Abstract.
Subjects sometimes fail to suppress a reflexive saccade towards the flashed stimulus in an anti-saccade task. Here, we studied how error rates in the anti-saccade task varied as a function of saccadic probability. Ten subjects performed 200 anti-saccade trials for each of three saccade-direction probability conditions (20%, 50%, and 80%). We found that as the likelihood of a saccade in a given direction increased, the percentage of pro-saccade errors also increased for stimulus presentations in this direction. These results provide support for the hypothesis that errors in the anti-saccade task are the result of an increased level of motor preparation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ability to suppress reflexive responses in favour of voluntary behaviour is a hallmark of executive control. It is often tested by the anti-saccade task which requires subjects to look away from a suddenly appearing peripheral stimulus and towards its mirror position in the opposite visual field (Hallett 1978; Hallett and Adams 1980; Munoz and Everling 2004a). Successful performance of this task requires suppression of the visual grasp reflex, an orienting eye movement towards a flashed visual stimulus in the periphery (Hess et al. 1946). Errors in the anti-saccade task occur more frequently when the initial fixation stimulus is removed 200 ms (gap period) prior to the presentation of the peripheral stimulus (Forbes and Klein 1996; Fischer and Weber 1997).
The superior colliculus (SC) and frontal eye fields (FEF) contain discrete populations of fixation and saccade neurons (Munoz and Everling 2004b) that modulate their activity during the gap period. Fixation neurons are tonically active during visual fixation, decrease their activity during the gap period, and pause for saccades (Dorris and Munoz 1995; Everling et al. 1998b). Reciprocally, saccade neurons are inactive during fixation, display a low-frequency increase in activity during the gap period (Dorris et al. 1997), and burst for saccades into their response field. Both types of neurons are thought to be responsible for reflexive short-latency saccades, as predicted by the fixation disengagement (Reuter-Lorenz et al. 1991; Sommer 1994; Dorris and Munoz 1995) and oculomotor preparation (Pare and Munoz 1996; Dorris et al. 1997) hypotheses. The fixation disengagement hypothesis contends that a gap-related disengagement of ocular fixation facilitates preparatory motor processes which lead to a general reduction in saccadic reaction times. The oculomotor preparation hypothesis proposes that these preparatory processes are spatially localized on the saccadic motor map and that short-latency saccades occur when the phasic visual response that is initiated by the visual stimulus cumulates with preparatory activity and reaches the threshold for generation of a reflexive saccade (Sommer 1994; Edelman and Keller 1996; Dorris et al. 1997). In the anti-saccade task, these short-latency saccades are errors because they are directed towards the stimulus instead of away from it.
Single neuron recording in monkeys have demonstrated that pro-saccade errors in the anti-saccade task are preceded by a high level of preparatory activity of saccade neurons in the SC (Everling et al. 1998a) and FEF (Everling and Munoz 2000). It has further been demonstrated that the level of saccade preparation in the SC is positively correlated with the probability of saccade direction (Basso and Wurtz 1998; Dorris and Munoz 1998). Based on these findings, we hypothesized that error rates would be higher in the anti-saccade task when the stimulus was presented at the location of high saccade probability (Fig. 1A, B). This finding would provide support for the hypothesis that errors in the anti-saccade task are the result of an increase in motor preparation (Munoz and Everling 2004a).
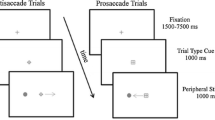
Schematic of the three saccade-direction probability conditions (A) and hypothesized associated levels of preparatory neural activity contralateral to the stimulus (B). A The visual fixation period was randomized between 1,000 and 1,500 m, and the gap duration was 200 ms. Three different anti-saccade probability conditions were tested. B A high probability of saccade direction leads to a higher level of preparatory activity. This allows the phasic visual activity to pass the saccade trigger threshold
Materials and methods
Subjects
Ten participants (seven male, three female), aged 20–57 years (mean ± SD, 26.3±11.0), were paid for their participation as subjects in this experiment and gave written, informed consent prior to their inclusion in this study. All subjects had normal or corrected-to-normal vision. None of the subjects reported any neurological or psychiatric disorders. The procedures in this study were approved by the University Research Ethics Board for Health Sciences Research and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Electrophysiological recording procedure
Horizontal eye movements were recorded using an electrooculogram (EOG) in a bipolar montage by placing Ag-AgCl skin electrodes at the outer canthi of both eyes. A ground electrode was placed just above the eyebrows in the centre of the forehead. Subjects wore the electrodes for approximately 10 min before the start of the first task condition in order to minimize EOG drift. Eye position signals were sampled at 1,000 Hz, low-pass filtered at 30 Hz and stored for off-line analysis by the Neuroscan 4.2 (Neurosoft Inc.) system.
Behavioural task
Participants were seated comfortably in a dark room at a distance of 80 cm from a 21-inch computer monitor. Two Pentium PCs running the CORTEX program (Dr Robert Desimone, NIMH) were used to control the experiment. All experimental stimuli were presented on a computer monitor situated directly in front of the subject. Each trial began with the presentation of a white fixation point (0.2°) in the centre of the dark monitor screen. The fixation point remained on the screen for a randomized period of 1,000–1,500 ms, followed immediately by a period of 200 ms when the screen was blank (Fig. 1A). This was followed by the presentation of a white stimulus (filled circle, 1°), either 8° to the left or 8° to the right of the central fixation point (Fig. 1A), for 500 ms. Probability of left-right stimulus presentation was 20–80%, 50–50%, 80–20%; tested in three separate blocks of trials. Subjects were instructed to maintain fixation during the fixation and gap periods, and to look away from the peripheral stimulus when it appeared to the mirror location in the opposite visual field as quickly as possible (Fig. 1A). The central fixation point reappeared 1,000 ms following removal of the stimulus, indicating the start of the next trial. Subjects performed 200 trials for each of the 3 saccade direction conditions, for a total of 600 trials per subject. The order of conditions was pseudo-randomized between subjects to include all possible permutations in this study. Subjects were not explicitly informed of the saccade direction probability in each condition. They were, however, informed that saccade-direction probability would vary across trial blocks. Subjects were given several minutes of rest between each condition.
Data analysis
In an off-line analysis, saccade onsets were automatically identified by a custom-written computer program using MATLAB (Mathworks). The onset of a saccade was identified as the time when horizontal eye velocity first exceeded 30°/s following stimulus presentation. Trials with saccadic reaction times (SRTs) <80 ms were excluded as anticipations and trials with SRTs >400 ms were excluded as no-response trials. Additionally all trials were visually inspected for blinks, which were also excluded from further analysis, and correct identification of onset of the saccade.
Statistical comparisons for error rates and SRTs were conducted using repeated measures analysis of variance (ANOVA) followed by multiple Bonferroni-corrected paired t -tests. All values are reported as means ± standard error of the mean.
Results
Saccadic reaction times
To investigate the effect of saccade-direction probability on the reaction times of correct anti-saccades, we performed a two-way repeated measures ANOVA with the factors probability (20%, 50%, or 80%) and saccade direction (left or right). The analysis revealed a significant main effect for probability ( F (2,9)=11.29, P <0.001) but not for saccade direction ( F (1,9)=0.13, P =0.73) or for the interaction ( F (2,9)=0.20, P =0.82). We therefore combined leftward and right anti-saccades (Fig. 2A). Across subjects, mean SRT of correct anti-saccades was 244.4±9.8 ms, 231.3±11.6 ms, and 223.2±10.6 ms in the 20%, 50%, and 80% saccade direction probability condition, respectively. Paired comparisons between the three conditions using Bonferroni-corrected t -tests demonstrated significant effects between the 20% and 50% probability condition ( t (9)=2.89, P <0.05) and the 20% and 80% probability condition ( t (9)=4.47, P <0.001). This finding demonstrates an effect of saccade-direction probability on the SRTs of anti-saccades.
Anti-saccadic reaction times (A) and error rates (B) for all subjects ( crosses) and the sample average ( bars). Reaction times were shorter for anti-saccades towards the high saccade-probability direction (A) but subjects generated more errors when the stimulus was presented at the side of high saccade-probability (B)
One might expect from the motor preparation hypothesis that the reaction time of pro-saccade errors would also depend on the saccade direction probability. We tested this prediction initially by averaging all leftward and rightward pro-saccade errors for each subject. We then compared the effect of saccade-direction probability on the reaction times of pro-saccade errors using a one-way repeated measures ANOVA with the factor probability (20%, 50%, or 80%). The ANOVA showed no differences for the reaction times of pro-saccade errors between the three probability conditions ( F (2,8)=1.29, P =0.3). The mean SRT of pro-saccade errors in the 20%, 50%, and 80% condition was 123.4±14.0 ms, 140.9±6.3 ms, and 143.3±6.8 ms, respectively. Pairwise Bonferroni-correct t -tests also showed no differences ( P >0.05). In a second analysis, we averaged all leftward and rightward saccades from all ten subjects for the three saccade probability conditions. Figure 3 shows the cumulative distribution of pro-saccade errors obtained from the ten subjects. This analysis also did not show any significant differences between the three probability conditions when we included all reaction times in the analysis (ANOVA, F (2,496)=1.97, P =0.3). A closer inspection of the distribution revealed, however, that subjects generated pro-saccade errors slightly faster in the 80% probability condition when we look at short-latency saccades with reaction times under 160 ms (insert in Fig. 3). This range of reaction times corresponds to the rising part of the cumulative SRT distribution and represents about 80% of all pro-saccade errors in each condition. An ANOVA with the factor probability (20%, 50%, or 80%) was significant for these reaction times ( F (2,416)=6.36, P =0.002). Paired post-hoc comparisons between the three conditions using Bonferroni-corrected t -tests demonstrated significant effects between the 20% and 50% probability condition ( t (9)=3.53, P =0.001) and the 20% and 80% probability condition ( t (9)=2.53, P <0.05). A cutoff of 140 ms that is commonly used to classify express saccades in humans (Munoz et al. 1998) also showed significant differences ( F (2,331)=4.29, P =0.015). This finding shows a slight reduction for short-latency pro-saccade error reaction times in the 80% saccade direction probability condition.
Error rates
We compared the effects of saccade-direction probability on the proportion of pro-saccade errors by computing a two-way ANOVA with the factors saccade-direction probability (20%, 50%, or 80%) and direction (left or right). The analysis yielded a main effect for probability ( F (2,9)=20.93, P <0.001) but not for direction ( F (1,9)=2.20, P =0.19) or for the interaction ( F (2,9)=3.32, P =0.06). We therefore combined leftward and rightward saccades. Figure 2B shows the percentage of errors from individual subjects ( crosses) and across subjects ( bars) for the three saccade-direction probability conditions. Error rates increased with increasing saccade-direction probability (4.4±1.4% for 20% probability, 10.9±3.1% for 50% probability, and 23.2±4.5% for 80% probability). Post-hoc paired comparisons (Bonferroni-corrected t -tests) showed significant differences between the 20% and 50% probability condition ( t (9)=6.58, P <0.001) and between the 20% and 80% probability condition ( t (9)=4.3, P <0.001).
Finally, we computed a one-way ANOVA with the factor experimental block (first, second, or third experimental block) to test for a possible order effect on the percentage of pro-saccade errors, irrespective of saccade-direction probability, and found this to be non-significant ( F (2,9)=2.26, P =0.13).
Discussion
We have shown that the rate of pro-saccade errors in the anti-saccade task is modulated by saccade-direction probability. Stimulus presentations at the side of high saccade-direction probability evoked significantly more pro-saccade errors than stimulus presentation at the side of low saccade-direction probability.
Previous research has focused on the influence of the status of fixation on performance of the anti-saccade task. A robust finding is that the introduction of a temporal gap of 200 ms between disappearance of the initial fixation stimulus and peripheral stimulus presentation leads to significantly more errors in the task (Fischer and Weber 1997; Bell et al. 2000). In the present study, the gap period was identical and thus reduction of the fixation signal was equal across the three saccade-direction probability conditions. Therefore a reduced fixation signal cannot be solely responsible for errors in the anti-saccade task.
The motor preparation hypothesis contends that pro-saccade errors in the anti-saccade task result from an increased level of preparatory activity which renders the phasic stimulus-related burst more likely to reach threshold (Munoz and Everling 2004a). Here, we modulated the level of preparatory activity by varying the probability that subjects had to generate a saccade to either the left or right side. The effect of this increased saccade motor preparation was evident in the reduction of anti-saccade reaction times and the slight reduction of short-latency pro-saccade errors with increasing saccade-direction probability. Conversely, the percentage of pro-saccade errors increased with increasing saccade-direction probability. The present results support the hypothesis that the level of preparatory activity is crucial for the performance of the anti-saccade task.
How relevant is our finding for the interpretation of pro-saccade errors in the anti-saccade task in clinical populations? The well-documented deficit of patients with frontal lobe lesions or schizophrenia in performing the anti-saccade task (for reviews see Everling and Fischer 1998; Broerse et al. 2001) is usually interpreted as the result of a disturbance in their oculomotor fixation system and not as a result of an increased saccade preparation. However, Walker et al. (1998) reported that a patient with a prefrontal lesion, who was completely unable to suppress pro-saccade errors on anti-saccade trials, never made these glances towards the stimuli under identical conditions when he was simply required to maintain fixation. This finding raises the possibility that the problem of frontal lobe patients in suppressing pro-saccade errors in the anti-saccade task may not be the result of a generalized loss of ocular fixation but rather an abnormally increased level of preparatory saccade activity in tasks that require both the suppression of reflexive saccades and the generation of voluntary saccades.
References
Basso MA, Wurtz RH (1998) Modulation of neuronal activity in superior colliculus by changes in target probability. J Neurosci 18:7519–7534
Bell AH, Everling S, Munoz DP (2000) Influence of stimulus eccentricity and direction on characteristics of pro- and antisaccades in non-human primates. J Neurophysiol 84:2595–2604
Broerse A, Crawford TJ, den Boer JA (2001) Parsing cognition in schizophrenia using saccadic eye movements: a selective overview. Neuropsychologia 39:742–756
Dorris MC, Munoz DP (1995) A neural correlate for the gap effect on saccadic reaction times in monkey. J Neurophysiol 73:2558–2562
Dorris MC, Munoz DP (1998) Saccadic probability influences motor preparation signals and time to saccadic initiation. J Neurosci 18:7015–7026
Dorris MC, Pare M, Munoz DP (1997) Neuronal activity in monkey superior colliculus related to the initiation of saccadic eye movements. J Neurosci 17:8566–8579
Everling S, Fischer B (1998) The antisaccade: a review of basic research and clinical studies. Neuropsychologia 36:885–899
Edelman JA, Keller EL (1996) Activity of visuomotor burst neurons in the superior colliculus accompanying express saccades. J Neurophysiol 76:908–926
Everling S, Munoz DP (2000) Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. J Neurosci 20:387–400
Everling S, Dorris MC, Munoz DP (1998a) Reflex suppression in the anti-saccade task is dependent on prestimulus neural processes. J Neurophysiol 80:1584–1589
Everling S, Pare M, Dorris MC, Munoz DP (1998b) Comparison of the discharge characteristics of brain stem omnipause neurons and superior colliculus fixation neurons in monkey: implications for control of fixation and saccade behavior. J Neurophysiol 79:511–528
Fischer B, Weber H (1997) Effects of stimulus conditions on the performance of antisaccades in man. Exp Brain Res 116:191–200
Forbes K, Klein RM (1996) The magnitude of the fixation offset effect with endogenously and exogenously controlled saccades. J Cogn Neurosci 8:344–352
Hallett PE (1978) Primary and secondary saccades to goals defined by instructions. Vision Res 18:1279–1296
Hallett PE, Adams BD (1980) The predictability of saccadic latency in a novel voluntary oculomotor task. Vision Res 20:329–339
Hess WR, Burgi S, Bucher V (1946) Motor function of tectal and tegmental area. Monatsschr Psychiatr Neurol 112:1–52
Munoz DP, Everling S (2004a) Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci 5:218–228
Munoz DP, Schall JD (2004b) Concurrent, distributed control of saccade initiation in the frontal eye field and superior colliculus. In: Hall WC, Moschovakis AK (eds) The superior colliculus: new approaches for studying sensorimotor integration. CRC Press, Boca Raton, pp 55–82
Munoz DP, Broughton JR, Goldring JE, Armstrong IT (1998) Age-related performance of human subjects on saccadic eye movement tasks. Exp Brain Res 121:391–400
Pare M, Munoz DP (1996) Saccadic reaction time in the monkey: advanced preparation of oculomotor programs is primarily responsible for express saccade occurrence. J Neurophysiol 76:3666–3681
Reuter-Lorenz PA, Hughes HC, Fendrich R (1991) The reduction of saccadic latency by prior offset of the fixation point: an analysis of the gap effect. Percept Psychophys 49:167–175
Sommer MA (1994) Express saccades elicited during visual scan in the monkey. Vision Res 34:2023–2038
Walker R, Husain M, Hodgson TL, Harrison J, Kennard C (1998) Saccadic eye movement and working memory deficits following damage to human prefrontal cortex. Neuropsychologia 36:1141–1159
Acknowledgements.
We thank Matthew Brown for his assistance in data analysis. We also thank two anonymous reviewers for very helpful comments on the manuscript. This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) and the National Alliance for Research on Schizophrenia and Depression (NARSAD). S.E. is a Canadian Institutes of Health Research New Investigator and an EJLB Research Scholar.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koval, M.J., Ford, K.A. & Everling, S. Effect of stimulus probability on anti-saccade error rates. Exp Brain Res 159, 268–272 (2004). https://doi.org/10.1007/s00221-004-2104-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-004-2104-x