Abstract
Using near infrared spectroscopy and repetitive transcranial magnetic stimulation (rTMS), we studied interhemispheric interactions between bilateral motor and sensory cortices in humans. RTMS consisted of a triple-pulse burst (50 Hz) repeated every 200 m for 2 s (10 bursts, 30 pulses); one kind of theta burst TMS (TBS) (Huang et al. in Neuron 45:201–206, 2005). The hemoglobin concentration changes were recorded at the right prefrontal cortex, premotor area (PM), primary hand motor area (M1) and primary sensory area (S1) during and after TBS over the left PM, M1 and S1 or sham stimulation in eight normal volunteers. In addition, motor evoked potentials (MEPs) to TMS over the right M1 were recorded from the left first dorsal interosseous muscle after the conditioning TBS over left S1. TBS over PM induced a significant oxy-Hb decrease at the contralateral PM. TBS over M1 elicited a significant oxy-Hb decrease at the contralateral S1, and TBS over S1 significant oxy-Hb decreases at the contralateral M1 and S1. MEPs to TMS of the right M1 were significantly suppressed by the conditioning TBS over the left S1. These results suggest that there are mainly inhibitory interactions between bilateral PMs and bilateral sensorimotor cortices in humans. Those are partly compatible with the previous findings. In addition to between the primary motor cortices, bilateral connection is requisite for smooth bimanual coordination between the sensory cortices or premotor cortices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To investigate the interactions between two distant cortical areas in humans, several non-invasive methods have been used. They are neuroimaging methods, electrophysiological methods including transcranial magnetic stimulation (TMS) over two sites (paired stimulation method) and their combinations. Paired TMS techniques were first applied to see the connections, such as between bilateral motor cortices (Ferbert et al. 1992), cerebello-motor cortical (Ugawa et al. 1995) connections. The inhibitory interaction between bilateral primary motor cortices (M1) was demonstrated by paired TMS over the bilateral M1s (Ferbert et al. 1992). Similar paired TMS also showed interactions between premotor areas (PMs) and bilateral M1s (Civardi et al. 2001; Mochizuki et al. 2004a) and between motor related areas and the contralateral primary sensory cortex (S1) (Mochizuki et al. 2004b). These physiological studies can evaluate interactions between the sensorimotor area and other areas, but can hardly evaluate interactions between other areas (e.g., between bilateral PMs) because we have no good physiological measures to evaluate the excitability of areas other than the sensorimotor cortex.
Recent neuroimaging studies could show some functional connections between two distant cortical areas other than sensorimotor cortices. Positron emission tomography (PET) revealed regional cerebral blood flow (rCBF) changes at bilateral motor related areas including the supplementary motor area, PM and cerebellum elicited by repetitive TMS over M1 or PM (Siebner et al. 2000; Chouinard et al. 2003). These results suggest some tight connections between the stimulated area and areas showing rCBF changes. However, one major limitation of PET study is due to the irradiation of the subjects. Near-infrared spectroscopy (NIRS) is one of alternative noninvasive methods to see cerebral functional changes. It has four distinct advantages over preexisting techniques: high signal-to-noise ratios for single events, noninterference with magnetic field changes elicited by TMS, higher time resolution than PET and the lack of irradiation. This technique estimates hemoglobin (Hb) concentration changes by measuring reflected light, based on the differences in absorption spectra between oxy- and deoxy-Hb (Jöbsis 1977; Chance et al. 1988; Villringer et al. 1993). In previous studies we successfully recorded Hb concentration changes by a single pulse TMS (Noguchi et al. 2003; Mochizuki et al. 2006). In the present paper, using combination of NIRS and TMS, we studied the interhemispheric interactions between bilateral motor and sensory cortical areas in humans.
Subjects and methods
Subjects
Eight healthy volunteers (two women and six men, 28–53 years old) participated in this study. All subjects were right handed based on the Edinburgh Handedness Inventory (Oldfield 1971) and they all gave written informed consent to participate in the study. The experimental procedures used here were approved by the Ethics Committee of the University of Tokyo and were carried out in accordance with the Declaration of Helsinki.
Electromyographic recordings
Surface electromyograms (EMGs) were recorded from the bilateral first dorsal interosseous muscles (FDIs) with 9 mm diameter, Ag–AgCl surface cup electrodes. The active electrode was placed over the muscle belly and the reference electrode over the metacarpophalangeal joint of the index finger. Responses were amplified with an amplifier (Biotop, NEC Medical Systems, Japan) through filters set at 100 Hz and 3 kHz, then recorded with a sampling rate of 10 kHz and stored by a computer (Signal Processor DP-1200, NEC Medical Systems, Japan) for later off-line analysis of the data.
TMS
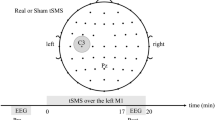
TMS was delivered with a figure-of-eight-shaped coil (outer diameter of each wing was 7 cm) connected to a Magstim 200 magnetic stimulator or a Magstim Super Rapid Package (The Magstim Co. Ltd, Whitland, UK). The coil was positioned over the left PM, M1 or S1. M1 was defined as the “hot spot” where stimulation evoked the largest MEP in the right FDI. In two of the subjects, that position was confirmed to be over the primary motor cortex by the neuronavigation system (Spetzger et al. 1995; Boroojerdi et al. 1999). PM is proved to be located 2–3 cm anterior site from M1 by PET study (Fink et al. 1997). To minimize stimulation effect spreading by TMS, we kept the distance between two stimulation sites as long as possible, and defined PM at a 3 cm anterior site from M1. In our previous paper (Enomoto et al. 2001), the S1 demonstrated by functional magnetic resonance images was located at 2 cm posterior to C3 of the International 10–20 system, the recording site for SEP. We selected a parietal lobe at a 3 cm posterior, 3 cm lateral site from M1. It was a bit posterior to the recording site for SEP. However, we have confirmed it to be a posterior part of somatosensory cortex, and we defined it as S1 (Fig. 1). At 2 cm away from the coil, the magnetic field elicited by single pulse TMS decreased to the half of that provoked just underneath the coil (Bohning et al. 1997). So, 3 cm apart from the coil may be long enough for excluding the stimulation spread. In fact, repetitive TMS (rTMS) at an intensity of 90% AMT could activate PM (2.5 cm anterior from M1) and M1 separately (Gerschlager et al. 2001).
Locations of TBS and NIRS recording sites. TBS was applied over left PM, M1 or S1. NIRS recordings were made at four measurement points. Each measurement pair of emitter (open circle) and detector (open square) was located on medio-lateral line at a distance of 3 cm. Measurement points (midpoints) were arranged on right PFC, PM, M1 and S1
The coil was oriented to induce medially directed currents in the brain when monophasic TMS was delivered, and medially directed currents at the first phase when delivering biphasic TMS. The intensity was adjusted to be 80 and 100% of the active motor threshold (AMT) at M1 for biphasic conditioning TMS. We defined the AMT as the lowest intensity that evoked five small responses (about 100 μV) in a series of ten stimuli when the subject made a 5% maximal voluntary contraction (about 50 μV). Sham stimulation was performed with two coils. One non-discharging coil was positioned at the midpoint between left M1 and PM, and the other was positioned 10 cm above the head and the same currents as real stimulation were induced in it to make sound. The holder of the first coil was touched to the edge of the other one for delivering vibration to the subject when giving a stimulus. As the conditioning stimulus, theta burst TMS (TBS) was used since it has good modulation effects on the stimulated site (Huang et al. 2005).
One train of TBS consisted of triple-pulse bursts (50 Hz) repeated every 200 m for 2 s (10 bursts, 30 pulses). TBS over three sites and sham stimulation were applied at an intensity of 80 or 100% AMT. Eight different stimulation conditions (four sites of stimulation × two intensities) were done in all the subjects. Each stimulation condition consisted of two sessions. Then, in total, 16 sessions (two sessions × eight conditions) were done in one subject. Each session consisted of ten trains of TBS (total 100 bursts, 300 pulses). Each train was given at random inter-train intervals of 34–36 s. We cannot completely exclude the possibility of some accumulative effects even at this interval of 34–36 s. But continuous NIRS recording showed that in most subjects Hb concentration changes by TBS returned to the baseline before the next TBS. In addition, since all experiments were done in the same manner and compared the results between different stimulation conditions, we can safely say that some difference should be present between different stimulation conditions. The interval between the end of a session and the onset of next session was 15 min, which was long enough for the Hb concentration changes to return to the baseline. The order of sessions was counterbalanced within and across subjects. At most, six sessions were done on one experimental day (1,800 pulses at most). In one subject, all the experiments were performed on 3 days separated by a week or more.
To see some adverse effect and cope with it immediately, we recorded electroencephalograms continuously and one doctor observed the subject carefully during whole the experiments. We also sometimes talked with the subject in the experiments to check their condition.
NIRS measurement
We used a NIRS system (ETG-A1; Hitachi Medical Corporation, Tokyo, Japan) having four emitters and four detectors, and the four measurement points (midpoints between emitters and detectors) were placed on the right PM, M1, S1 and a site at 3 cm anterior from PM [which is corresponding to prefrontal cortex (PFC)] (Fig. 1). The homologous positions in the contralateral hemisphere were also marked as the stimulated sites. Near-infrared laser diodes with two wavelengths, 790 and 830 nm, were used as the light sources, and transmittance data of the light beams were obtained every 500 m. We calculated concentrations of oxy-hemoglobin (oxy-Hb) and deoxy-hemoglobin (deoxy-Hb) from the transmittance data. In this study, each event period ranged from 5 s before the TBS onset to 30 s after the end of TBS. Under each condition, the average Hb concentration changes were obtained from the results of two sessions and they were used in statistical analyses.
Additional physiological experiment
In the main experiments, we observed a large oxy-Hb decrease and small deoxy-Hb increase at the right M1 after TBS over the left S1 (see “Results”, third column in Figs. 3, 5). To confirm this finding electrophysiologically, we examined the excitability of the right M1 after TBS over the left S1 using a randomized conditioning-test design reported previously (Hanajima et al. 2001).
In this experiment, the test stimulus was a single pulse, monophasic TMS over the right M1 with a figure-of-eight-shaped coil (outer diameter of each wing was 7 cm). The coil was oriented to induce anteriorly directed currents in the brain. The intensity of the test stimulus was adjusted to evoke an MEP of approximately 0.2–0.5 mV peak to peak in the relaxed left FDI. The conditioning stimulus was TBS over the left S1. TBS was composed of triple-pulse bursts repeated every 200 ms for 2 s (10 bursts, 30 pulses) at an intensity of 80% AMT. Three conditions [the test stimulus (15 trials) or conditioning TBS given alone (5 trials), and the test stimulus preceded by the conditioning TBS by an interval of 200 m from the onset of the last burst (15 trials)] were intermixed randomly in one block. Data were analyzed off-line after the experiments.
The amplitude of each single MEP from the left FDI in each condition was measured in order to compare amplitudes of the control and conditioned MEPs in the same block. We calculated the ratio of the mean amplitude of the conditioned MEP to that of the control MEP in each subject. The order of two blocks (TBS over S1 and sham stimulation) was counterbalanced across subjects. This additional experiment was performed 1 week or longer interval after the last day of the main experiments.
Statistical analysis
In the main experiments, the 95% confidence interval was calculated for each time point of oxy- and deoxy-Hb changes in each condition. We obtained the mean Hb concentration changes by averaging Hb data from 5 to 20 s after a TBS pulse in each condition, and used these values in statistical comparisons. In each NIRS recording point, two-way analysis of variance (ANOVA) [with factors of stimulation type (three sties and sham stimulation) and INTENSITY (80 and 100%)] was performed on the mean Hb concentration changes. When an ANOVA test showed significant effects, we further performed post-hoc analyses with Scheffe’s method for the significant effects. In the additional experiment, we used a paired t-test for comparison. The statistical significance was set at P = 0.05.
Results
Neither abnormal electroencephalograms nor any adverse effects were noted during or after the experiments in any subjects.
Figures 2, 3, 4 and 5 show averaged relative Hb-concentration changes and the 95% confidence intervals at right PFC (Fig. 2), PM (Fig. 3), M1 (Fig. 4) or S1 (Fig. 5) when TBS was applied over left PM, M1 or S1 or when sham stimulation was given.
NIRS recordings at right PFC. TBS was applied over left PM (first column), M1 (second column), S1 (third column) or sham stimulation (fourth column) at 80% AMT a or 100% AMT b. The first and third rows show oxy-Hb concentration changes, and the second and fourth deoxy-Hb. The ground averaged data (n = 8) for the four stimulation conditions are separately shown. The averages are depicted by thick lines and the 95% confidence intervals thin lines. No significant changes were evoked by any stimulation
NIRS recordings at right PM. Figure settings are all the same as Fig. 2. The PM stimulation evoked significant reduction of oxy Hb whereas no other stimulations elicited any changes
NIRS recordings at right M1. Figure settings are all the same as Fig. 2. Significant oxy-Hb decrease was observed in the left PM (80% AMT) and S1 (80 and 100% AMT) stimulation conditions
NIRS recordings at right S1. Figure settings are all the same as Fig. 2. Significant oxy-Hb decreases were evoked by the left M1 (80 and 100% AMT) and S1 (80 and 100% AMT) stimulations
No significant changes in oxy-Hb concentration (longer than 15 s) were observed at right PFC (Fig. 2). At right PM, significant oxy-Hb decreases were observed in the left PM stimulation condition (80 and 100% AMT) (Fig. 3). At an intensity of 80% AMT, oxy-Hb began to decrease after the TBS onset and returned to the baseline around 25 s later at the right PM (Fig. 3a, first column). The oxy-Hb significantly decreased as compared to the baseline 4–25 s after the TBS onset, as shown by the 95% confidence lines (the upper thin line was lower than zero), but the deoxy-Hb did not change significantly. No significant changes were seen when TBS was applied over the other sites. At right M1, significant oxy-Hb decreases were observed in the left PM (80% AMT) and S1 (80 and 100% AMT) stimulation conditions (Fig. 4). At the right S1, significant decreases were observed in the left M1 (80 and 100% AMT) and S1 (80 and 100% AMT) stimulation conditions (Fig. 5). Sham stimulation induced no significant changes in oxy-Hb. No significant changes were observed in deoxy-Hb in any conditions.
Comparisons of mean oxy-Hb concentration changes between four sites of stimulation (stimulation SITE) or TBS intensities (INTENSITY) at each recording site showed some significant effects (SITE effects of 80% AMT TBS were summarized in Fig. 6). At the right PM, two-way ANOVA showed a significant stimulation SITE effect [SITE, F (3, 56) = 7.38, P < 0.001; INTENSITY, F (1, 56) = 3.59, P = 0.06] without any significant SITE × INTENSITY interaction [F (3, 56) = 0.62, P = 0.61] and post-hoc analysis revealed that TBS over left PM induced stronger oxy-Hb decrease than the other three stimulation sites (P < 0.05). At the right M1, there were significant SITE and INTENSITY effects [SITE, F (3, 56) = 3.95, P = 0.01; INTENSITY, F (1, 56) = 4.79, P = 0.03] without any significant SITE × INTENSITY interaction [F (3, 56) = 0.23, P = 0.88]. Post-hoc analysis disclosed that TBS over left S1 induced deeper oxy-Hb reduction than sham stimulation (P = 0.03) whereas its effect did not significantly differ from those evoked by TBS over left PM (P = 0.07) or M1 (P = 0.15). The significant INTENSITY effect meant that TBS effect at an intensity of 80% AMT was larger than that at 100% AMT. At the right S1, there was a significant SITE effect [SITE, F (3, 56) = 5.98, P = 0.001; INTENSITY, F (1, 56) = 0.03, P = 0.86] without any significant SITE × INTENSITY interaction [F (3, 56) = 0.14, P = 0.94]. TBS over the left M1 or S1 induced more prominent oxy-Hb decrease than sham stimulation. At the right PFC, there were no significant effects or interactions [SITE, F (3, 56) = 1.34, P = 0.34; INTENSITY, F (1, 56) = 3.30, P = 0.08; SITE × INTENSITY, F (3, 56) = 1.47, P = 0.23].
Mean changes of relative oxy-Hb concentrations (averages of Hb concentration values from 5 to 20 s after the onset of TBS at 80% AMT) at right PFC, PM, M1 and S1. Concentration changes elicited by TBS over left PM are denoted by filled columns, M1 by longitudinal stripe columns, S1 by oblique stripe columns, and sham stimulation by non-filled columns. At PM, the contralateral PM stimulation induced a significant oxy-Hb decrease. At M1, the contralateral S1 stimulation induced a significant oxy-Hb decrease; the contralateral M1 or S1 stimulation evoked a significant oxy-Hb decrease at S1. Error bars indicate standard errors. Asterisks indicate statistically significant changes (*P < 0.05, **P < 0.01, one-way ANOVA and post-hoc analysis with Scheffe’s method)
The result of the additional physiological experiment was shown in Fig. 7. The MEP inhibition by TBS over S1 (28%) was significantly larger than that by sham stimulation (1%) (paired t-test: P = 0.01). This indicates that the right M1 was functionally suppressed after TBS over the left S1 in parallel with its oxy-Hb concentration reduction.
Comparisons of the effects on MEPs from the left FDI evoked by TMS over right M1 between the conditioning TBS over left S1 and sham stimulation. The ratios were the average values of conditioned MEP/control MEP. TBS over left S1 induced a significant MEP decrease even though sham stimulation did not induce significant MEP changes. Error bars indicate standard errors
Discussion
The results of our present study can be summarized as follows. (1) TBS over PM induced a significant oxy-Hb decrease at the contralateral PM. (2) TBS over M1 induced significant oxy-Hb decrease at the contralateral S1 and TBS over S1 significant oxy-Hb decrease at the contralateral M1 and S1. (3) 80% AMT TBS over S1 induced larger oxy-Hb decrease at the contralateral hemisphere than that by 100% AMT TBS. (4) MEPs were also suppressed by conditioning TBS over the contralateral S1.
Oxy-Hb decrease
Three different patterns of NIRS changes have been reported in natural brain activation and TMS stimulation. First, large oxy-Hb increase and small deoxy-Hb decrease were observed in natural brain activation (Chance et al. 1988; Villringer et al. 1993; Kleinschmidt et al. 1996) or after low frequency rTMS (0.25 Hz; 30 stimuli) over the NIRS probes at an intensity of 100% of the maximal stimulator output, which was above the resting motor threshold (Oliviero et al. 1999). This pattern is usually called as activation. Another is large oxy-Hb decrease and small deoxy-Hb increase (Wenzel et al. 2000; Fabbri et al. 2003). This pattern is usually called as deactivation. The other one is small oxy-Hb change and large deoxy-Hb decrease. This pattern is observed only after a single pulse TMS under the center of the coil (Mochizuki et al. 2006). The pattern observed in the present study, large oxy-Hb decrease and small deoxy-Hb increase, is almost the same as that seen in natural deactivation among the above three patterns. Deoxy-Hb concentration, however, did not show any significant changes in the present results. This discrepancy might be due to the lower sensitivity of deoxy-Hb changes than oxy-Hb changes in NIRS measurement (Madsen and Secher 1999). We conclude that some deactivation must occur at the sensori-motor cortices after TBS over the contralateral sensori-motor cortices.
Some Hb concentration changes seemed to start a bit before the stimulation (e.g., in Fig. 4). Similar results are sometimes seen in some experiments. In the study of NIRS recording before and during gait (Miyai et al. 2001), oxy-Hb increase at supplementary motor areas started before gait. One explanation for this phenomenon is that Hb concentration changes begin when subjects can expect the start of the experiments. In our experiments, even the start of stimulation was randomized in time, randomization was not much enough to escape the expectation. It may partly explain the early start of Hb changes.
Interhemispheric connection
In animal studies, motor related areas and sensory cortex have direct connections with the contralateral homotopic and non-homotopic areas through the corpus callosum (Karol and Pandya 1971; Jenny 1979; Marconi et al. 2003). In humans, direct homotopic or non-homotopic connections have also been suggested to be present between bilateral primary motor and sensory cortices (Ferbert et al. 1992; Hanajima et al. 2001; Mochizuki et al. 2004a, b). The interhemispheric connections between other areas (e.g., PM to the contralateral PM) have not been studied physiologically because of the lack of detection methods for the function of those areas. Using a combination of NIRS and TMS, this study has physiologically proved that PM has a functional connection with the contralateral PM (directly or indirectly) in humans and that motor and sensory cortices interacted densely with the contralateral motor and sensory cortices.
The excitability changes underneath the coil most probably explain the inhibition of the contralateral hemisphere after a short train of TMS through some interhemispheric interactions. The contralateral changes may be evoked without any changes under the coil. However, it is not plausible because we showed some changes under the coil in a previous paper (Mochizuki et al. 2006).
Intensity of TBS
The effect of 80% AMT TBS over S1 was larger than that of 100% AMT TBS. Münchau et al. (2002) reported that slow repetitive TMS over PM at an intensity of 80% AMT evoked intercortical facilitatory effects on the ipsilateral M1, but repetitive TMS at an intensity of 90% AMT had no effects. They speculated that these two areas have mutual facilitatory and inhibitory connections and stimulation at a higher intensity might evoke a mixture of effects that cancel out each other at 80% AMT. In another paired pulse TMS study (Civardi et al. 2001), single pulse TMS over PM at 90% AMT induced an inhibitory effect on ipsilateral M1, but when TMS was 110% AMT this inhibitory effect was changed to facilitatory one. In our study, 100% AMT TBS might have also evoked a mixture of facilitatory and inhibitory effects on the contralateral side and masked the inhibitory effect observed at 80% AMT TBS.
Physiological meaning
Between bilateral M1s the predominant connection is inhibitory. When one M1 is active, the contralateral M1 must be deactivated (Ferbert et al. 1992; Schambra et al. 2003; Plewnia et al. 2003). This connection plays critical roles in bimanual movements. Our study has shown the physiological inhibitory connection between bilateral PMs in humans for the first time. This connection must much contribute to the interactions between both hemispheres in bimanual and one-hand movements. Our results also demonstrated dense inhibitory connections between bilateral M1s and S1s. S1 was strongly influenced by the ipsilateral M1 (Enomoto et al. 2001). Even though we cannot conclude whether the bilateral sensori-motor cortical connections are direct one or indirect one from the present results, our findings indicate dense interactions between bilateral primary motor and sensory cortices. Smooth, fine and coordinated bimanual movements must be guaranteed by combination of powerful bilateral PM connection and dense bilateral sensori-motor cortical connections.
In conclusion we found the interhemispheric inhibitory connections between bilateral PM, M1 and S1 using TBS and NIRS in humans, and these connections would be requisite for bimanual coordinated movements in humans.
References
Bohning DE, Pecheny AP, Epstein CM, Speer AM, Vincent DJ, Dannels W, George MS (1997) Mapping transcranial magnetic stimulation (TMS) fields in vivo with MRI. Neuroreport 8:2535–2538
Boroojerdi B, Foltys H, Krings T, Spetzger U, Thron A, Töpper R (1999) Localization of the motor hand area using transcranial magnetic stimulation and functional magnetic resonance imaging. Clin Neurophysiol 110:699–704
Chance B, Leigh JS, Miyake H, Smith DS, Nioka S, Greenfeld R, Finander M, Kaufmann K, Levy W, Young M, Cohen P, Yoshioka H, Boretsky R (1988) Comparison of time-resolved and -unresolved measurements of deoxyhemoglobin in brain. Proc Natl Acad Sci USA 85:4971–4975
Chouinard PA, van Der Werf YD, Leonard G, Paus T (2003) Modulating neural networks with transcranial magnetic stimulation applied over the dorsal premotor and primary motor cortices. J Neurophysiol 90:1071–1083
Civardi C, Cantello R, Asselman P, Rothwell JC (2001) Transcranial magnetic stimulation can be used to test connections to primary motor areas from frontal and medial cortex in humans. Neuroimage 14:1444–1453
Enomoto H, Ugawa Y, Hanajima R, Yuasa Y, Mochizuki H, Terao Y, Shiio Y, Furubayashi T, Iwata NK, Kanazawa I (2001) Decreased sensory cortical excitability after 1 Hz rTMS over the ipsilateral primary motor cortex. Clin Neurophysiol 112:2154–2158
Fabbri F, Henry ME, Renshaw PF, Nadgir S, Ehrenberg BL, Franceschini MA, Fantini S (2003) Bilateral near-infrared monitoring of the cerebral concentration and oxygen-saturation of hemoglobin during right unilateral electro-convulsive therapy. Brain Res 992:193–204
Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD (1992) Interhemispheric inhibition of the human motor cortex. J Physiol 453:525–546
Fink GR, Frackowiak RSJ, Pietrzyk U, Passingham RE (1997) Multiple nonprimary motor areas in the human cortex. J Neurophysiol 77:2164–2174
Gerschlager W, Siebner HR, Rothwell JC (2001) Decreased corticospinal excitability after subthreshold 1 Hz rTMS over lateral premotor cortex. Neurology 57:449–455
Hanajima R, Ugawa Y, Machii K, Mochizuki H, Terao Y, Enomoto H, Furubayashi T, Shiio Y, Uesugi H, Kanazawa I (2001) Interhemispheric facilitation of the hand motor area in humans. J Physiol 531:849–859
Huang YZ, Edwards MJ, Runis E, Bhatia KP, Rothwell JC (2005) Theta burst stimulation of the human motor cortex. Neuron 45:201–206
Jenny AB (1979) Commissural projections of the cortical hand motor area in monkeys. J Comp Neurol 188:137–146
Jöbsis FF (1977) Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 198:1264–1267
Karol EA, Pandya DN (1971) The distribution of the corpus callosum in the rhesus monkey. Brain 94:471–486
Kleinschmidt A, Obrig H, Requardt M, Merboldt KD, Dirnagl U, Villringer A, Frahm J (1996) Simultaneous recording of cerebral blood oxygenation changes during human brain activation by magnetic resonance imaging and near-infrared spectroscopy. J Cereb Blood Flow Metab 16:817–826
Madsen PL, Secher NH (1999) Near-infrared oximetry of the brain. Prog Neurobiol 58:541–560
Marconi B, Genovesio A, Giannetti S, Molinari M, Caminiti R (2003) Callosal connections of dorso-lateral premotor cortex. Eur J Neurosci 18:775–788
Miyai I, Tanabe H, Sase I, Eda H, Oda I, Konishi I, Tsunazawa Y, Suzuki T, Yanagida T, Kubota K (2001) Cortical mapping of gait in humans: a near-infrared spectroscopic topography study. Neuroimage 14:1186–1192
Mochizuki H, Huang YZ, Rothwell JC (2004a) Interhemispheric interaction between human dorsal premotor and cotralateral primary motor cortex. J Physiol 561:331–338
Mochizuki H, Terao Y, Okabe S, Furubayashi T, Arai N, Iwata NK, Hanajima R, Kamakura K, Motoyoshi K, Ugawa Y (2004b) Effects of motor cortical stimulation on the excitability of contralateral motor and sensory cortices. Exp Brain Res 158:519–526
Mochizuki H, Ugawa Y, Terao Y, Sakai KL (2006) Cortical hemoglobin-concentration changes under the coil induced by single-pulse TMS in humans: a simultaneous recording with near-infrared spectroscopy. Exp Brain Res 169:302–310
Münchau A, Bloem BR, Irlbacher K, Trimble MR, Rothwell JC (2002) Functional connectivity of human premotor and motor cortex explored with repetitive transcranial magnetic stimulation. J Neurosci 22:554–561
Noguchi Y, Watanabe E, Sakai KL (2003) An event-related optical topography study of cortical activation induced by single-pulse transcranial magnetic stimulation. Neuroimage 19:156–162
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Oliviero A, Di Lazzaro V, Piazza O, Profice P, Pennisi MA, Della Corte E, Tonali P (1999) Cerebral blood flow and metabolic changes produced by repetitive magnetic brain stimulation. J Neurol 246:1164–1168
Plewnia C, Lotze M, Gerloff C (2003) Disinhibition of the contralateral motor cortex by low-frequency rTMS. Neuroreport 14:609–612
Schambra HM, Sawaki L, Cohen LG (2003) Modulation of excitability of human motor cortex (M1) by 1 Hz transcranial magnetic stimulation of the contralateral M1. Clin Neurophysiol 114:130–133
Siebner HR, Peller M, Willoch F, Minoshima S, Boecker H, Auer C, Drzezga A, Conrad B, Bartenstein P (2000) Lasting cortical activation after repetitive TMS of the motor cortex: a glucose metabolic study. Neurology 54:956–963
Spetzger U, Laborde G, Gilsbach JM (1995) Frameless neuronavigation in modern neurosurgery. Minim Invasive Neurosurg 38:163–166
Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I (1995) Magnetic stimulation over the cerebellum in humans. Ann Neurol 37:703–713
Villringer A, Planck J, Hock C, Schleinkofer L, Dirnagl U (1993) Near infrared spectroscopy (NIRS): a new tool to study hemodynamic changes during activation of brain function in human adults. Neurosci Lett 154:101–104
Wenzel R, Wobst P, Heekeren HH, Kwong KK, Brandt SA, Kohl M, Obrig H, Dirnagl U, Villringer A (2000) Saccadic suppression induces focal hypooxygenation in the occipital cortex. J Cereb Blood Flow Metab 20:1103–1110
Acknowledgments
Part of this work was supported by Research Project Grant-in-aid for Scientific Research No 17590865 (RH), No 16500194 (YU) from the Ministry of Education, Science, Sports and Culture of Japan, grants for the Research Committee on rTMS treatment of movement disorders, the Ministry of Health and Welfare of Japan (17231401), the Research Committee on dystonia, the Ministry of Health and Welfare of Japan, a grant from the Committee of the Study of Human Exposure to EMF, Ministry of Public Management, Home Affairs, Post and Telecommunications and grants from Life Science foundation of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mochizuki, H., Furubayashi, T., Hanajima, R. et al. Hemoglobin concentration changes in the contralateral hemisphere during and after theta burst stimulation of the human sensorimotor cortices. Exp Brain Res 180, 667–675 (2007). https://doi.org/10.1007/s00221-007-0884-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-007-0884-5