Abstract
The purpose of the present study was to determine whether the soleus H-reflex is modulated with changes in the level of postural threat during walking. H-reflexes were tested at four points in the step cycle when subjects walked in 5 conditions representing different levels of postural threat. H-reflexes were significantly increased in amplitude at heelstrike in conditions of increased postural threat compared to normal treadmill walking with only minimal changes in H-reflex amplitude at other step cycle points. Conversely when subjects walked while holding stable handles, to decrease postural threat, the amplitude of the H-reflex was significantly smaller at heelstrike and midstance compared to normal walking. The changes in the amplitude of the H-reflex between walking conditions were not accompanied by changes in ongoing electromyographic activity or movements. Our findings suggest that the amplitude of the reflex is adjusted in a phase-specific manner, related to the postural uncertainty of the task. These adaptations in reflex amplitude may be related to changes in the amplitude of corrective responses following perturbations during walking. The adaptations in the amplitude of the H-reflex specific to heelstrike may be important in the control of foot placement at ground contact.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In a recent study we observed that subjects who received a forward push while walking on a treadmill generated a burst of activity in the soleus (SOL) muscle of the stance leg (Misiaszek and Krauss 2005). This burst of activity in SOL was part of a larger whole-body corrective response to regain stability following the perturbation. Given that the burst of activity in SOL followed a dorsiflexion disturbance in the ankle joint motion, this balance corrective response in SOL may be derived from a stretch-related reflex comparable to the reflexes observed with mechanical disturbances applied to the foot during walking (Sinkjaer et al. 1996). This would indicate that a portion of the corrective response evoked with a forward push at the torso is generated by reflexive mechanisms. In the same study, we also observed that the burst generated in SOL was substantially increased if the subjects walked with their arms voluntarily folded across the chest. The implication is that the corrective responses in the muscles of the legs are facilitated when postural threat is increased by the restriction of arm use. Consequently, if the burst of activity in SOL is derived from a stretch-related reflex then the expectation would be that SOL stretch reflexes would be facilitated when subjects walk with their arms restricted, compared to walking normally.
The H-reflex, evoked in SOL by electrical stimulation of the tibial nerve, is the electrical analogue of the stretch reflex and a means by which the excitability of the stretch-evoked reflex can be assessed in humans (recently reviewed in Misiaszek 2003). Consequently, we hypothesize that the SOL H-reflex will be facilitated during walking when there is an increased threat to postural stability, compared to normal walking. We also suggest that the amplitude of the SOL H-reflex should be scaled to the level of postural threat, showing higher amplitudes in the most unstable condition. Such an effect was noted by Schneider and Capaday (2003) who demonstrated that the SOL H-reflexes of subjects walking backward were substantially larger at midswing if the subjects walked without support. However, the H-reflex at midswing decreased immediately when subjects held safety handrails, suggesting the postural uncertainty of the novel task led to the facilitated reflex. In contrast, Llewellyn et al. (1990) demonstrated that the SOL H-reflex was suppressed at comparable SOL EMG activation when subjects walked on a narrow beam compared to walking on a treadmill, suggesting that increased postural threat decreases excitability of the monosynaptic component of the stretch reflex. In the present study we provide evidence to suggest that the SOL H-reflex is facilitated in a phase-specific manner when walking with an increased postural threat. A portion of this work has been published as an abstract (Krauss and Misiaszek 2005).
Materials and methods
Subjects and protocol
A total of twenty subjects (age 20–34) participated in this study. Subjects reported no history of neurological, metabolic or cardiovascular disease, and had not experienced musculoskeletal injury, back pain, or concussion in the previous six months. All subjects provided written informed consent in a protocol approved by the University of Alberta Health Research Ethics Board.
In this study we compared the amplitude of the SOL H-reflex between five walking conditions. The intention of the H-reflex stimulation was to compare the reflex during periods of steady walking, but when the threat to stability was altered. It was not feasible to perform all five conditions in one experimental session. Consequently, the study was divided into two parts. Ten subjects participated in each part. For both parts, subjects walked on a motorized treadmill at a self-selected comfortable walking speed (range 0.9–1.1 m/s). H-reflexes were elicited at four points of the step cycle: heelstrike, midstance, toe-off, and midswing of the right leg (Fig. 1c).
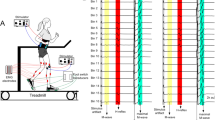
Schematic of the experimental set-up. a Subjects walked on a motorized treadmill. Subjects walked either with their arms swinging freely or folded across their chest. In some conditions (AP, Arms Crossed AP) perturbations were applied to the torso using a continuous cable system attached to the subject’s waist. The experimenter perturbed the subject either forward or backward by rotating the drum controlling the cable system forward or backward. b In the Handles condition subjects held stable handles while they walked. The handles were mounted to the front of the treadmill frame, adjusted to a height forming a 90 degree angle at the subject’s elbows. c H-reflexes were elicited in blocks of stimuli at heelstrike, midstance, toe-off, and midswing of the step cycle for all walking conditions. The order of presentation was randomized across walking conditions and subjects
In Part I, subjects walked in four conditions representing increasing levels of postural threat: (1) normally with the arms free (Normal), (2) with the arms voluntarily folded across the chest (Arms Crossed), (3) while receiving perturbations in the anterior and posterior directions applied to the torso (AP), and (4) while receiving perturbations to the torso and with their arms voluntarily folded across the chest (Arms Crossed AP). In the conditions with the arms crossed, subjects loosely held their arms across the chest, and were free to release their arms if it was necessary to recover from a perturbation. The order of presentation of the walking conditions was randomized across subjects. Subjects were informed before an AP or Arms Crossed AP trial that they could expect perturbations. Perturbations were manually applied in both the forward and backward directions using a continuous cable system that was attached to the subject at the waist using a padded belt (Fig. 1a). Perturbations occurred at a frequency of approximately 1 every 30 s throughout the step cycle, with a force ranging between 10 and 30% of the subject’s body weight. Perturbation timing and direction were unpredictable to the subjects. The perturbations were utilized to create a sense of uncertainty and the responses to the perturbations were not analyzed (see Misiaszek and Krauss (2005) for analysis of responses to perturbations). We were investigating how the sense of postural threat affects the H-reflex amplitude and were not interested in H-reflex modulation during a perturbation. Therefore, H-reflexes were evoked in step cycles occurring at least 3 steps after a perturbation; hence, reflexes were elicited during periods of steady walking between perturbations. Safety railings mounted at the sides and front of the treadmill were available for subjects to use throughout all conditions; however, none of the subjects used the safety railings.
In Part II subjects walked (1) normally with the arms free (Normal) and (2) while holding stable handles (Handles) to reduce postural threat. The handles were mounted on the treadmill frame in front of their body. The height of the handles was adjusted so that subjects could maintain approximately a 90° angle at the elbow when holding onto the handles (Figure 1b). Perturbations were not applied in the Handles condition.
Nerve stimulation
H-reflexes were evoked in SOL of the right leg by applying a 1 ms square wave pulse (Grass S88 stimulator) to the tibial nerve using a bipolar stimulating electrode placed on the skin over the popliteal fossa. To ensure that both increases and decreases in the amplitude of the H-reflex could be observed we used a stimulus intensity that produced an H-reflex on the ascending limb of the recruitment curve, while also producing a small, but stable M-wave (resulting from the direct activation of the α-motoneurons in the mixed nerve) of approximately 5% of the maximum M-wave amplitude (Mmax). Stimulus intensity (the amplitude of the M-wave) was monitored online throughout the experiment using an oscilloscope. The amplitude of Mmax has been shown to vary across the step cycle (Simonsen et al. 1995). Consequently, Mmax was obtained for each point in the step cycle and the current required for achieving a 5% Mmax stimulus was appropriately adjusted. Therefore, the applied current was different for each point in the step cycle tested. As a result, H-reflex stimulation occurred in blocks with the stimulus delivered at one point of the step cycle at a time. Approximately 20 H-reflexes with M-waves of 5% Mmax were elicited for each point of the step cycle with an additional 10–15 H-reflexes randomly interspersed at other points in the step cycle to prevent predictability. Stimuli were manually triggered by the experimenter, and step cycle timing was monitored by viewing heel and toe contact data, obtained from custom-made sensors, displayed on an oscilloscope. Stimuli were elicited at least 5 s apart. The order of presentation of the stimulation points was randomized across walking conditions and across subjects.
Recording and data acquisition
Electromyographic (EMG) recordings were obtained for the SOL and tibialis anterior (TA) muscles of the right leg. Recordings were made using pairs of Ag/AgCl disposable surface electrodes (A10012, Vermed) placed over the bellies of the muscles. Ground electrodes were placed over the tibia. Signals were pre-amplified and bandpass filtered (30 Hz to 3 kHz, Grass Model P511). Electrogoniometers (Biometrics) were placed across the right knee and ankle joints to record joint angles during walking. Heel and toe contact data were measured using custom-made sensors affixed to the insole of the right shoe. All signals were digitized at 3000 Hz using a 12-bit analog–digital converter (PCI-MIO-16E-4, National Instruments) and saved to computer using custom-written software (LabView 5.1, National Instruments) for later analysis.
Data analysis
The twenty stimuli evoked at each point in the step cycle for a walking condition were sorted offline. Those stimuli that occurred within approximately a 10% window of the step cycle phase and with an M-wave of 4–6% Mmax were retained for further analysis. The timing of the stimulus within the step cycle was calculated from the stimulus artifact. (Consequently, the H-reflex itself may have fallen outside the boundaries of the analysis window. However, this is irrelevant to the between-tasks comparisons of interest of this study as any influence this may have on the analysis would be consistent across the conditions as the same binning procedure was utilized throughout.) The 10% windows around each point of the step cycle of interest were estimated from the average control steps for each walking condition. The specific percentage of the step cycle where each event occurred varied across subjects and this was reflected in the exact placement of the bins that were analyzed (except for heelstrike, which was defined as 0% of the step cycle). This typically resulted in 15–20 reflexes for each point in the step cycle, for each walking condition, being included in the analysis for each subject. Peak-to-peak amplitudes of the H-reflex and M-wave were measured from individual traces and were normalized to Mmax for that point in the step cycle. Normalized H-reflexes and M-waves were averaged for each subject and the average values were used for statistical analysis.
The background EMG activity in SOL and TA EMG was determined by calculating the mean activity for a 50 ms window prior to the stimulus artifact for each H-reflex retained for the analysis (see above). To do this, the SOL and TA EMG signals were digitally full-wave rectified and then low-pass filtered at 50 Hz with a 4th order dual-pass Butterworth filter. These values were then normalized to the maximum rectified and filtered EMG observed over the step cycle of 30 control steps (i.e. steps that did not receive a stimulus) taken from the Normal condition, for each subject. The normalized EMG values were then averaged for each subject and used in the subsequent statistical analysis. The average knee and ankle angles were calculated for the 50 ms window preceding the stimulus artifact for each subject and were used in the subsequent statistical analysis.
In this study we were interested in comparisons between walking conditions at each point in the step cycle, and not across different points in the cycle. Therefore, planned comparisons were used to test our hypotheses. The error term used in the planned comparisons was estimated from the residual error of a two-way repeated measures analysis of variance. Separate analyses were performed for each of the measured variables (M-wave amplitude, H-reflex amplitude, SOL EMG, TA EMG, ankle angle, knee angle). In addition, separate analyses were performed for Parts I and II of the study. Statistical significance was set at P < 0.05.
Results
The amplitude of the SOL H-reflex was modulated over a step cycle of treadmill locomotion (Fig. 2a), consistent with the phase-dependent modulation of reflex amplitude that has been noted previously (Capaday and Stein 1986). This general pattern of modulation (largest reflexes during stance and smallest during swing) was similar for all five walking conditions tested.
a Group averaged H-reflex amplitudes for the unstable walking conditions (Arms Crossed, AP, and Arms Crossed AP) are shown with the Normal condition for each point in the step cycle tested. Each bar represents the mean (N = 10). Error bars represent one standard error. Group averaged M-wave amplitudes are shown by the symbols (closed diamond Normal; closed square Arms Crossed; open triangle AP; open circle Arms Crossed AP). b Average (n = 30) electromyography and goniometer traces from control steps during Arms Crossed AP (thick lines) and Normal walking (thin lines). Dotted lines represent heelstrike and toe-off respectively. c Group averaged (N = 10) EMG amplitude in the SOL and TA muscles. Each bar represents the average amplitude of the background EMG for the 50 ms immediately preceding the stimulus delivery, averaged across trials and across subjects. Error bars represent one standard error. Asterisks indicate significant difference compared to the Normal condition for that point in the step cycle (planned comparison, P < 0.05)
H-reflex amplitude in unstable environments
In Part I, subjects were asked to walk on a treadmill in four conditions of increasing postural threat: Normal, Arms Crossed, AP, and Arms Crossed AP. The conditions represented various combinations of task constraints including restricting arm swing and/or experiencing periodic and unpredictable perturbations at the torso. Despite these changes in the task, the walking pattern of the legs was consistent across all four walking conditions. This is exemplified in Fig. 2b depicting average (n = 30) data for two of the walking conditions for one subject; Normal is the thin trace which is generally obscured by the overlaid thicker trace representing the Arms Crossed AP condition. Specific analysis of the EMG levels in SOL and TA at the four points of the step cycle studied indicated no significant differences between tasks, with one exception (Fig. 2c). The SOL activity at toe-off was significantly lower during the AP condition than during the Arms Crossed condition (P < 0.05). Ankle and knee angles were not different between tasks at any of the step cycle points studied. There were no differences in step cycle duration or speed of movement across tasks.
The group averaged H-reflex amplitudes are shown in Fig. 2a for each of the points in the step cycle and the four walking conditions. The group averaged M-wave amplitudes are also plotted in Fig. 2a as the symbols. Planned comparisons of the average H-reflex amplitudes were made between conditions for each point in the step cycle. At heelstrike all conditions of decreased stability (Arms Crossed, AP, and Arms Crossed AP) resulted in significantly larger H-reflexes, as compared to the Normal condition. The H-reflex amplitude at heelstrike in the unstable conditions was approximately 22% larger than during Normal walking. No differences were found between the three decreased stability conditions. At other points in the step cycle the H-reflex amplitude was relatively unaltered by the change in walking conditions, with only two additional differences detected. H-reflexes at midstance during the Arms Crossed condition were significantly decreased, compared to Normal. In addition, H-reflexes at toe-off during the AP condition were reduced, compared to Normal. No other differences were identified. The amplitude of the M-wave was specifically controlled to be 5% Mmax for all subjects and all points in the step cycle. Consequently, no differences were identified in the M-wave amplitudes.
H-reflex amplitude with increased stability
In Part II of these experiments subjects were asked to hold stable handles while walking. The muscle activity and movement patterns of the legs did not change noticeably when subjects walked while holding stable handles placed in front of them. Average traces for the control step EMG and goniometer signals are shown in Fig. 3b for one subject, with the thin traces representing the Normal condition and the overlaid thick traces representing the Handles condition. The group averaged activity of SOL and TA at each point in the step cycle studied for both walking conditions are depicted in Fig. 3c. No differences were detected in the EMG amplitudes between conditions at any of the points of the step cycle. In addition, no differences in the ankle or knee angles were identified between walking conditions.
a Group averaged H-reflex amplitudes for the Handles walking condition are shown with the Normal condition for each point in the step cycle tested. Each bar represents the mean (N = 10). Error bars represent one standard error. Group averaged M-wave amplitudes are shown by the symbols (closed diamond Normal; open circle Handles). b Average (n = 30) electromyography and goniometer traces from control steps during Handles (thick lines) and Normal walking (thin lines) . Dotted lines represent heelstrike and toe-off respectively. c Group averaged (N = 10) EMG amplitude in the SOL and TA muscles. Each bar represents the average amplitude of the background EMG for the 50 ms immediately preceding the stimulus delivery, averaged across trials and across subjects. Error bars represent one standard error. Asterisks indicate significant difference compared to the Normal condition for that point in the step cycle (planned comparison, P < 0.05)
The amplitude of the H-reflex was reduced (P < 0.05) during the Handles condition, compared with Normal, at both the heelstrike and midstance points of the step cycle (Fig. 3a). At heelstrike, the H-reflex amplitude was nearly 40% lower when subjects held Handles compared to Normal walking. The H-reflex amplitude in the Handles condition at midstance was 10% lower than in the Normal condition. No difference in reflex amplitude was observed between conditions at either the toe-off or midswing points in the step cycle. In addition, no differences were identified in the amplitudes of the M-waves as these were controlled throughout the experiment.
Discussion
In this study we showed that changing the context within which a person walked (by varying the level of postural threat) led to significant changes in the amplitude of the SOL H-reflex. However, the changes in H-reflex excitability were not generalized across the step cycle, but consisted of increases and decreases in amplitude specific to a few points of the step cycle. In particular, reflexes recorded at heelstrike appeared to be most susceptible to context-dependent adaptation. The H-reflex amplitude at heelstrike increased by 22% when subjects walked in less stable conditions and was 40% smaller when subjects walked in a more stable condition (holding handles), compared to walking normally on the treadmill.
The modulation of the H-reflex at heelstrike fits with our hypothesis. That is, H-reflexes were larger when walking in conditions that threatened stability. However, we did not find any difference in H-reflex amplitude between the AP and the Arms Crossed AP conditions. This is in direct contrast to our previous finding that the amplitude of the corrective response in SOL following perturbations was significantly increased when the subjects also crossed their arms (Misiaszek and Krauss 2005). In fact, in that study crossing the arms had the effect of more than doubling the amplitude of the evoked response in SOL at midstance, whereas in this study the H-reflex at midstance was largely unchanged between walking conditions. Consequently, the larger evoked response observed in SOL in our previous study is not related to changes that influence the H-reflex. Although we cannot exclude the possibility that changes in fusimotor drive acting on the simple stretch reflex might contribute to the larger corrective response in SOL, it is likely that other pathways, such as long-loop functional stretch reflexes or group II afferent pathways may be involved (Sinkjaer et al. 2000).
Nevertheless, the clear finding of this study was that the amplitude of the H-reflex was modified with the walking context. This was most apparent for reflexes evoked at heelstrike. Other points also may show influences, for instance at midstance the H-reflex was smaller when subjects held stable handles, consistent with the results at heelstrike. However, the unstable conditions AP and Arms Crossed AP did not produce significant increases in H-reflex amplitudes at midstance. At this point in the step cycle the H-reflex is relatively large during the Normal walking task and the ongoing EMG activity is substantial. Therefore, it is possible that the H-reflex was less sensitive to increases in amplitude because the reflex was close to saturation. However, this does not seem to be the case as some subjects showed reflexes that were clearly larger and others that were clearly smaller suggesting that the H-reflex remained sufficiently sensitive.
The context-dependent changes in reflex amplitude we observed at heelstrike are reminiscent of the context-dependent changes in reflex amplitude described by Schneider and Capaday (2003). In that study, the authors noted that H-reflexes were substantially increased only during the swing phase of backward walking in subjects unaccustomed to the task. However, immediately upon grasping safety rails the H-reflexes during the swing phase were suppressed. The authors suggested that the increase in the H-reflex was related to the uncertainty of the task, associated with foot placement and stability. In our study, the H-reflex amplitude at midswing was unaltered between conditions. However, our subjects were walking forward and therefore likely had more confidence in the foot placement than the subjects in the Schneider and Capaday (2003) study. We suggest that in our study the larger H-reflexes at heelstrike during the unstable walking tasks and the smaller H-reflexes when subjects hold handles is similarly related to task uncertainties, in particular, the threat of losing balance. In this context, the increased H-reflex amplitude in less stable walking conditions may serve to assist in controlling ankle angle or joint stiffness at ground contact, when the new base of support is being established. It is also important to note that in our study we sampled only four points in the step cycle. Therefore, we cannot say that changes in the H-reflex between tasks are restricted to heelstrike per se. It is possible that changes in reflex amplitude also occurred in late swing or early stance.
An important finding in this study is the specificity of the influences of the walking task on the amplitude of the H-reflex. The amplitude of the H-reflex was not generally facilitated or inhibited over the whole step cycle. Rather, the observed changes were primarily observed at heelstrike in our study. Recently, using the same experimental paradigms as used in the present study, but studying cutaneous reflexes we found that the amplitudes of these reflexes were also adapted at specific points in the step cycle (Haridas et al. 2005). For example, cutaneous reflexes elicited in TA with stimulation of the distal tibial nerve were facilitated during the less stable walking conditions, but only at ipsilateral and contralateral heel-strike. Changing the walking tasks did not influence the amplitude of this reflex at the other points in step cycle studied. Cutaneous responses in other muscles each revealed their own patterns of facilitation or suppression between the various walking tasks, indicating that each reflex pathway was uniquely regulated by the change in walking context and was not subject to a generalized facilitation or inhibition. Together these findings suggest that reflexes can be regulated in a very specific manner, according to the context of the task being performed and at specific points in the step cycle. This “phase-specific” context-dependent regulation of reflex excitability is likely achieved by cortical influences. Bretzner and Drew (2005) found that motor cortical influences on the excitability of cutaneous reflexes in the walking cat showed a high degree of specificity. These authors suggested that these cortical influences may be important to specifically regulate reflex excitability to stabilize movements. In addition, Pijnappels et al. (1998) demonstrated that cortical inputs can lead to phase-dependent influences on cutaneous reflex excitability. Thus, cortical influences are capable of producing phase-specific changes in reflex excitability, such as those observed here for the SOL H-reflex.
Ours is not the first study to examine the influence of walking in an unstable environment on the amplitude of the SOL H-reflex. Llewellyn et al. (1990) found that subjects walking on a narrow beam generally suppressed the H-reflex at matched SOL EMG levels, compared to walking on a treadmill. Thus, it appears that the results of Llewellyn et al. (1990) are in direct contrast to our current findings. However, this difference is likely related to the very different environmental and task constraints imposed by the two studies. Beam walking consists of greater co-contraction of TA and SOL to stabilize the ankle joint and a longer double support phase than normal treadmill walking. Llewellyn et al. (1990) did not report the same phase-dependent specific regulation of H-reflex amplitude, but rather a generalized decrease, and this may be due to differences in the walking pattern between beam walking and treadmill walking. In addition, the major threat to stability introduced by walking on a narrow beam is related to medial-lateral stability and the constraint imposed on medial-lateral placement of the feet. In this context, the electrically evoked H-reflex may itself be a source of instability (Earles et al. 2000). Consequently, the suppression of the H-reflex during beam walking may have been related to the technique employed. We cannot rule out the possibility that the instability evoked by the H-reflex in our study led to similar suppression to reduce the induced disturbance (which might account for the smaller H-reflex noted at midstance in the Arms Crossed condition). However, if this occurred then the facilitation of the reflex we observed occurred in spite of this competing influence.
We have shown here and previously (Haridas et al. 2005) that reflexes are specifically regulated as a function of threat to stability during walking. These context-dependent adaptations in reflexes coincide with context-dependent adaptations of the corrective responses to balance disturbances during walking (Misiaszek et al. 2000; Misiaszek and Krauss 2005; Rietdyk and Patla 1998). We cannot state with certainty whether these events are directly related. However, Rietdyk and Patla (1998) noted that the magnitude of corrective response as a function of threat to stability was modulated in a complex manner, with specific adaptations occurring in different muscles. One possible means of accomplishing this complex modification of a functional response is by the cumulative integration of specific adaptations to a number of simpler responses. Thus, the specific adaptations observed in the amplitudes of the H-reflex, and cutaneous reflexes (such as those described in Haridas et al. 2005), along with adaptations that likely occur in other reflexive responses (such as the functional stretch reflex, force feedback contributions and others) may occur to dynamically weight the expression of each of the related motor responses across the step cycle to meet the specific needs of the task constraints. This would be akin to adjusting ‘motor membership functions’ (Prochazka 1996) as a way to optimize balance corrective responses to meet the changing needs of a shifting environment (Misiaszek 2006).
References
Bretzner F, Drew T (2005) Motor cortical modulation of cutaneous reflex responses in the hindlimb of the intact cat. J Neurophysiol 94:673–87
Capaday C, Stein RB (1986) Amplitude modulation of the soleus H-reflex in the human during walking and standing. J Neurosci 6:1308–1313
Haridas C, Zehr EP, Misiaszek JE (2005) Postural uncertainty leads to dynamic control of cutaneous reflexes from the foot during human walking. Brain Res 1062:48–62
Earles DR, Koceja DM, Shively CW (2000) Environmental changes in soleus H-reflex excitability in young and elderly subjects. Int J Neurosci 105:1–13
Krauss EM, Misiaszek JE (2005) Soleus H reflex modulation during walking in an unstable environment. Soc Neurosci Abstr: Program No. 869.10
Llewellyn M, Yang JF, Prochazka A (1990) Human H-reflexes are smaller in difficult beam walking than in normal treadmill walking. Exp Brain Res 83:22–28
Misiaszek JE (2006) Neural control of walking balance: IF falling THEN react ELSE continue. Exerc Sport Sci Rev 34:128–134
Misiaszek JE (2003) Early activation of arm and leg muscles following pulls to the waist during walking. Exp Brain Res 151:318–329
Misiaszek JE, Krauss EM (2005) Restricting arm use enhances compensatory reactions of leg muscles during walking. Exp Brain Res 161:474–485
Misiaszek JE, Stephens MJ, Yang JF, Pearson KG (2000) Rapid whole-leg corrective reaction to a perturbation of the torso during walking in humans. Exp Brain Res 131:511–523
Pijnappels M, Van Wezel BM, Colombo G, Dietz V, Duysens J (1998) Cortical facilitation of cutaneous reflexes in leg muscles during human gait. Brain Res 787:149–153
Prochazka A (1996) The fuzzy logic of visuomotor control. Can J Physiol Pharmacol 74:456–462
Rietdyk S, Patla AE (1998) Context-dependent reflex control: some insights into the role of balance. Exp Brain Res 119:251–259
Schneider C, Capaday C (2003) Progressive adaptation of the soleus H-reflex with daily training at walking backward. J Neurophysiol 89:648–656
Simonsen EB, Dyhre-Poulsen P, Voigt M (1995) Excitability of the soleus H reflex during graded walking in humans. Acta Physiol Scand 153:21–32
Sinkjaer T, Andersen JB, Ladouceur M, Christensen LOD, Nielsen JB (2000) Major role for sensory feedback in soleus EMG activity in the stance phase of walking in man. J Physiol 523:817–827
Sinkjaer T, Andersen JB, Larsen B (1996) Soleus stretch reflex modulation during gait in humans. J Neurophysiol 76:1112–1120
Acknowledgements
This work was supported by funding to JEM from the Natural Sciences and Engineering Research Council of Canada (NSERC). EMK was supported by a scholarship from NSERC. The authors wish to thank Dr. D. Collins for his helpful comments on a preliminary draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krauss, E.M., Misiaszek, J.E. Phase-specific modulation of the soleus H-reflex as a function of threat to stability during walking. Exp Brain Res 181, 665–672 (2007). https://doi.org/10.1007/s00221-007-0962-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-007-0962-8