Abstract
The soleus H-reflex modulation pattern was investigated during stepping following transspinal stimulation over the thoracolumbar region at 15, 30, and 50 Hz with 10 kHz carry-over frequency above and below the paresthesia threshold. The soleus H-reflex was elicited by posterior tibial nerve stimulation with a single 1 ms pulse at an intensity that the M-wave amplitudes ranged from 0 to 15% of the maximal M-wave evoked 80 ms after the test stimulus, and the soleus H-reflex was half the size of the maximal H-reflex evoked on the ascending portion of the recruitment curve. During treadmill walking, the soleus H-reflex was elicited every 2 or 3 steps, and stimuli were randomly dispersed across the step cycle which was divided in 16 equal bins. For each subject and condition, the soleus M-wave and H-reflex were normalized to the maximal M-wave. The soleus background electromyographic (EMG) activity was estimated as the linear envelope for 50 ms duration starting at 100 ms before posterior tibial nerve stimulation for each bin. The gain was determined as the slope of the relationship between the soleus H-reflex and the soleus background EMG activity. The soleus H-reflex phase-dependent amplitude modulation remained unaltered during transspinal stimulation, regardless frequency, or intensity. Similarly, the H-reflex slope and intercept remained the same for all transspinal stimulation conditions tested. Locomotor EMG activity was increased in knee extensor muscles during transspinal stimulation at 30 and 50 Hz throughout the step cycle while no effects were observed in flexor muscles. These findings suggest that transspinal stimulation above and below the paresthesia threshold at 15, 30, and 50 Hz does not block or impair spinal integration of proprioceptive inputs and increases activity of thigh muscles that affect both hip and knee joint movement. Transspinal stimulation may serve as a neurorecovery strategy to augment standing or walking ability in upper motoneuron lesions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinal stimulation has gained prominent attention in the scientific community, with research investigations spanning from animals to humans, and from mathematical modeling to intraspinal, epidural, and transspinal (transcutaneous spinal cord) delivery of electrical current for restoring locomotor, respiratory, and bladder function in upper motoneuron lesions (Dimitrijevic et al. 1998; Harkema et al. 2011; Minassian et al. 2004, 2016; Rattay et al. 2000; Angeli et al. 2014; Moraud et al. 2016; Knikou 2013; Knikou et al. 2015; Knikou and Murray 2019). In humans, epidural or transspinal stimulation may activate similar neuronal circuits or pathways (Hofstoetter et al. 2018), while it is likely that both produce transsynaptic activation of motoneurons based on the characteristics of stimulation, e.g. intensity, frequency, single pulse vs. pulse train. Epidural and transspinal stimulation at different frequencies is used to promote recovery of motor function and improve clinical signs of pathological muscle tone (Hofstoetter et al. 2014; Sayenko et al. 2019).
Reflexes were once thought of as stereotyped responses of minimal contribution or importance to movement (Zehr and Stein 1999). Muscle reflexes that convey information from proprioceptors are modulated in a task- and phase-dependent manner (Capaday and Stein 1986, 1987; Llewellyn et al. 1990; Knikou et al. 2009b, 2011; Knikou and Mummidisetty 2011; Knikou 2012; Mummidisetty et al. 2013), while a proportion of soleus muscle force during the stance phase of gait in humans is attributed to the soleus Hoffmann (H-) reflex (Yang et al. 1991). These results along with the phase-dependent modulation of primary afferent depolarization and presynaptic inhibition of monosynaptic reflex transmission (Menard et al. 2002; Rudomin 2009), support the important role of muscle reflexes in the function of spinal locomotor networks.
A functional link exists between transspinal stimulation, muscle reflexes, and spinal locomotor centers. Our thesis is supported by the 1) phase-dependent modulation of leg transspinal evoked potentials (TEPs) in healthy subjects and their abnormal modulation in people with spinal cord injury (Courtine et al. 2007; Hofstoetter et al. 2008; Dy et al. 2010; Zaaya et al. 2021; Pulverenti et al. 2019, 2022), 2) summation of TEPs with motor evoked potentials (MEPs) and soleus H-reflexes at specific time intervals (Knikou 2014; Knikou and Murray 2018), 3) step-like leg EMG burst activity induced by transspinal stimulation in healthy subjects and children with spinal cord injury when gravity is eliminated (Shapkova and Schomburg 2001; Gorodnichev et al. 2012), and 4) entrainment of previously silent muscles during assisted stepping and low frequency transspinal stimulation in four persons with clinically complete spinal cord injury (Minassian et al. 2016).
The development of therapeutic strategies for locomotor recovery in neurological disorders needs to take into consideration that interventions should promote and not interfere with spinal integration of proprioceptive feedback (Formento et al. 2018). The temporal summation between the soleus H-reflex and the soleus TEP, and the parallel soleus TEP and H-reflex depression, when transspinal is delivered after or before stimulation of group Ia afferents (Knikou 2013; Knikou and Murray 2018), supports the possibility that transspinal stimulation may block or decrease afferent volleys traveling to the spinal cord during walking. This hinders the use of transspinal stimulation during assisted stepping and minimizes the possibility of augmenting further the locomotor training benefits by tonic noninvasive transspinal stimulation. Therefore, the main objective of this study was to establish the effects of transspinal stimulation over the thoracolumbar region at 15, 30, and 50 Hz at sub- and suprathreshold paresthesia intensities on the soleus H-reflex phase-dependent amplitude modulation and locomotor muscle activity in healthy human subjects. We hypothesized that transspinal stimulation at suprathreshold paresthesia intensity, regardless frequency, produces soleus H-reflex and locomotor muscle activity depression throughout the step cycle, consistent to our recent observations with a single 1 ms transspinal stimulation at 1.3 times soleus TEP threshold (Pulverenti et al. 2019; Islam et al. 2021).
Materials and methods
Participants
Ten (7 males, 3 females) healthy adults aged 23 to 47 years (27.6 ± 7.7; mean ± SD) without evidence of past or present neurological, orthopedic or any systemic disorder participated in the study. All subjects gave their written informed consent before participating in the study that was performed in compliance with the Declaration of Helsinki. The experimental protocol was approved by the Institutional Review Board (IRB) of the City University of New York (IRB Number 2022–0003-CSI).
Surface EMG
Following standard preparation (skin was dry shaved, abraded, and cleaned with alcohol), single differential bipolar surface electrodes (common mode rejection ratio > 100 dB at 40 Hz, input impedance > 100,000 MΩ) with fixed inter-electrode distance of 2 cm (MA411n, Motion Lab Systems Inc., Baton Rouge, LA) were used to record myoelectric signals from both legs during walking at a self-selected speed on a motorized treadmill from the vastus medialis (VM), vastus lateralis (VL), gracilis (GRC), medial hamstrings (MH), medial gastrocnemius (MG), soleus (SOL), and tibialis anterior (TA) muscles. Surface electrodes were secured with Tegaderm transparent film (3 M Healthcare, St. Paul, MN, USA). All EMG signals during stepping were low-pass filtered with a cutoff frequency of 1,000 Hz (MA-300, Motion Lab Systems Inc., Baton Rouge, LA), sampled at 2,000 Hz using a 16-bit data acquisition card (NI-PCI-6225, National Instruments, Austin, TX) and saved in a personal computer for off-line analysis.
Transspinal stimulation
With subjects seated at the edge of a treatment table, the Thoracic 10 spinous process was identified via palpation and anatomical landmarks. A single reusable self-adhered cathode electrode (10.2 × 5.1 cm2, Uni-Patch, Massachusetts, USA) was placed at midline along the vertebrae equally between the left and right paravertebral sides covering from Thoracic 10 to Lumbar 1–2 vertebral levels. A pair of interconnected anode electrodes (same type as the cathode) was placed on either side of the iliac crests (Knikou 2013, 2014; Knikou et al. 2015; Skiadopoulos et al. 2022).
The position of the cathodal stimulating electrode was based on the shape of the right and left SOL TEP at increasing intensities, concomitant presence of SOL TEPs in the right and left legs at low intensities, and presence of SOL TEP depression in response to paired transspinal stimuli delivered via a constant current stimulator (DS7A or DS7AH, Digitimer, Welwyn Garden City, UK) triggered by a 1401 data acquisition interface running Spike 2 (CED Ltd., Cambridge, UK). After the optimal location was identified, the electrode was affixed to the skin via Tegaderm transparent film (3M Healthcare, Minnesota, USA), and maintained under pressure via a custom-made pad. With subjects’ supine, and knee joints flexed at 30°, ankles supported in a neutral position, and legs maintained in midline via external support, the stimulation intensity (46.5 ± 23.3 mA) that a TEP of 100 µV for the right SOL muscle was noted on the oscilloscope was termed as TEP threshold (Knikou 2013; Knikou and Murray 2018).
Soleus H-reflex during stepping
The soleus H-reflex from the right leg was elicited and recorded according to methods we have utilized extensively in human subjects at rest and during stepping (Knikou 2008; Knikou et al. 2009a, b, 2011; Mummidisetty et al. 2013; Knikou and Mummidisetty 2011, 2014; Islam et al. 2021).
With the subject seated, monophasic 1 ms square pulses were triggered by a data acquisition interface (1401 plus, Cambridge Electronics Design Ltd., UK) and delivered via a constant current stimulator (DS7A, Digitimer Ltd., UK) to the right posterior tibial nerve at the popliteal fossa via a hand-held monopolar stainless-steel electrode used as a probe. The anode was a stainless-steel plate of 4 cm2 placed proximal to the patella. When a soleus H-reflex could be evoked without an M-wave at the lowest stimulation intensity and the M-wave had a similar shape to the H-reflex at increasing intensities, the monopolar probe electrode was replaced by a pre-gelled disposable electrode (SureTrace, Conmed, NY) that was maintained under pressure via a custom-made pad and pre-wrap.
Then, each subject stood on the treadmill and the soleus H-reflex and M-wave recruitment curves were assembled. Approximately 80 stimuli in total were delivered at 0.2 Hz to assemble the soleus H-reflex and M-wave recruitment curve during standing (Knikou et al. 2009b; 2011). Stimuli corresponding to the M-wave and H-reflex recruitment curve, and the associated peak-to-peak amplitude of the responses (M-wave and H-reflex) were saved and retrieved by the customized LabVIEW software program as reference values utilized later during treadmill walking.
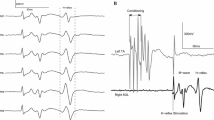
The amplitude of the M-wave and H-reflex as a percentage of the maximal M-wave is one of the most important factors in human electrophysiological studies (Knikou 2008). Due to shifts of the recording and stimulating electrodes, the maximal M-wave varies significantly across the step cycle phases (Simonesen and Dyhre-Poulsen 1999). To counteract this methodological limitation, during treadmill walking a supramaximal stimulus was delivered 80 ms after the test H-reflex stimulus at the same bin of the step cycle (Fig. 1B). This maximal M-wave was used to normalize the soleus M-wave and H-reflex of the same sweep. During treadmill walking, for each double stimuli (test H-reflex and maximal M-wave) delivered at each bin, the customized LabVIEW software measured the peak-to-peak amplitude of the M-wave and maximal M-wave and estimated the most optimal stimulation intensity. This stimulation intensity is based on the intensities and amplitudes from the M-wave and H-reflex recruitment curve during standing, and on the amplitude of the M-waves (0 to 15% of the maximal M-wave) and H-reflexes (50% of the maximal H-reflex or 20 to 30% of the maximal M-wave) elicited 80 ms after the test stimulus, both evoked on the on the ascending limb of the recruitment curve (Knikou et al. 2009a). Stimulation to the posterior tibial nerve was delivered randomly across the step cycle that was divided real-time into 16 equal bins based on the right heel and toe transducer signals registering heel contact and toe off (HLT100C, TSD111; Biopac Systems Inc., Goleta, CA, USA). Bin 1 corresponds approximately to heel contact, bin 8 to stance-to-swing initiation, bin 9 to swing initiation, and bins 10–16 to the swing phase (Knikou et al. 2009a, 2009b, 2011). At least 5 reflexes within the acceptance criteria were recorded at each bin of the step cycle, and approximately 560 H-reflexes were recorded during walking with and without transspinal stimulation for each subject.
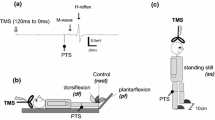
Experimental protocol. A.Transspinal and posterior tibial nerve stimulation along with surface EMG electrodes and foot switch transducers during walking on a motorized treadmill at self-selected speed. B. Representative raw waveform averages of the soleus H-reflex evoked throughout the step cycle that was divided into 16 equal bins. The first test stimulus (grey boxes) evoked the soleus M-wave (yellow boxes) and H-reflex (red boxes) followed 80 ms after by a supramaximal stimulus that evoked a maximal M-wave (green boxes) in each bin of the step cycle. Soleus H-reflexes were evoked randomly at each bin of the step cycle and were accepted when the M-wave ranged from 0 to 15% of the maximal M-wave evoked 80 ms after the test stimulus at the same bin, and the H-reflex was evoked on the ascending limb of the recruitment curve and was approximately 50% of the maximal H-reflex. At least 5 accepted H-reflexes were recorded at each bin, while approximately a total of 560 H-reflexes were recorded under control conditions and following transspinal stimulation for each subject
Soleus H-reflexes were recorded separately without (control) and during transspinal stimulation delivered at 15, 30, and 50 Hz with 10 kHz carry-over frequency (DS8R, Digitimer Ltd., Welwyn Garden City, UK) at subthreshold and suprathreshold paresthesia intensities. The paresthesia threshold was established during standing with 50 Hz transspinal stimulation since this frequency was noted by subjects to produce the most discomfort. Paresthesia threshold corresponded to the stimulation intensity that produced sensation spreading to paraspinal muscles. Sub- and suprathreshold stimulation was delivered at 0.96 (103.1 ± 61.37 mA) and 2.53 (180.37 ± 54.8 mA) times paresthesia threshold intensities, respectively. The intensity at suprathreshold level was adjusted when the subject reported great discomfort. Multiples of paresthesia threshold intensity were used to replicate stimulation protocols utilized in neurological disorders to promote recovery of sensorimotor function (Skiadopoulos et al. 2022).
Data analysis
The soleus M-wave and H-reflex evoked from the right posterior tibial nerve during treadmill walking were measured as peak-to-peak amplitude and were normalized to the associated maximal M-wave. Data were subjected to Shapiro–Wilk’s test for normal distribution and Mauchly’s test of sphericity for homogeneity of variances. When sphericity was not established, the Greenhouse–Geisser correction statistic was used. Repeated measures ANOVA (rmANOVA) with frequency, intensity, and bins as the main factors were performed to establish the main effects of transspinal stimulation on the phase-dependent soleus H-reflex amplitude modulation during treadmill walking.
The right SOL background EMG activity for each bin was estimated from the mean value of the linear EMG envelope of 50 ms duration (band-pass filtered 20–500 Hz, rectified, low-pass filtered at 20 Hz) at 100 ms before posterior tibial nerve stimulation (Knikou et al. 2009a, 2011, 2015). The right soleus background EMG activity was normalized to the maximum peak-to-peak amplitude of the SOL EMG during the walking bout that a control or conditioned H-reflex was also recorded. For each subject and reflex condition, the mean amplitude of the control and/or conditioned soleus H-reflexes was plotted on the y-axis against the homonymous normalized background EMG activity on the x-axis at each bin of the step cycle, and a least squares linear regression was fitted to the data. The slope and intercept from the linear regression were grouped based on transspinal stimulation frequency and intensity and statistically significant differences were established with an rmANOVA.
To establish whether transspinal stimulation influenced locomotor muscle activity, EMG signals of SOL, MG, TA, MH, VL, VM, and GRC muscles were filtered with a 4th-order Butterworth band-pass filter with lower cutoff frequency of 20 Hz and higher cutoff frequency of 500 Hz in LabVIEW software. After full-wave rectification linear EMG envelopes were obtained with a 4th-order Butterworth low-pass filter with cutoff frequency of 20 Hz, and the mean EMG amplitude across all steps was determined. The mean EMG amplitude was normalized to the associated maximal locomotor EMG. This analysis was performed separately for each muscle during treadmill walking with and without transspinal stimulation. For each subject, muscle and condition, the integrated EMG was calculated. Mauchly’s test for sphericity assumption was applied. Repeated measures ANOVA with Greenhouse–Geisser correction when the sphericity assumption was violated was applied to the data followed by post hoc Holm-Sidak tests to establish statistically significant differences on the EMG under control conditions and following transspinal stimulation at different frequencies and intensities. In all statistical tests, significant differences were established a α = 0.05 confidence level. Results are presented as mean values along with the standard error of the mean (SEM).
Results
Soleus H-reflex modulation during transspinal stimulation at 15, 30, and 50 Hz
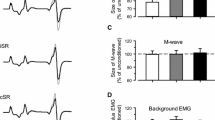
The average walking speed was 1.02 ± 0.09 m/s. The step cycle duration was not significantly different between the right and left legs with a mean duration of 1.252 ± 0.188 and 1.218 ± 0.117 s (p = 0.11), respectively. The overall average amplitudes of the soleus H-reflexes under control conditions and during subthreshold and suprathreshold transspinal stimulation are shown in Fig. 2A, B, respectively. The soleus H-reflexes for all cases are shown as percentages of the maximal M-wave evoked 80 ms at each bin after the test stimulus. Two-way rmANOVA at 7 × 16 levels (7: conditions; 16: bins of step cycle) on the normalized soleus H-reflexes grouped for each bin and conditions across subjects showed that the soleus H-reflex was not significantly different under control conditions and during 15, 30, and 50 Hz subthreshold and suprathreshold transspinal stimulation (F6, 960 = 1.044, p = 0.395). The soleus H-reflex was significantly different across the bins of the step cycle (F15, 960 = 93.23, p < 0.001), but an interaction between bins and conditions was not found (F90, 960 = 0.24, p = 1.0). The corresponding soleus M-waves (Fig. 2C, D) remained unchanged during control walking and following transspinal stimulation (F6, 960 = 0.89, p = 0.65). These results suggest that transspinal stimulation at 15, 30, and 50 Hz does not affect the physiological phase-dependent amplitude modulation of the soleus H-reflex in healthy subjects.
Soleus H-reflex phase-dependent amplitude modulation during transspinal stimulation. Soleus H-reflexes A, B. and associated M-waves C, D. amplitudes from all subjects as a percentage of the maximal M-wave evoked by a supramaximal stimulus 80 ms after the test H-reflex stimulus. For all cases, recordings are shown under control conditions and following subthreshold and suprathreshold transspinal stimulation at 15, 30, and 50 Hz with 10 kHz carry-over frequency. The bins of the step cycle are indicated as well as the step cycle as a percentage. Error bars for pool data are not indicated for clarity purposes
Relationship between soleus H-reflex and background EMG activity
The background EMG activity of the right soleus muscle as a function of the step cycle from all subjects is illustrated in Fig. 3. The soleus background EMG activity is shown under control conditions and during 15, 30, and 50 Hz transspinal stimulation at subthreshold and suprathreshold intensities. Two-way ANOVA at 7 × 16 levels (7: conditions, 16: bins of the step cycle) showed that the soleus background EMG activity was significantly different across conditions (F6, 920 = 2.11, p = 0.049). Holm-Sidak pairwise multiple comparisons showed that a significant difference was present only between suprathreshold 15 Hz and subthreshold 30 Hz (t = 3.33, p < 0.001).
Soleus background EMG activity, normalized to the maximum soleus locomotor EMG, from all subjects is plotted against the bin number of the step cycle when the H-reflex was evoked without (control condition; black lines) and with subthreshold A. and suprathreshold B. transspinal stimulation. The bins of the step cycle are indicated as well as the step cycle as a percentage. Error bars for pool data are not indicated for clarity purposes
The soleus EMG background activity was linearly related to the soleus H-reflex amplitude (Fig. 4A) under control conditions (R2 = 0.91; y = 78.51x—0.902) and during all transspinal stimulations, for example at 30 Hz subthreshold (R2 = 0.93; y = 82.45x – 1.07) and 30 Hz suprathreshold (R2 = 0.91; y = 76.81x – 3.26). The overall amplitude of the slope (Fig. 4B) computed from the linear regression line fitted to the soleus H-reflex amplitude plotted against the soleus background EMG activity at each bin of the step cycle for each subject and condition separately was not significantly different across conditions (Kruskal–Wallis; H = 3.049, df = 6, p = 0.8). A similar result was found for the intercept (Fig. 4C) of the linear regression line (one-way ANOVA; F6,59 = 0.146, p = 0.989).
Linear relationship between background soleus EMG activity and H-reflex amplitude modulation. A. The overall mean amplitude from all subjects of the soleus H-reflex expressed as a percentage of the maximal M-wave is plotted against the soleus background EMG activity. For all graphs the 16 points correspond to the 16 bins of the step cycle. B, C. The overall amplitude of slope and intercept from all subjects tested resulting from the linear relationship between the mean amplitude of the soleus H-reflex and EMG background activity during walking on a motorized treadmill, fitted for each subject and condition separately
EMG activation profiles during transspinal stimulation at 15, 30, and 50 Hz
Figure 5 summarizes the linear EMG envelopes from all subjects during treadmill walking under control conditions (black lines) and during transspinal stimulation for the left and right knee and ankle muscles. The linear EMG envelopes are grouped separately for subthreshold and suprathreshold transspinal stimulation at 15, 30 and 50 Hz. Overall, the SOL, MG, and TA EMG amplitudes were not significantly different among conditions tested (p > 0.05), while the EMG amplitude of thigh/knee muscles varied significantly from control EMG values during suprathreshold transspinal stimulation. Specifically, an increase in motor output was evident in right VM at suprathreshold 30 Hz (one-way ANOVA; F6,47 = 3.517, p = 0.006 followed by Holm-Sidak), right VL at suprathreshold 50 Hz (one-way ANOVA; F6,44 = 3.34, p = 0.008), left VM at suprathreshold 30 and 50 Hz (F6, 30 = 13.14, p < 0.05), and left VL at suprathreshold 30 and 50 Hz (F6,24 = 13.96, p < 0.05). To conclude, transspinal stimulation facilitates motor activity of knee extensor muscles while the EMG profile of distal ankle muscles remains unchanged.
Mean linear EMG envelopes from all subjects of the left and right SOL, MG, TA, MH, VM, GRC and VL muscles during control stepping (black lines) are shown superimposed to the EMGs recorded during transspinal stimulation at 15, 30, and 50 Hz transspinal stimulation at subthreshold and suprathreshold paresthesia intensities. The step cycle and EMG amplitudes are shown as a percentage of the total step cycle duration and associated maximum locomotor EMG activity
Discussion
In this study, we provide evidence on the actions of transspinal stimulation at 15, 30, and 50 Hz on spinal locomotor networks in healthy humans. Transspinal stimulation when delivered above or below paresthesia levels resulted in no significant effects on the soleus H-reflex phase-dependent modulation profile. This finding was evident regardless of stimulation frequency or intensity. In addition, an unaltered soleus background EMG activity, slope, and intercept of the linear relationship between background EMG activity and soleus H-reflex amplitude was present. Moreover, transspinal stimulation had differential effects on the locomotor muscle activity of thigh and ankle muscles. Locomotor EMG activity did not alter in the distal ankle muscles while excitatory effects were evident in the more proximal to the stimulation site knee extensor muscles.
Our findings differ from the generalized depression of neuronal excitability we have recently reported during 1 ms single pulse transspinal conditioning stimulation (Pulverenti et al. 2019; Islam et al. 2021). The difference may be attributed to the fact that single pulse transspinal stimulation at suprathreshold intensities produces long-lasting inhibition on motoneuron excitability (Knikou and Murray 2018). Specifically, the soleus H-reflex, TA flexion reflex, bilateral ankle MEPs, and bilateral knee and ankle EMG activity are all depressed following single 1 ms or pulse train transspinal conditioning stimulation at supra motor threshold intensities (Pulverenti et al. 2019; Zaaya et al. 2020; Islam et al. 2021). Maintenance of a physiological soleus H-reflex modulation profile during walking (Fig. 2) suggests that transspinal stimulation at these frequencies and intensities does not block or decrease the afferent volleys traveling to the spinal cord during walking and thereby the physiological spinal integration of proprioceptive afferent signals. Appropriate engagement of spinal neuronal circuits at each step cycle phase is reflected by the phase-dependent amplitude modulation of the soleus H-reflex. Therefore, transspinal stimulation at 15, 30, and 50 Hz delivered above and below paresthesia levels can be delivered during walking when used as a treatment strategy in upper motoneuron lesions.
Further, a significant new finding was the increase in activity of bilateral VL and VM muscles throughout the step cycle (Fig. 5). The increase in EMG activity clearly suggests that transspinal stimulation may be used as a neuromodulatory strategy to promote standing and walking abilities in people with upper motoneuron lesions. Transspinal stimulation over the thoracolumbar enlargement produces bilateral leg extension of paralyzed or paretic muscles and improves gait kinematics and locomotor EMG activity during weight-supported walking in people with spinal cord injury (Hofstoetter et al. 2015). Thus, it is likely that transspinal stimulation activates the neural circuits for standing and walking. Indeed, recent data show that stand training with transspinal stimulation at varying frequencies (0.2 to 30 Hz) in persons with spinal cord injury, promotes self-assisted standing and upright trunk posture with minimal or no external assistance (Rath et al. 2018; Sayenko et al. 2019).
The mechanism(s) by which VL and VM locomotor EMG activity increases is not well understood. We may attribute this effect to 1) activation of excitatory or inhibition of inhibitory local neuronal pathways, 2) changes in membrane potentials of alpha motoneurons, 3) potentiation of synaptic transmission, and 4) changes in descending motor drive and ascending sensory convergence. The latter is supported by the summation between soleus H-reflex, soleus/TA MEPs and TEPs, and modulation of the afferent-mediated MEP facilitation (Knikou 2014; Knikou et al. 2015; Knikou and Murray 2018) but cannot explain the selective increase of activity in VL/VM bilaterally. The cathodal electrode was placed at Thoracic 10 and covered based on body/trunk height up to Lumbar 1/2. Consequently, the non-significant effect on ankle EMG activity cannot be attributed to the position of the cathodal electrode, since at this position TEPs in ankle muscles are readily evocable (Knikou 2013, 2014; Skiadopoulos et al. 2022). Transspinal stimulation at 2.5 times paresthesia intensities excited cutaneous afferents over multiple segments affecting indirectly activity of different classes of spinal interneurons. In the future it is worth investigating the amplitude modulation of the quadriceps H-reflex alone and following superficial peroneal nerve conditioning stimulation while transspinal stimulation is delivered at rest and during walking. Cutaneous stimulation of the superficial peroneal nerve at rest produces biphasic facilitation of the quadriceps H-reflex, due to activation of lumbar propriospinal neurons, which reverses to inhibition during contraction due to Ib inhibitory interneurons (Marchand-Pauvert et al. 2002). This conditioning reflex protocol, although complex, will establish directly possible mechanisms associated with the increase in EMG activity we observed in this study.
Limitations of the study
We investigated here the modulatory effects of only 2 different intensities relative to the paresthesia threshold. Further experiments are needed at stimulation intensities where TEPs in knee and ankle muscles are evoked. Moreover, these experiments need to be repeated in people with spinal cord injury because the injured spinal cord may respond differently. Understanding better the underlying mechanisms of action will enable us to develop targeted stimulation protocols to maximize the recovery of standing and walking abilities of these individuals.
Conclusion
We demonstrated that transspinal stimulation at 15, 30 and 50 Hz below and above paresthesia did not affect the soleus H-reflex phase amplitude modulation or the EMG activity of ankle muscles in able-bodied individuals. An increase in EMG activity was evident for the knee extensor muscles bilateral. Further studies are required to determine the underlying mechanisms of action of transspinal stimulation in spinal locomotor networks, and whether differential actions are present in individuals with spinal cord injury.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Change history
15 March 2024
In this article, the family name of the author Abdullah M. Sayed Ahmad was incorrect. This has been corrected.
References
Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ (2014) Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 137(Pt 5):1394–1409. https://doi.org/10.1093/brain/awu038
Capaday C, Stein RB (1986) Amplitude modulation of the soleus H-reflex in the human during walking and standing. J Neurosci 6(5):1308–1313. https://doi.org/10.1523/JNEUROSCI.06-05-01308.1986
Capaday C, Stein RB (1987) Differences in the amplitude of the human soleus H reflex during walking and running. J Physiol 392(1):513–522. https://doi.org/10.1113/jphysiol.1987.sp016794
Courtine G, Harkema SJ, Dy CJ, Gerasimenko YP, Dyhre-Poulsen P (2007) Modulation of multisegmental monosynaptic responses in a variety of leg muscles during walking and running in humans. J Physiol 582(3):1125–1139. https://doi.org/10.1113/jphysiol.2007.128447
Dimitrijevic MR, Gerasimenko Y, Pinter MM (1998) Evidence of spinal central pattern generator in humans. Ann N Y Acad Sci 860:360–376. https://doi.org/10.1111/j.1749-6632.1998.tb09062.x
Dy CJ, Gerasimenko YP, Edgerton VR, Dyhre-Poulsen P, Courtine G, Harkema SJ (2010) Phase-dependent modulation of percutaneously elicited multisegmental muscle responses after spinal cord injury. J Neurophysiol 130(5):2808–2820. https://doi.org/10.1152/jn.00316.2009
Formento E, Minassian K, Wagner F, Mignardot JB, Le Goff-Mignardot CG, Rowald A, Bloch J, Micera S, Capogrosso M, Courtine G (2018) Electrical spinal cord stimulation must preserve proprioception to enable locomotion in humans with spinal cord injury. Nat Neurosci 21(12):1729–1741. https://doi.org/10.1038/s41593-018-0262-6
Gorodnichev RM, Pivovarova EA, Puhov A, Moiseev SA, Savochin AA, Moshonkina TR, Chsherbakova NA, Kilimnik VA, Selionov VA, Kozlovskaya IB, Edgerton VR, Gerasimenko YP (2012) Transcutaneous electrical stimulation of the spinal cord: a noninvasive tool for the activation of stepping pattern generators in humans. Hum Physiol 38(2):158–167. https://doi.org/10.1134/S0362119712020065
Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman RG, Edgerton VR (2011) Effect of epidural stimulation of the lumbrosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. The Lancet 377(9781):1938–1947. https://doi.org/10.1016/S0140-6736(11)60547-3
Hofstoetter US, Minassian K, Hofer C, Mayr W, Rattay F, Dimitrijevic MR (2008) Modification of reflex responses to lumbar posterior root stimulation by motor tasks in healthy subjects. Artif Organs 32(8):644–648. https://doi.org/10.1111/j.1525-1594.2008.00616.x
Hofstoetter US, McKay WB, Tansey KE, Mayr W, Kern H, Minassian K (2014) Modification of spasticity by transcutaneous spinal cord stimulation in individuals with incomplete spinal cord injury. J Spinal Cord Med 37(2):202–211. https://doi.org/10.1179/2045772313Y.0000000149
Hofstoetter US, Freundl B, Binder H, Minassian K (2018) Common neural structures activated by epidural and transcutaneous lumbar spinal cord stimulation: elicitation of posterior root-muscle reflexes. PLoS ONE 13(1):e0192013. https://doi.org/10.1371/journal.pone.0192013
Islam MA, Pulverenti TS, Knikou M (2021) Neuronal actions of transspinal stimulation on locomotor networks and reflex excitability during walking in humans with and without spinal cord injury. Front Hum Neurosci 15:620414. https://doi.org/10.3389/fnhum.2021.620414
Knikou M (2008) The H-reflex as a probe: pathways and pitfalls. J Neurosci Methods 171(1):1–12. https://doi.org/10.1016/j.jneumeth.2008.02.012
Knikou M (2012) Function of group IB inhibition during assisted stepping in human spinal cord injury. J Clin Neurophysiol 29(3):271–277. https://doi.org/10.1097/WNP.0b013e318257c2b7
Knikou M (2013) Neurophysiological characterization of transpinal evoked potentials in human leg muscles. Bioelectromagnetics 34(8):630–640. https://doi.org/10.1002/bem.21808
Knikou M (2014) Transpinal and transcortical stimulation alter corticospinal excitability and increase spinal output. PLoS ONE 9(7):e102313. https://doi.org/10.1371/journal.pone.0102313
Knikou M, Mummidisetty CK (2011) Reduced reciprocal inhibition during assisted stepping in human spinal cord injury. Exp Neurol 231(1):104–112. https://doi.org/10.1016/j.expneurol.2011.05.021
Knikou M, Mummidisetty CK (2014) Locomotor training improves premotoneuronal control after chronic spinal cord injury. J Neurophysiol 111(11):2264–2275. https://doi.org/10.1152/jn.00871.2013
Knikou M, Murray LM (2018) Neural interactions between transspinal evoked potentials and muscle spindle afferents in humans. J Electromyogr Kinesiol 43:174–183. https://doi.org/10.1016/j.jelekin.2018.10.005
Knikou M, Murray LM (2019) Repeated transspinal stimulation decreases soleus H-reflex excitability and restores spinal inhibition in human spinal cord injury. PLoS ONE 14(9):e0223135. https://doi.org/10.1371/journal.pone.0223135
Knikou M, Angeli CA, Ferreira CK, Harkema SJ (2009a) Soleus H-reflex modulation during body weight support treadmill walking in spinal cord intact and injured subjects. Exp Brain Res 193(3):397–407. https://doi.org/10.1007/s00221-008-1636-x
Knikou M, Angeli CA, Ferreira CK, Harkema SJ (2009b) Soleus H-reflex gain, threshold, and amplitude as function of body posture and load in spinal cord intact and injured subjects. Int J Neurosci 119(11):2056–2073. https://doi.org/10.1080/00207450903139747
Knikou M, Hajela N, Mummidisetty CK, Xiao M, Smith AC (2011) Soleus H-reflex phase-dependent modulation is preserved during stepping within a robotic exoskeleton. Clin Neurophysiol 122(7):1396–1404. https://doi.org/10.1016/j.clinph.2010.12.044
Knikou M, Dixon L, Santora D, Ibrahim MM (2015) Transspinal constant-current long-lasting stimulation: a new method to induce cortical and corticospinal plasticity. J Neurophysiol 114(3):1486–1499. https://doi.org/10.1152/jn.00449.2015
Llewellyn M, Yang JF, Prochazka A (1990) Human H-reflexes are smaller in difficult beam walking than in normal treadmill walking. Exp Brain Res 83:22–28. https://doi.org/10.1007/BF00232189
Marchand-Pauvert V, Nicolas G, Burke D, Pierrot-Deseilligny E (2002) Suppression of the H reflex in humans by disynaptic autogenetic inhibitory pathways activated by the test volley. J Physiol 542(Pt 3):963–976. https://doi.org/10.1113/jphysiol.2002.021683
Menard A, Leblond H, Gossard JP (2002) Sensory integration in presynaptic inhibitory pathways during fictive locomotion in the cat. J Neurophysiol 88(1):163–171. https://doi.org/10.1152/jn.2002.88.1.163
Minassian K, Jilge B, Rattay F, Pinter MM, Binder H, Gerstenbrand F, Dimitrijevic MR (2004) Stepping-like movements in humans with complete spinal cord injury induced by epidural stimulation of the lumbar cord: electromyographic study of compound muscle action potentials. Spinal Cord 42(7):401–416. https://doi.org/10.1038/sj.sc.3101615
Minassian K, Hofstoetter US, Danner SM, Mayr W, Bruce JA, McKay WB, Tansey KE (2016) Spinal rhythm generation by step-induced feedback and transcutaneous posterior root stimulation in complete spinal cord-injured individuals. Neurorehabil Neural Repair 30:233–243. https://doi.org/10.1177/1545968315591706
Moraud EM, Capogrosso M, Formento E, Wenger N, DiGiovanna J, Courtine G, Micera S (2016) Mechanisms underlying neuromodulation of spinal circuits for correcting gait and balance deficits after spinal cord injury. Neuron 89(4):814–828. https://doi.org/10.1016/j.neuron.2016.01.009
Mummidisetty CK, Smith AC, Knikou M (2013) Modulation of reciprocal and presynaptic inhibition during robotic-assisted stepping in humans. Clin Neurophysiol 124(3):557–564. https://doi.org/10.1016/j.clinph.2012.09.007
Pulverenti TS, Islam MA, Alsalman O, Murray LM, Harel NY, Knikou M (2019) Transspinal stimulation decreases corticospinal excitability and alters the function of spinal locomotor networks. J Neurophysiol 122(6):2331–2343. https://doi.org/10.1152/jn.00554.2019
Pulverenti TS, Zaaya M, Grabowski E, Grabowski M, Knikou M (2022) Brain and spinal cord paired stimulation coupled with locomotor training facilitates motor output in human spinal cord injury. Front Neurol 13:1000940. https://doi.org/10.3389/fneur.2022.1000940
Rath M, Vette AH, Ramasubramaniam S, Li K, Burdick J, Edgerton VR, Gerasimenko YP, Sayenko DG (2018) Trunk stability enabled by noninvasive spinal electrical stimulation after spinal cord injury. J Neurotrauma 35:2540–2553. https://doi.org/10.1089/neu.2017.5584
Rattay F, Minassian K, Dimitrijevic MR (2000) Epidural stimulation of posterior structures of the human lumbrosacral cord: 2. quantitative analysis by computer modeling. Spinal Cord 38(8):473–489. https://doi.org/10.1038/sj.sc.3101039
Rudomin P (2009) In search of lost presynaptic inhibition. Exp Brain Res 196(1):139–151. https://doi.org/10.1007/s00221-009-1758-9
Sayenko D, Rath M, Ferguson AR, Burdick J, Havton L, Edgerton VR, Gerasimenko Y (2019) Self-assisted standing enabled by non-invasive spinal stimulation after spinal cord injury. J Neurotrauma 36(9):1435–1450. https://doi.org/10.1089/neu.2018.5956
Shapkova EY, Schomburg ED (2001) Two types of motor modulation underlying human stepping evoked by spinal cord electrical stimulation (SCES). Acta Physiol Pharacol Bulg 26(3):155–157
Skiadopoulos A, Pulverenti TS, Knikou M (2022) Physiological effects of cathodal electrode configuration for transspinal stimulation in humans. J Neurophysiol 128(6):1663–1682. https://doi.org/10.1152/jn.00342.2022
Yang JF, Stein RB, James KB (1991) Contribution of peripheral afferents to the activation of the soleus muscle during walking in humans. Exp Brain Res 87(3):679–687. https://doi.org/10.1007/BF00227094
Zaaya M, Pulverenti TS, Islam MA, Knikou M (2020) Transspinal stimulation downregulates activity of flexor locomotor networks during walking in humans. J Electromyogr Kinesiol 52:102420. https://doi.org/10.1016/j.jelekin.2020.102420
Zaaya M, Pulverenti TS, Knikou M (2021) Transspinal stimulation and step training alter function of spinal networks in complete spinal cord injury. Spinal Cord Ser Cases 7(1):55. https://doi.org/10.1038/s41394-021-00421-6
Zehr E, Stein RB (1999) What functions do reflexes serve during human locomotion? Prog Neurobiol 58(2):185–205. https://doi.org/10.1016/s0301-0082(98)00081-1
Funding
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) of the National Institutes of Health (NIH) under Grant Number R01HD100544 and by the New York State Department of Health (NYSDOH), Spinal Cord Injury Research Board (SCIRB) under Contracts C35594GG and C38333GG awarded to Maria Knikou. The funding sources were not involved in study design, data collection, data analysis, data interpretation, or decision to publish.
Author information
Authors and Affiliations
Contributions
AMS: performed experiments, analyzed data, and approved the final version of the manuscript.
MR, YA, MM, JF, SKS, AS: performed experiments, and approved the final version of the manuscript.
MK: conception and design of research, performed experiments, analyzed data, prepared figures, interpreted results of experiments, wrote the first draft of the manuscript, approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of the City University of New York. All participants provided their written informed consent to participate in this study.
Additional information
Communicated by Bill J Yates.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sayed Ahmad, A.M., Raphael, M., Han, J.F. et al. Soleus H-reflex amplitude modulation during walking remains physiological during transspinal stimulation in humans. Exp Brain Res 242, 1267–1276 (2024). https://doi.org/10.1007/s00221-024-06779-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-024-06779-x