) Control of mental process. In: Hove BV (ed) Unsolved mysteries of the mind. Erlbaum, UK, pp 93–148
Monsell S (2003) Task switching. Trends Cogn Sci 7:134–140
Munoz DP, Everling S (2004) Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci 5:218–228
Munoz DP, Schall JD (2004) Concurrent, distributed control of saccade initiation in the frontal eye field and superior colliculus. In: Hall WC, Moscovakis A (eds) The superior colliculus: new approaches for studying sensorimotor integration. CRC Press, Boca Raton, pp 55–82
Munoz DP, Broughton JR, Goldring JE, Armstrong IT (1998) Age-related performance of human subjects on saccadic eye movement tasks. Exp Brain Res 121:391–400
Paré M, Hanes DP (2003) Controlled movement processing: superior colliculus activity associated with countermanding saccades. J. Neurosci 23:6480–6489
Prince SJD, Pointon AD, Cumming BG, Parker AJ (2002) Quantitative analysis of the responses of V1 neurons to horizontal disparity in dynamic redom-dot stereograms. J Neurophysiol 87:191–208
Rogers RD, Monsell S (1995) Costs of a predictable switch between simple cognitive tasks. J Exp Psychol Gen 124:207–231
Schiller PH, True SD, Conway JL (1980) Deficits in eye movements following frontal eye-field and superior colliculus ablations. J Neurophysiol 44:1175–1189
Schuch S, Koch I (2003) The role of response selection for inhibition of task sets in task shifting. J Exp Psychol Hum Percept Perform 29:92–105
Sinha N, Brown JT, Carpenter RH (2006) Task switching as a two-stage decision process. J Neurophysiol 95:3146–3153
Spector A, Biederman I (1976) Mental set and mental shift revisited. Am J Psychol 89:669–670
Wylie GR, Javitt DC, Foxe JJ (2004) The role of response requirements in task switching: dissolving the residue. Neuroreport 15:1079–1087
Yeung N, Nystrom LE, Aronson JA, Cohen JD (2006) Between-task competition and cognitive control in task switching. J Neurosci 26:1429–1438
Acknowledgments
These studies were funded by the Canadian Institutes of Health Research, the Canada Research Chair Program, and the Ontario Graduate Scholarship program. We wish to thank N. Alahyane, I. Armstrong, D. Brien, B. Coe, C. Green, R. Hakvoort-Schwerdtfeger, R. Marino, A. Peltsch and B. White for comments on early versions of the manuscript. We also wish to thank two anonymous reviewers for their suggestions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cameron, I.G.M., Watanabe, M. & Munoz, D.P. Contrasting instruction change with response change in task switching. Exp Brain Res 182, 233–248 (2007). https://doi.org/10.1007/s00221-007-0983-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-007-0983-3