Abstract
Functional near-infrared spectroscopy is a noninvasive optical imaging technique to register brain activity. It utilizes near-infrared light to evaluate the oxygenated (HbO) and deoxygenated hemoglobin concentration. Here, we used HbO and HbR to analyze the oxygen saturation and electromyographic signals to study muscle activity during the single left- and right-hand movements. Sixteen right-handed volunteers participated in the experiment. During the active phase of the experiment, the subject was asked to perform movements with his left or right hand according to the screen instructions. There were 40 total hand movement trials (20 for each hand) that were performed in random order. The oxygen saturation increased contralaterally, peaking at about 6 s post-command onset and then decreased, reaching baseline level at 12 s. The maximal amplitudes appeared in the primary motor (M1) cortex in the hemisphere contralateral to the performing limb. In the left hemisphere, the right hand induced a higher response than the left hand. In the right hemisphere, the response amplitude remains similar for both hands. We hypothesized that the right hand being a dominant hand in the group may require additional neuronal recruitment in the contralateral M1 cortex.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Functional near-infrared spectroscopy (fNIRS) is a powerful tool of noninvasive optical imaging technique to register brain activity [1,2,3,4,5]. It employs near-infrared light to detect changes in oxygenated (HbO) and deoxygenated (HbR) hemoglobin levels due to hemodynamic brain activity and the rapid delivery of oxygenated blood to active cortical areas through a neurovascular coupling [1, 4, 6]. FNIRS has the same physiological basis as functional magnetic resonance imaging (fMRI), so both technologies provide interrelated data [7, 8]. At the same time, fNIRS has many advantages: portability, ease of use, real-time monitoring, low sensitivity to motor artifacts, higher temporal resolution, the possibility of separate registration of both deoxyhemoglobin and oxyhemoglobin changes, radiation-less environment [9, 10].
FNIRS neuroimaging technique is widely used in various applications [11], for example, in hybrid multimodal brain-computer interfaces (usually, in BCIs for recognition of motor imagery and control commands formation, implemented with simultaneous EEG registration) [12,13,14,15,16,17], for assessing the level of attention (in particular, for neurofeedback implementation) [18], in the studies of aspects of human memory [19, 20], in investigations and diagnosis of various cognitive disabilities and brain disorders (e.g., Alzheimer’s disease, schizophrenia, dyslexia, Parkinson’s disease, epilepsy, childhood disorders, post-neurosurgery dysfunction) [10, 21] and other scientific and clinical applications [10, 22,23,24,25]. When fNIRS is used to record the hemodynamic response, a long-term cognitive load is given on the subject in most experimental paradigms [10]. This is due to the presence of a relatively long time delay (usually more than 2 s temporal period) between the activation of a certain area of the cerebral cortex and the appearance of the hemodynamic response in it. Moreover, the hemodynamic response becomes more pronounced during repeated executions of the experimental task, reflecting increased oxygen consumption by the involved neuronal ensemble. In particular, when analyzing motor activity using fNIRS, a series of the same movements (e.g., the so-called tapping task) is usually performed within one experimental session [26,27,28].
However, the noted long-term repetitive motor activity is not typical for real human behavior. More urgent problems are analyzing the hemodynamic response features to a single repetition of the movement (single-trial analysis) and its relationship with the electromyographic (EMG) signal [29]. For example, in the brain-computer interfaces (BCIs), it is essential to recognize single movements rather than a series of repetitive movements. Moreover, a detailed study of single motions in terms of hemodynamic response is important for a better understanding of the mechanisms in the cerebral cortex during the preparation and execution of a movement. Here, a detailed study of the temporal dynamics of the ongoing processes is especially important, both by analyzing electromyographic (EMG) signals and by analyzing changes in the deoxyhemoglobin and oxyhemoglobin levels. These issues are poorly studied at present.
Thus, revealing specific features of brain cortex activity related to single motions of limbs is essential not only for basic neuroscience research but also for medical applications to improve post-traumatic and post-stroke patients’ quality of life using BCI for rehabilitation [30, 31]. Another problem here is the diagnosis of age-related degradations in neural processes associated with changes in the brain’s neurochemical and structural properties [32]. It determines the cognitive and motor performance decline during a daily activity of elderly adults. Upper limbs represent the most important and most used part of the human motor system; thus, the degradation of its functioning with age, including decreasing reaction time and over-activation of the brain’s motor and prefrontal area [33, 34], is the most prominent. Several mechanisms are supposed to provide a compensatory mechanism to overcome age-related structural changes in the human brain [35, 36]. They lead to maintain the performance of executed motor actions, and simultaneously these mechanisms demand more neuronal resources and, therefore, slows the motor response of elderly patients. In our previous works [37, 38], using the whole-scalp electroencephalography (EEG) analysis, we have shown that the age-related slowing down in the motor initiation before the dominant hand movements is accompanied by the increased theta activation within the sensorimotor area and reconfiguration of the theta-band functional connectivity in elderly adults. It seems to us that the use of analysis of the hemodynamic response of the brain of patients when performing single motor exercises with the upper limbs may provide additional prognostic possibilities for diagnosing age-related changes in the brain [39, 40].
In this paper, we investigate the execution by a subject of single movements by the hands. As a basis, we carry out EMG analysis during motor activity. Using the fNIRS technique, we investigate the temporal hemodynamic response features in the brain’s motor cortex to single movements performed. We pay special attention to investigating differences in the analyzed characteristics when performing movements with the left and right hand.
2 Materials and methods
2.1 Participants
Sixteen healthy volunteers (age: 20–45 years, males) participated in the experiment. All of the participants were right-handed, amateur practitioners of physical exercises, and non-smokers. The subjects were conditionally healthy: no diagnosed diseases of the musculoskeletal or nervous system, no prescribed medications. Prior to the experiment, the participants were asked to maintain a healthy lifestyle for a least 48 h, including 8 h night rest, prohibited alcohol consumption, limited caffeine consumption, and moderate physical activity.
The experiments were held in the first half of the day (mainly from 11 am to 2 pm) in a room with a sufficient natural lighting level. Before the beginning of the experiment, the volunteers were instructed about its goals and methods, along with possible inconveniences. The participants were able to ask any related questions and receive detailed answers. After all explanations, each participant filled and signed the blank of informed written consent before participating in the experiment. The experimental procedure was performed following the Helsinki’s Declaration and approved by the local Ethics Committee of the Innopolis University.
2.2 Experimental equipment
The fNIRS signals were recorded with the “NIRScout” device by “NIRx” (Germany). The NIRScout system uses 8 sources and 8 detectors to record hemodynamic response in the cortex with a sampling rate of 7.8125 Hz. Optodes are placed noninvasively on the subject’s scalp by mounting to the sockets of the special cap by “EASYCAP” (Germany). FNIRS channels were formed by “source-detector” pairs that were placed close enough to each other. The optimal distance between source and detector is about 3 cm due to the specific traveling path of near-infrared light in cortex’s tissue: the light diffuses in the tissue and at a depth of approximately 3 cm is reflected toward the detector, forming a so-called banana-shaped path.
In our experiments, we placed optodes in the primary motor cortex area (M1), as shown in Fig. 1a. Figure 1a illustrates our montage with overall 23 fNIRS channels. Additionally, 8 EEG electrodes locations according to the international scheme “10-10” are shown as the reference for a better understanding of optodes’ placements.
EMG signals were recorded with POLY channels of the encephalograph “Encephalan-EEGR-19/26” by “Medicom-MTD” (Russia). In our study, we used special EMG sensors with two recording electrodes and one ground electrode. Such a scheme allows recording the envelope of EMG signal with a sampling rate of 250 Hz (Fig. 1e demonstrates a typical raw EMG signal). EMG envelope is related to muscular contractions, and in our experiment, it was used to assess hand movements, while high-frequency EMG component was filtered (with a low-pass filter with cutoff frequency—1 Hz). EMG electrodes were placed noninvasively on the forearm muscle on each of the subject’s hands, as shown in Fig. 1b. Before electrode mounting, the skin was treated with abrasive gel “NuPrep” to ensure good conductivity and high quality of recorded EMG signals. As recording electrodes, we used disposable, pre-gelled adhesive electrodes “Sticktrode” by “Neuroelectrics” (Spain).
All experimental work was carried out in the Neuroscience and Cognitive Technology Laboratory of the Innopolis University.
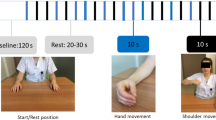
a Locations of fNIRS sources (marked with red) and detectors (marked with blue) on the subject’s head in the area of the primary motor cortex (shaded area); 23 fNIRS channels (grey circles with channel numbers) across motor cortex (shaded area) and 8 EEG electrodes (FCz, C3, Cz, C4, CP3, CPz, CP4, Pz) according to “10-10” scheme (black circles with channel names), which are shown as the reference points; b Placement of EMG recording electrodes (turquoise) and ground electrode (black); c General scheme of the experiment; d Detailed scheme of one trial with corresponding on-screen commands; e A typical raw signal from the EMG sensor. A significant increase in the amplitude of the EMG signal makes it possible to identify the moment of movement reliably
2.3 Experimental procedure
The experiment was performed as follows. The subject was sitting in a comfortable chair with hands placed on armrests. The monitor was placed in front of the subject’s eyes at a distance of 70–80 cm for demonstration of visual commands.
The experimental design is schematically shown in Fig. 1c. Each experiment began and ended with a 3-min recording of background brain activity, during which the subject was instructed to relax and make no hand movements. During the active phase of the experiment, the subject was asked to perform movements with his left or right hand according to the screen instructions. There were 40 total hand movement trials (20 for each hand) that were performed in random order.
Each hand movement trial consisted of several phases accompanied by specific commands on the monitor, as illustrated by Fig. 1d. The trial started with the fixation of the subject’s attention—a bright cross appeared on a black screen for 2 seconds and acted as a signal for the subject to prepare for the trial. Attention fixation was followed by a visual cue: the cross stayed on the screen, and the left- or right-oriented arrow appeared on the top of it for 1.5 seconds. During this phase, the subject was informed that the left- or right-hand movement (correspondingly) was required. The next phase was motor execution: the arrow disappeared from the screen, but the cross stayed for 5 seconds. During this time interval, the subject performed the required hand movement. Hand movement consisted of bending and unbending of fingers to the palm’s center, similar to clenching of the imaginary ball. The trial ended with rest: the cross disappeared, and the black screen was shown for 15 seconds. During this phase, the subject rested and waited for the next command. The duration of the experiment for one subject was about 21 minutes.
2.4 Data acquisition and analysis
FNIRS technology commonly implements laser light with two wavelengths: \(\lambda _1 = 785\) nm and \(\lambda _2 = 850\) nm [41]. Near-infrared lights with these parameters can pass through skin, bone, and water, but is highly absorbed by oxyhemoglobin and deoxyhemoglobin, respectively. In our experimental setup for the fNIRS recording, the light sources and detectors were placed on the scalp, and dual-wavelength light was transmitted through the skin, skull, and top layer of the cerebral cortex.
Raw fNIRS data need to be preprocessed to be used as an indicator of changes in the tissues’ oxygenation. Since oxyhemoglobin and deoxyhemoglobin have different light absorption properties, we calculated changes in the reflected dual-wavelength light using the modified Beer–Lambert law [42]. As a result, we obtained two characteristic measures HbO and HbR, which reflect the speed of relative changes in oxyhemoglobin and deoxyhemoglobin concentration levels, correspondingly. More convenient for analyzing the level of saturation of the cerebral cortex with oxygen is the following characteristic, called oxygen saturation SaO2:
As with most experimental data, fNIRS signals are often exposed to internal and external noises, whose characteristic frequencies lie in the fNIRS frequency band. These include physiological artifacts such as Mayer waves (\(\sim 0.1\) Hz), respiration (\(\sim 0.25\) Hz), and heartbeat (\(\sim 1\) Hz). According to common practice [14, 28], the band-pass filtering is sufficient for removing low-frequency physiological noise in fNIRS data in many cases. We applied a band-pass filter (\(0.01{-}0.1\) Hz) to pre-process experimental fNIRS data before all calculations based on the modified Beer–Lambert law. FNIRS data acquisition and pre-processing procedures were performed with “NIRScout” software.
For further analysis of EMG and fNIRS data, we used the trials described earlier. However, at the beginning of each trial, we added the 5-second fragment taken from the rest phase of the previous trial (or from the background activity in the case of the first trial). We used this additional fragment to perform a baseline correction procedure to eliminate the influence of any previous dynamics on the current trial.
For each subject, EMG and fNIRS trials were time-locked to the command and averaged across all trials corresponding to the left and right-hand movements. Thus, event-related EMG/fNIRS signals were obtained. On the group level, the 7-s EMG trials were tested against the baseline separately for the left and right hands. The 18-s fNIRS trials were corrected to the baseline as \((\mathrm{SaO}2-\mathrm{SaO}2_{base})/\mathrm{SaO}2_\mathrm{base}\) and contrasted between the left-hand and right-hand movements.
The statistical testing used the paired samples two-tailed t-test to compare EMG or fNIRS amplitudes at each time point [43]. In this case, we deal with the multiple comparisons problem (MCP) [44, 45]. This problem originates from the fact that the effect of interest is evaluated at a large number of time points. The MCP involves that, due to a large number of statistical comparisons (i.e., one per time point), it is impossible to control the so-called family-wise error rate (FWER) through the standard statistical procedures that operate at the level of single points. To solve the MCP, we have used the cluster-based permutation test [44]. All values that passed a threshold (\(p=0.005\)—for EMG data, \(p=0.025\)–for fNIRS data) were collected to the positive and negative clusters. The significance of these clusters was tested via the nonparametric cluster-based statistics with \(N=65536\) randomizations.
3 Results and discussion
First of all, we analyzed the EMG dataset. Fig. 2 shows group-mean event-related EMG signals for left and right hand movements. The carried out statistical analysis revealed the time clusters where the event-related EMG signal differs significantly from background EMG activity for the group of subjects (see horizontal red bars in Fig. 2). In other words, all subjects in the time range defined by the cluster perform a movement, while the left boundary of the cluster corresponds to the group-mean moment of the movement beginning, and the right one — to the moment of the movement completion. One can see that the movement with the right hand lasts a bit longer than the left hand one.
EMG signals (group-mean ± SE (standard error)) for left and right hand movement; \(t=3.5\) s is the moment when the command to start the movement is given. The data are averaged over all 16 subjects. The amplitude of EMG signals is normalized to the maximum amplitude in the group of subjects. The horizontal bold red line indicates the time cluster where the event-related EMG signal differs significantly from background EMG activity for the group of subjects. Here, ‘*’ indicates \(p<10^{-4}\)
Time dependencies (group-mean ± SE) of cerebral tissue oxygen saturation during left (blue curves) and right (black curves) hand movement for all 23 fNIRS channels. The data are averaged over all 16 subjects. Vertical dashed lines indicate the moment when the command to start the movement is given. The channel number is indicated in the upper right corner of each distribution. The horizontal bold red line indicates the time cluster for the given channel where the oxygen saturation during the right hand movement differs significantly from the oxygen saturation during left hand movement for the group of subjects. Here, ‘*’ indicates \(p<0.05\) and ‘**’ indicates \(p<0.01\)
To investigate the differences between hemodynamic responses in the motor cortex M1 during right and left hands movements, we obtained the corresponding time dependencies of oxygen saturation for all 23 fNIRS channels (see Fig. 3 where group-mean event-related oxygen saturation dependencies are shown). The carried out statistical analysis revealed the time clusters on specific channels where the event-related oxygen saturation for the right hand movement differs significantly from the event-related oxygen saturation for the left hand movement for the group of subjects (see horizontal red bars in Fig. 3). In the left hemisphere in the neighborhood of EEG channel C3 (see the channels 1 and 7 in Fig. 3), the oxygen saturation is significantly higher during the right hand movement. At the same time, there are no significant differences in the right hemisphere.
Analysis of the temporal dynamics of the oxygen saturation dependencies in Fig. 3 shows that a significant difference is established on channels 1 (\(p=0.013\)) and 7 (\(p=0.0068\)), approximately 2.5 seconds after the command to start the movement. Significant differences disappear from 10.5 to 12 seconds (depending on the channel) after the command.
In summary, we analyzed the muscle EMG-activity and the hemodynamic fNIRS-response during a single movement with left and right hands. The EMG signals enhanced their amplitude for approximately \(0.4-1.5\) s post-command onset, reflecting the movement execution. In terms of the oxygen saturation, hemodynamic response increased contralaterally, peaking at about 6 s post-command onset and then decreased, reaching baseline level at 12 s. These results coincide with the literature: the most reliable cortical hemodynamic response following motor stimulation consists of an increase in HbO, coupled with a decrease in HbR [9]. Similarly, oxygen saturation grows when the HbO increases and HbR decreases (see Eq. 1). According to Roy and Sherrington [46], this is a typical hemodynamic response in activated tissue, reflecting the demand of recruited neurons for oxygenated blood and the washout of deoxygenated waste products.
Considering the fNIRS data from the multiple sensors, we confirmed that the response distribution is regionally specific. Visual inspection of the black and blue curves in Fig. 3 indicates that the maximal responses appear in the hemisphere contralateral to the performing limb. The black curve corresponding to the right-hand has the most pronounced peak at the sensors 1, 7, 6 in the vicinity of the C3 EEG sensor position. On the contrary, the blue curve corresponding to the left-hand has the pronounced peak at the sensors 5, 10, 11 in the vicinity of the C4 EEG sensor position. According to recent work [47], these locations coincide with the primary motor cortex (M1).
Interestingly, in the left hemisphere, the right hand induced a higher response than the left hand. In the right hemisphere, the response amplitude remains similar for both hands. We hypothesized that the right hand being a dominant hand in the group may require additional neuronal recruitment in the contralateral M1 cortex. On the one hand, the magnitude of the response may be modulated by the motor task’s frequency, intensity, or complexity. On the other hand, the longitudinal training improves laterality toward contralateral M1 activation during the left- and right-hand movements [9]. Thus, we suppose that the dominant hand (here, the right hand for all the subjects) receives extensive training during daily activities. As a result, it may result in a growing neuronal ensemble of the motor cortex that subserves dominant hand movements.
4 Conclusions
In this paper, we conducted a detailed study of the oxygen saturation in the motor cortex, using the fNIRS technique, when a human performs single movements with the upper limbs. We discussed the relationship between the observed hemodynamic response and EMG data, particularly with differences in left and right hand movements. We discovered that the oxygen saturation increases contralaterally and reaches the maximum at about 6 s after the command onset and then decreases, reaching baseline level at 12 s. The maximal amplitudes appeared in the primary motor (M1) cortex in the hemisphere contralateral to the performing limb. In the left hemisphere, the right hand induced a higher response than the left hand. In the right hemisphere, the response amplitude remains similar for both hands. We hypothesize that the right hand being dominant in the group may require additional neuronal recruitment in the contralateral M1 cortex. The consequence is faster execution of the movement with the right hand.
Data Availability Statement
This manuscript has associated data in a data repository. [Authors’ comment: Kurkin, S.A.; Badarin, Artem; Maximenko, V.A.; Grubov, Vadim V.; Hramov, Alexander E. (2021): fNIRS dataset for the analysis of oxygen saturation in the primary motor cortex during a single hand movement. Figshare. Dataset. https://doi.org/10.6084/m9.figshare.13530863.v1].
References
A. Villringer, J. Planck, C. Hock, L. Schleinkofer, U. Dirnagl, Neurosci. Lett. 154, 101 (1993)
Y. Hoshi, M. Tamura, J. Appl. Physiol. 75, 1842 (1993)
A. Villringer, B. Chance, Trends Neurosci. 20, 435 (1997)
A.F. Abdelnour, T. Huppert, Neuroimage 46, 133 (2009)
T. Myllylä, V. Korhonen, V. Kiviniemi, V. Tuchin, Experimental studies with selected light sources for NIRS of brain tissue: quantifying tissue chromophore concentration, in Optical Techniques in Neurosurgery, Neurophotonics, and Optogenetics II (International Society for Optics and Photonics, 2015), Vol. 9305, p. 93051S
E.N. Lazareva, V.V. Tuchin, J. Biomed. Opt. 23, 035004 (2018)
X. Cui, S. Bray, D.M. Bryant, G.H. Glover, A.L. Reiss, Neuroimage 54, 2808 (2011)
T.J. Huppert, R.D. Hoge, S.G. Diamond, M.A. Franceschini, D.A. Boas, Neuroimage 29, 368 (2006)
D.R. Leff, F. Orihuela-Espina, C.E. Elwell, T. Athanasiou, D.T. Delpy, A.W. Darzi, G.Z. Yang, Neuroimage 54, 2922 (2011)
M.A. Rahman, A.B. Siddik, T.K. Ghosh, F. Khanam, M. Ahmad, J. Digital Imaging pp. 1–18 (2020)
M. Ferrari, V. Quaresima, Neuroimage 63, 921 (2012)
S. Zhang, Y. Zheng, D. Wang, L. Wang, J. Ma, J. Zhang, W. Xu, D. Li, D. Zhang, Neurosci. Lett. 655, 35 (2017)
S. Ahn, S.C. Jun, Front. Hum. Neurosci. 11, 503 (2017)
N. Naseer, K.S. Hong, Front. Hum. Neurosci. 9, 3 (2015)
V. Kaiser, G. Bauernfeind, A. Kreilinger, T. Kaufmann, A. Kübler, C. Neuper, G.R. Müller-Putz, Neuroimage 85, 432 (2014)
S. Fazli, J. Mehnert, J. Steinbrink, G. Curio, A. Villringer, K.R. Müller, B. Blankertz, Neuroimage 59, 519 (2012)
A.E. Hramov, V.A. Maksimenko, A.N. Pisarchik, Phys. Rep. (2021). https://doi.org/10.1016/j.physrep.2021.03.002
A.M. Marx, A.C. Ehlis, A. Furdea, M. Holtmann, T. Banaschewski, D. Brandeis, A. Rothenberger, H. Gevensleben, C.M. Freitag, Y. Fuchsenberger et al., Front. Hum. Neurosci. 8, 1038 (2015)
D. Talamonti, C.A. Montgomery, D.P. Clark, D. Bruno, NeuroImage 222, 117223 (2020)
S. Jahani, A.L. Fantana, D. Harper, J.M. Ellison, D.A. Boas, B.P. Forester, M.A. Yücel, Sci. Rep. 7, 1 (2017)
P.M. Arenth, J.H. Ricker, M.T. Schultheis, Clin. Neuropsychol. 21, 38 (2007)
X. Cui, D.M. Bryant, A.L. Reiss, Neuroimage 59, 2430 (2012)
W.C. Su, M.L. Culotta, M.D. Hoffman, S.L. Trost, K.A. Pelphrey, D. Tsuzuki, A.N. Bhat, Front. Hum. Neurosci. 14, 57 (2020)
E.A. Genina, A.N. Bashkatov, D.K. Tuchina, P.A. Dyachenko, N. Navolokin, A. Shirokov, A. Khorovodov, A. Terskov, M. Klimova, A. Mamedova et al., Biomed. Opt. Express 10, 5182 (2019)
O. Semyachkina-Glushkovskaya, A. Abdurashitov, A. Dubrovsky, D. Bragin, O. Bragina, N. Shushunova, G. Maslyakova, N. Navolokin, A. Bucharskaya, V. Tuchind et al., J. Biomed. Opt. 22, 121719 (2017)
A.R. Anwar, M. Muthalib, S. Perrey, A. Galka, O. Granert, S. Wolff, U. Heute, G. Deuschl, J. Raethjen, M. Muthuraman, Brain Topogr. 29, 645 (2016)
A. Rahimpour, L. Pollonini, D. Comstock, R. Balasubramaniam, H. Bortfeld, J. Neurosci. Methods p. 108790 (2020)
A.E. Hramov, V. Grubov, A. Badarin, V.A. Maksimenko, A.N. Pisarchik, Sensors 20, 2362 (2020)
A. von Lühmann, A. Ortega-Martinez, D.A. Boas, M.A. Yücel, Front. Hum. Neurosci. 14, 30 (2020)
U. Chaudhary, N. Birbaumer, A. Ramos-Murguialday, Nat. Rev. Neurol. 12, 513 (2016)
P. Chholak, G. Niso, V.A. Maksimenko, S.A. Kurkin, N.S. Frolov, E.N. Pitsik, A.E. Hramov, A.N. Pisarchik, Sci. Rep. 9, 1 (2019)
R.D. Seidler, J.A. Bernard, T.B. Burutolu, B.W. Fling, M.T. Gordon, J.T. Gwin, Y. Kwak, D.B. Lipps, Neurosci. Biobehav. Rev. 34, 721 (2010)
F.A. Sorond, Y. Cruz-Almeida, D.J. Clark, A. Viswanathan, C.R. Scherzer, P. De Jager, A. Csiszar, P.J. Laurienti, J.M. Hausdorff, W.G. Chen et al., J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 70, 1526 (2015)
C. Maes, J. Gooijers, J.J.O. de Xivry, S.P. Swinnen, M.P. Boisgontier, Neurosci. Biobehav. Rev. 75, 234 (2017)
N.S. Ward, Ageing Res. Rev. 5, 239 (2006)
P.A. Reuter-Lorenz, D.C. Park, Neuropsychol. Rev. 24, 355 (2014)
N.S. Frolov, E.N. Pitsik, V.A. Maksimenko, V.V. Grubov, A.R. Kiselev, Z. Wang, A.E. Hramov, PLoS ONE 15 (2020)
A.N. Pavlov, E.N. Pitsik, N.S. Frolov, A. Badarin, O.N. Pavlova, A.E. Hramov, Sensors 20, 5843 (2020)
C. Zich, S. Debener, A.K. Thoene, L.C. Chen, C. Kranczioch, Neurobiol. Aging 49, 183 (2017)
C.C. Lin, J.W. Barker, P.J. Sparto, J.M. Furman, T.J. Huppert, Exp. Brain Res. 235, 1247 (2017)
H. Ayaz, P.A. Shewokis, A. Curtin, M. Izzetoglu, K. Izzetoglu, B. Onaral, J. Vis. Exp. p. e3443 (2011)
W.B. Baker, A.B. Parthasarathy, D.R. Busch, R.C. Mesquita, J.H. Greenberg, A. Yodh, Biomed. Opt. Express 5, 4053 (2014)
S. Tak, J.C. Ye, Neuroimage 85, 72 (2014)
E. Maris, R. Oostenveld, J. Neurosci. Methods 164, 177 (2007)
V. Maksimenko, A. Kuc, N. Frolov, S. Kurkin, A. Hramov, Sci. Rep. 11, 1 (2021)
C.S. Roy, C.S. Sherrington, J. Physiol. 11, 85 (1890)
T.L. Rich, B.T. Gillick, Brain Sci. 9, 69 (2019)
Acknowledgements
The reported study was funded by Russian Foundation for Basic Research and National Natural Science Foundation of China according to the research project No 19-52-55001. A.H. thanks the President program for support of Russian Leading Scientific Schools (Grant NSH-2594.2020.2). S.K. is supported by the President Program (Grant MD-1921.2020.9).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kurkin, S., Badarin, A., Grubov, V. et al. The oxygen saturation in the primary motor cortex during a single hand movement: functional near-infrared spectroscopy (fNIRS) study. Eur. Phys. J. Plus 136, 548 (2021). https://doi.org/10.1140/epjp/s13360-021-01516-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epjp/s13360-021-01516-7