Abstract
This study addresses the question of how posture and movement are oriented with respect to the direction of gravity. It is suggested that neural control levels coordinate spatial thresholds at which multiple muscles begin to be activated to specify a referent body orientation (RO) at which muscle activity is minimized. Under the influence of gravity, the body is deflected from the RO to an actual orientation (AO) until the emerging muscle activity and forces begin to balance gravitational forces and maintain body stability. We assumed that (1) during quiet standing on differently tilted surfaces, the same RO and thus AO can be maintained by adjusting activation thresholds of ankle muscles according to the surface tilt angle; (2) intentional forward body leaning results from monotonic ramp-and-hold shifts in the RO; (3) rhythmic oscillation of the RO about the ankle joints during standing results in body swaying. At certain sway phases, the AO and RO may transiently overlap, resulting in minima in the activity of multiple muscles across the body. EMG kinematic patterns of the 3 tasks were recorded and explained based on the RO concept that implies that these patterns emerge due to referent control without being pre-programmed. We also confirmed the predicted occurrence of minima in the activity of multiple muscles at specific body configurations during swaying. Results re-affirm previous rejections of model-based computational theories of motor control. The role of different descending systems in the referent control of posture and movement in the gravitational field is considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many intentional movements of humans and animals require a transfer of stable balance from one body posture to another. Although body postures are associated with the tendency to equalize muscle and external torques, the choice between different postures is not directly defined by torques, forces, EMG levels or other kinetic or kinematic variables describing the motor outcome. According to the notion of an equilibrium state defined in physics or an attractor point defined in dynamic systems theory, system parameters, rather than kinematic and kinetic variables, pre-determine where, in the spatial domain, balance can be achieved (Andronov et al. 1966; Feldman 2015).
Parametric control was demonstrated empirically in humans and animals (Matthews 1959; Asatryan and Feldman 1965; Feldman and Orlovsky 1972; Feldman 2015) who found that shifts in balance and intentional movement in general are controlled by changing a specific parameter, the threshold position of body segments at which muscles begin to be activated. This implies that in response to changes in the threshold position, EMG activity, muscle forces and kinematics emerge, without any pre-programming, and move the body to a new equilibrium position to which stable balance is re-addressed. This also implies that by shifting balance in spatial coordinates, the nervous system converts posture-stabilizing to movement-producing mechanisms, thus solving the classical posture–movement problem. This approach was also used to explain how various single- and multi-joint motor actions (grip force production, pointing from sitting or standing, reach-to-grasp and sit-to-stand movements, jumping, walking, pushing against a wall, head rotation, and body rotations in ballet) are controlled and how redundancy in the number of degrees of freedom is used in goal-directed movements (Mattos et al. 2011, 2015; Feldman 2015; Tomita et al. 2016).
Parametric control can be seen at different levels of the motor hierarchy. For example, at the single-joint level, a muscle generates activity depending on the difference between its actual length (x) and its threshold length (λ) if x − λ > 0 (Matthews 1959; Asatryan and Feldman 1965; Feldman and Orlovsky 1972; Fig. 1a). This principle of muscle activation has been generalized to dynamic conditions in the form x − λ* > 0 by considering that the muscle activation threshold, λ*, decreases with the speed of muscle stretching. It is also affected by intermuscular afferent feedback, depends on reciprocal and co-facilitatory influences on agonist and antagonist MNs and the history-dependent state of MNs (Turpin et al. 2016). This inequality encompasses both central and reflex sources of muscle activation and relaxation. It also implies that MNs function in a spatial frame of reference in which the threshold length, a physiological parameter, plays the role of the origin (referent) point from which MNs begin to be recruited. In the supra-threshold range, length- and velocity-dependent active muscle force is generated (Fig. 1a). The MN pool is controlled by shifting the spatial threshold.
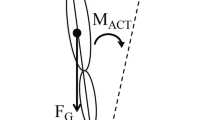
Referent control of motoneurons, arm and whole-body posture in gravitational field. a Motor units begin to be recruited when the difference between the actual (x) and threshold (λ*) muscle length becomes positive. When this happens, the number of recruited motor units and muscle force increase with this difference. Threshold component λ can be modified by central influences either directly on α-MNs or indirectly, via interneurons and γ-MNs. Threshold λ* is velocity-dependent (decreasing when the muscle is stretched at velocity v). Sensitivity (µ) of the threshold to velocity can be regulated by γ-dynamic MNs. b To balance the arm gravitational torque (arrow), the system specifies a threshold position (R) at which arm flexors begin to be activated. For each muscle, this threshold is reached when the muscle length (x) coincides with the respective threshold length (λ). Under the influence of the torque, the arm and flexor muscles are extended beyond their thresholds until active and passive muscle torques become sufficient to balance the external torque at some position (Q). If Q is different from the desired position, the R is adjusted until the desired position is reached. c Left panel, Actual orientation (AO) of the body relative to the vertical is the slope of the vector along the body from the ankle joint (solid line). Referent orientation (RO) is the body orientation (dashed line) at which multiple muscles reach their activation thresholds (or produce net zero torque if muscles are co-activated). It is assumed that during quiet standing, the RO is aligned with the vertical. Under the influence of gravitational torques the body leans somewhat forward to an AO at which body balance is achieved. c Right top and bottom panels, To intentionally lean the body forward, the system leans the RO forward, thus narrowing the gap between the initial AO and the new RO, which means that the activation threshold for antigravity muscle increases. As a consequence, the activity of these muscles decreases. Unbalanced gravitational torque leans the body until re-stretched and re-activated muscles begin to balance the increased gravitational torques at a new AO
These notions are illustrated in Fig. 1b for the control of arm posture in the gravitational field. To hold the arm at some position, the system shifts the threshold angle, R, at which flexor muscles begin to be activated. For a given flexor muscle, this occurs at a specific muscle length, λ. Under the influence of arm weight (gravitational torque), flexor muscles are stretched beyond the threshold angle, muscle activity increases until active and passive muscle forces begin to balance the arm torque at position Q. If for some reason the resulting arm position is unsatisfactory, the system adjusts the R to correct the error.
One of several ways of coordinating activation thresholds of multiple muscles is to specify a common spatial threshold called the referent configuration (RC) of the body such that all skeletal muscles reach their activation thresholds at this configuration. Changing the RC, the nervous system can control all muscles of the body as a coherent unit (St-Onge and Feldman 2004). RC can be changed by reciprocal influences on α-MNs of opposing muscle groups usually called flexors and extensors. These influences can be transmitted to α-MNs pre- or post-synaptically, via interneurons or γ-MNs (Feldman 2015). Reciprocal influences can be combined with co-facilitation of flexor and extensor MNs (C command), thus shifting activation thresholds from those defined by the RC (reciprocal command). In this case, the RC becomes located within a spatial range of body configurations at which muscles are co-activated and the RC becomes the body configuration at which muscles are active but generate net zero torques. Changes in multi-muscle activity depend on the difference between the actual configuration (AC) and RC. These ideas stemming from experimental findings comprise the referent control theory, an extension of the equilibrium-point hypothesis that implies empirical, i.e., non-computational control of motor actions (Feldman 2015; see “Discussion”).
The present study addresses the question of how parametric control is used to appropriately orient our actions in the gravitational field. The vertical direction at a given place on earth is aligned with the local gravity direction that changes when we travel from one distant location to another. Our motor actions are subconsciously adapted to the local vertical direction. It has been suggested that, for whole body actions, only those RCs that result in ACs that are appropriately oriented in the gravitational field are used to control posture and movement. To meet this requirement, the concept of referent body orientation (RO) with respect to the gravitational vertical has been introduced (Feldman 2015). As in other cases of referent control, in the absence of coactivation, multiple muscles across the body reach their activation thresholds at the RO. Let us assume that during quiet standing on a horizontal surface, the RO is aligned with the vertical direction (Fig. 1c, left panel). Since the centre of body mass is in front of the ankle joint during quiet standing (Winter 2009), there is a small gravitational torque causing the body to lean slightly forward from the RO. Stretched beyond their activation thresholds, ankle extensors will be activated, the body will lean until active and passive muscle torques begin to balance the gravitational torque, resulting in a small deviation of the AO of the body from the referent position. Thus, in quiet standing, the body leans slightly forward with respect to the vertical.
To intentionally lean the body forward, the system would have to lean the RO forward, bringing it closer to AO. Physiologically, this means raising the activation threshold for ankle extensors and other antigravity muscles. This would result in a drop in the activity of these muscles, and the unbalanced gravitational torque would start to lean the body forward while ankle extensors are re-stretched. Stretching would reactivate the extensor muscles until they begin to balance the increased gravitational torque at a new AO (Fig. 1c, right panel).
The aim of this study was to test the validity of the RO concept in the explanation of how posture and movement are controlled in several tasks.
In Task 1, subjects stood on a horizontal surface or on an ascending or descending ramp. When standing on inclined surfaces (ascending or descending ramps), RO and AO can remain unchanged but, to preserve balance, the local RC of the ankle should be changed resulting in a modification of ankle muscle EMG activity (Hypothesis 1).
In Task 2, subjects intentionally leaned forward while standing on a horizontal surface. As described above, to meet the task demand, it would be necessary to lean the RO forward (see Fig. 1c). As RO approaches AO, ankle extensor EMG activity would initially decrease and the unbalanced gravitational torque would start leaning the body forward. Then, re-stretched by the gravitational torque, extensor muscles would increase their activity until balance would be achieved at a leaned posture (Hypothesis 2).
In Task 3, subjects rhythmically leaned the body. To perform the task, subjects would have to sway the RO. Most of the time, AO would differ from RO but at some phase(s), RO and AO may transiently overlap, resulting in EMG minima in the activity of multiple muscles across the body with the depth of the minima defined by the level of muscle co-activation resulting from threshold shifts by a C command (Hypothesis 3).
While discussing results of this and related previous studies, we will also evaluate the feasibility of alternative theories that imply full or partial (in combination with referent control) pre-programming of motor commands and kinematics.
Methods
Subjects
Sixteen healthy young adults without neuro-musculoskeletal problems (age 22.8 ± 3.9 years, 7 males) participated in the study. All subjects were informed of the experimental procedures and signed consent forms approved by the local ethics committee, according to the Declaration of Helsinki.
Experimental procedures
To address Hypotheses 1–3, subjects performed the 3 motor tasks in a randomized order. In Task 1, subjects (n = 11; 5 males) stood with soft-soled shoes on a horizontal (ground) surface or on an ascending (toes up) or a descending (toes down) ramp surface. Surfaces were inclined by ± 18° and were 59.5 cm long. The order of surface presentation was block randomized across subjects. Arms were crossed on the chest and feet were placed at the preferred medio-lateral stance width, which was maintained in all conditions. Subjects were asked to maintain the same orthograde posture of quiet standing on each surface, without intentionally co-activating leg muscles. Ten trials (5 s each) were recorded in each condition.
In Task 2, subjects (n = 16) stood quietly for 3–4 s on a horizontal surface. They were instructed to lean forward at a self-paced speed as far as possible by rotating about the ankle joint (“ankle strategy”; Horak and Nashner 1986) without losing stability and to avoid lifting their heels off the ground to ensure plantarflexor lengthening. After maintaining the final position for 3–4 s, subjects returned to the initial position. Five trials were recorded per subject.
In Task 3, subjects (n = 11) swayed as an inverted pendulum about their ankles without lifting their toes or heels at a self-paced speed within their comfortable range to avoid involuntary reactions. Sways always started in the forward direction. Subjects performed 5 blocks of 5 trials on each of the 3 surfaces described in Task 1, with a rest period after each block (25 trials per platform orientation condition).
EMG activity levels during maximal isometric voluntary contractions (MVC) of ankle plantar flexors and dorsiflexors (gastrocnemius, GL; soleus, SOL; tibialis anterior, TA) were measured in standard muscle testing positions (Hislop and Montgomery 1995) and used to normalize EMGs in Task 1. MVCs were produced as rapidly as possible and maintained for at least 3 s. Data were collected for 5 s and the middle 3 s was analysed to avoid variations due to muscle shortening and lengthening associated with muscle contraction initiation and termination. The mean of 3 MVC maximal EMG levels, with a 1 min rest between trials, were recorded.
Data acquisition
Kinematics were recorded with a 2 camera-bar Optotrak Certus motion analysis system (Northern Digital Instruments, Waterloo, Ontario; sampling rate 100 Hz). Six markers were placed on the right superior–lateral acromion, mid-sternum, anterior–superior iliac crest, mid-patella, lateral malleolus (ankle), and toe. Two additional markers placed on a rod perpendicular to the floor served as a reference of the absolute vertical. Bipolar surface EMG signals were acquired using a Wireless EMG Cometa system with an operating bandwidth of 10–500 Hz, a common mode rejection ratio of 100 dB (true differential electrodes) and a system gain of 1000. EMG data were acquired from 11 right-sided muscles: TA, SOL, GL, biceps femoris (BF), semitendinosus (ST), rectus femoris (RF), vastus medialis (VM), external oblique (EO), iliopsoas (IP), tensor fasciae latae (TFL), and erector spinae (ES) as well as from one muscle (usually TA) of the left leg. Recordings were acquired using pre-gelled 10-mm diameter disposable silver/silver chloride electrodes (Ambu Blue Sensor P), placed according to SENIAM guidelines (Surface Electromyography for the Non-Invasive Assessment of Muscles) after cleaning the skin with alcohol. EMG signals were digitized (16 bit A/D board, ± 10 V range), sampled (2000 Hz), filtered (4th order, zero-lag Butterworth, 60–500 Hz), full-wave rectified and analysed using custom made Matlab programs.
Data analysis
Task 1 Primary outcome measures were changes in tonic EMG amplitudes in ankle muscles (SOL GL, TA) across support surfaces and body orientation relative to vertical (global deviation angle). For each muscle, average root mean square (RMS) EMG amplitudes in a 500 ms gliding window over the middle 3 s periods were calculated and normalised as a percentage of EMG activity level during MVC.
Body orientation in the sagittal plane relative to vertical was calculated as the angle formed by the referent markers (vertical vector) and the vector between the ankle and acromial markers, which was positive for forward leaning. Sagittal projections of the body configuration (stick diagrams) during standing on different surfaces were also determined. Mediolateral body orientation and rotation were not analysed since they were expected to be small and not changed with surface orientation in quiet standing (Horak and Nashner 1986; Winter 2009). Ankle angle was calculated between the vector going from the ankle marker to the toe marker and the vector going from the ankle to the knee. Maximal plantar flexion (toe pointing down) would be 180° and maximal dorsiflexion would be 0° (hypothetically if the great toe could touch the tibia).
Task 2 The primary outcome measure was the change in plantarflexor (GL, SOL) EMG activity from baseline during leaning. Mean baseline EMG in each trial was determined in a 500 ms window ending 1 s prior to movement onset. Movement onset was first identified as the point at which ankle angular velocity exceeded 10% of its maximal value. Then, an algorithm searched backwards in time for the first point at which the angular velocity exceeded the mean + 2SD of the baseline. Mean EMG amplitudes starting from the end of the baseline period were computed in each subsequent 10 ms time bin until the end of the trial. For each subject, mean EMG values were normalized to maximal EMG values in the same trial. The onset of an EMG decrease was detected when the EMG amplitude decreased from baseline for at least 50 ms (Perez and Rothwell 2015). The offset was defined as the time when EMG amplitude values returned to or exceeded the baseline level for at least 50 ms. The mean EMG amplitude during the silent period was subtracted from the mean baseline EMG value and the decrease was expressed as a percentage of baseline EMG.
Task 3 The amplitude of sway was defined as the difference between the extreme backward and forward body orientations in each sway cycle. The primary measure was the probability of a period of minimal EMG activity occurring across all recorded muscles (EMG minima) in a sway cycle. A sway cycle was defined as the period of oscillation from the initial to the next forward leaning position. To identify an EMG minimum, a MEAN function was computed that represented the mean normalized EMG value (as in Task 2) over all muscles computed in each 0.5 ms time bin from movement onset to offset. The curve was low-pass filtered at 1 Hz (zero lag; Butterworth 2nd order) and minima were identified as points corresponding to when the first derivative of the curve was zero, the second derivative was positive and the value of the minima was less than 5% of the maximal value. Minima were confirmed based on two other EMG functions: the MAX (Fig. 4c, d; St-Onge and Feldman 2004) and N-CURVE (not illustrated). The MAX function corresponded to the maximal normalized EMG value across all muscles in each time bin. Thus, EMG minima were identified as points at which the values of the MAX across all muscles were less than 5% of their EMG amplitude in each trial. The N-CURVE identified the number of muscles exceeding the threshold value. Body configuration (stick diagram) and orientation at the time of EMG minima were also determined as defined above (Task 1). We determined EMG minima when the body moved in each direction (forward and backward phases of the sway cycle). The probability of EMG minima occurring for each surface condition was computed as the total number of global EMG minima observed divided by the number of sway cycles.
Statistics
Data were verified for normality with Levene’s tests and for sphericity using Mauchly’s Test. Greenhouse-Geisser corrections were used when indicated. Initial significance levels of p < 0.05 were used for all tests, with Bonferroni corrections implemented for multiple comparisons.
For Hypothesis 1, repeated-measures ANOVAs compared differences in normalized amplitudes of baseline GL, SOL and TA EMG activity and body orientation across the 3 ramp conditions (repeated measures) with appropriate post-hoc tests. For Hypothesis 2, descriptive statistics were used to characterize patterns of EMG activity. For Hypothesis 3, repeated-measures ANOVAs compared the number of minima that occurred over the 25 cycles for each of the 3 ramp conditions with appropriate post-hoc tests. In addition, we compared differences in body orientations at the time of EMG minima for each direction using an ANOVA with two repeated measures (ramp condition and movement phase). Paired t-tests compared differences between mean orientations at times of minima for forward and backward movement phases for each ramp condition.
Results
EMG levels in ankle extensors but not the body orientation differed in quiet standing on different surfaces
During quiet standing (Task 1), body orientation with respect to the direction of gravity was maintained for different ramp inclinations (Fig. 2a), with no differences in global deviation angles (mean ± SD: 1.6 ± 1.3°, 1.8 ± 1.9° and 1.7 ± 1.5° for descending, horizontal and ascending ramps respectively, F 2,20 = 1.468, p = 0.254).
Quiet standing on horizontal or tilted surfaces. a Body orientation was maintained while tonic EMG activity of ankle extensors (GL and SOL) decreased and activity of ankle flexors (TA) remained unchanged when standing on descending, horizontal and ascending surfaces, in a representative subject. b Mean (+ SD) tonic EMG levels for ankle muscles for a group of subjects (n = 11), in % from maximal EMG level during MVC determined individually for each subject. c Qualitative explanation of findings in a and b. The same body orientation during standing on different surfaces was maintained by compensating the same gravitational torque (T) by passive and active ankle torques at the equilibrium points (filled circles). Passive ankle torque increased with rotation of the foot from plantarflexion to dorsiflexion. Extensor EMG levels and active torques (curves above the passive torque curve) increased with foot deflection (horizontal bars underneath the abscissa) from respective threshold angles (R D, R, R A). Ankle flexors may be silent in these conditions since they generate active torques (curves below the passive torque curve) outside the zone of extensor activation. The scheme shows that maintaining the same body orientation in different conditions necessitates appropriate resetting of the spatial activation thresholds of ankle muscles causing the observable changes in the muscle activation levels
GL EMG activity monotonically decreased from descending to horizontal to ascending ramp orientations (Fig. 2a, b). Mean GL EMG amplitude was 2.22 ± 1.27% MVC, 1.32 ± 0.40% and 1.06 ± 0.44% for the descending, horizontal and ascending ramps, respectively (F 2,20 = 8.134; p = 0.003). GL activity significantly differed by 0.26 ± 0.25% MVC between ascending and horizontal ramp conditions (t = − 3.434, p = 0.006), 0.90 ± 1.20% between horizontal and descending ramp conditions (t = − 2.496, p = 0.032) and by 1.16 ± 1.23% between the ascending and descending ramp conditions (t = − 3.128, p = 0.011).
For SOL, mean EMG amplitude also monotonically decreased and was 3.13 ± 1.44, 2.21 ± 1.43, and 1.79 ± 1.36% for the descending, horizontal and ascending ramps, respectively (F 2,20 = 19.567; p = 0.001). SOL activity differed by 0.42 ± 0.48% between the ascending and horizontal ramp conditions (t = − 2.874, p = 0.017), 0.91 ± 0.75% between the horizontal and descending ramp conditions (t = − 4.026, p = 0.002) and by 1.33 ± 0.87% between the ascending and descending ramp conditions (t = − 5.055, p = 0.001).
Dorsiflexor (TA) EMG activity did not vary between ramp conditions (descending: 1.16 ± 1.02%; horizontal: 0.75 ± 0.52%; ascending: 1.19 ± 0.91%; F 2,20 = 2.141, p = 0.144).
Forward leaning was accompanied by an initial decrease and later increase in ankle extensor activity
All 16 subjects showed a similar EMG activation pattern associated with forward leaning (Task 2)—a short-lasting decrease in GL and/or SOL EMG activity starting before leaning onset with a subsequent gradual increase in EMG activity during leaning (Fig. 3a). This pattern was seen in at least one ankle extensor in 91.9% of trials. The decrease in EMG activity occurred in both GL and SOL muscles in 67.6% of these trials. Extensor EMG activity decreased by an average of 68.1% (41.9–88.9%) in GL and 60.1% (26.2–88.2%) in SOL. The duration of the decrease was 270 ms (101–1129 ms) in GL and 328 ms (104–1000 ms) in SOL. No consistent pattern of modulation of TA activity occurred across trials or subjects. Although subjects were instructed to avoid intentional coactivation of ankle flexors and extensors while leaning the body, they involuntarily co-activated muscles in about 40% of all cycles (Fig. 3a) while in the other cycles, TA activity did not change.
Intentional body leaning. a Typically, extensor EMG levels decreased before and then were re-activated during body leaning. The ankle flexor muscle (TA) was active in this subject but could be silent in other subjects. b, c Qualitative explanation of why central changes in the referent orientation (RO, lower arrow) elicit body leaning (changes in AO). At the initial position (left body sketch) the body is in balance (left filled circle in b and c) such that passive and active extensor torques together compensate the gravitational torque (T), as in Fig. 2c. To lean the body, the system shifts the RO, thus increasing extensor activation thresholds (right open circle). As a consequence, the initial body position appears to be in the subthreshold zone, and therefore, extensor activity initially drops. Unbalanced gravitational torque leans the body and stretched extensor muscles are reactivated. The activity of these muscles increases until the increased gravitational torque becomes balanced at a new AO (right equilibrium point, filled circle in b). The ankle flexor muscle (TA) may remain silent if the system only shifts the RO (in b) but can elicit TA tonic activation by additionally shifting thresholds such that ankle muscles become co-activated at the final position (in c). As in Fig. 2c, muscle activation is proportional to the distance between AO and RO (horizontal bars on the abscissa)
EMG minima during swaying could be identified at different body orientations in all ramp conditions
Subjects swayed about the ankle joint with velocities ranging from 0.5 to 27º/s (mean 4.7 ± 8.8º/s) and amplitudes ranging from 15 to 50° (mean = 12.0 ± 7.32°; Task 3). EMG minima could be identified in all ramp conditions (Fig. 5a).
A greater number (N) of EMG minima per sway cycle was found in the ascending (N = 1.3 ± 0.4) and horizontal (N = 1.3 ± 0.3) ramp conditions compared to the descending ramp condition (N = 0.8 ± 0.4; F 2,20 = 10.006, p = 0.001; Fig. 5a).
In ~ 97% of sway cycles, EMG minima systematically occurred during forward and/or backward movement phases (Figs. 4a, 5a). There was no difference between the probability of EMG minima occurring in the forward or backward phases in each of the three ramp conditions (descending: 0.43 ± 0.15 during forward and 0.36 ± 0.23 during backward phase; horizontal: 0.58 ± 0.21 and 0.63 ± 0.25, respectively; ascending: 0.57 ± 0.04 and 0.66 ± 0.06, respectively).
Minimization of EMG activity of multiple muscles and respective body configurations during swaying on an ascending surface. a Vertical lines (0–7) are phases of swaying (top trace; up is leaning forward) at which EMG activity of 10 muscles across the body was minimised. b Respective body configurations. c Functions MAX and MEAN based on which EMG minima were identified. d Minima of these functions shown at a higher time scale for posture 7 (arrows)
The number of minima in EMG activity of muscles across the body during swaying at different surfaces. a Group data (mean + SD, per cycle) for EMG minima during forward or backward motion during swaying. b Possible relationship between RO and AO overlapping at points 0–7 for the example of swaying in Fig. 4
In the forward sway phase, minima occurred when the body was oriented at global deviations of 0.8 ± 5.3°, 3.1 ± 5.5°, and 4.3 ± 1.8° from the vertical, for the descending, horizontal and ascending ramp conditions, respectively. In the backward phase, minima occurred at mean deviations of 2.3 ± 4.7°, 5.9 ± 2.2° and 4.2 ± 5.2° for the descending, horizontal and ascending conditions, respectively. There was a significant main effect of direction (F 1,10 = 45.12, p < 0.001) but not ankle position (F 1.1,10.8 = 3.70, p = 0.079) on global deviations.
Discussion
During quiet standing (Task 1), tonic ankle extensor activity monotonically decreased from standing on descending to horizontal, to ascending surfaces, whereas the same body orientation was maintained, supporting previous findings (Mezzarane and Kohn 2007; Sasagawa et al. 2009) and Hypothesis (1). In Task 2, when subjects intentionally leaned forward while standing on a horizontal surface, ankle extensor activity initially decreased and then increased above the initial background level until the end of leaning, supporting the prediction following from Hypothesis (2). In Task 3 involving body swaying, we identified body postures at which EMG activity of multiple lower limb muscles was minimized. EMG minima occurred during forward and backward sway phases, at different body postures, thus validating Hypothesis (3). These results are consistent with the referent control theory, other aspects of which, including the RC concept, have been validated in previous studies (Feldman 2015). Despite the solid support, we do not claim that the theory is true or proven: According to the scientific methodology, a theory can only be confirmed if not refuted. The term “confirmed” in this context means that one can continue to use the theory if experimental results are consistent with it, although it can be modified or even refuted in the future.
A fundamental aspect of the tasks we analysed is the active transfer of balance from one posture to another. To explain how such a transfer is accomplished, it is necessary to indicate a physiological parameter(s) that the nervous system changes to shift balance from one posture to another. Therefore, results will be discussed first in the framework of referent control in which such parameters have explicitly been defined in the Introduction. Then, based on the present and previous findings, we will consider the feasibility of alternative theories that suggest that motor actions are controlled due to feed-forward computation and production of motor commands, possibly in combination with referent control (hybrid theories).
Standing on differently inclined surfaces
Since subjects maintained body orientation during standing on different surfaces, the gravitational torque remained constant across conditions. This means that the combined active and passive ankle torques produced the same net torque compensating the external, gravitational torque. During standing on the ascending ramp, GL and SOL were stretched compared to standing on the horizontal surface, potentially increasing the extensor ankle torque and tilting the body backward, thus changing the body orientation. Our results show, however, that the body orientation was preserved when subjects stood on the ascending ramp. In contrast, body configurations during standing on differently inclined surfaces were different due to changes in the ankle angle. Balance of torques and stability was characteristic of each of these configurations. This means that the system somehow transferred balance and stability from one body posture to another to stand on differently inclined surfaces while maintaining the body orientation.
To meet the above requirements, referent control of posture in Task 1 was likely accomplished by adjusting the threshold joint angle (R) at which MNs of ankle muscles began to be recruited and generated an active torque (Fig. 2c, curves diverged from the passive torque curve). Physiologically, in terms of referent control, this process can primarily be associated with a shift in the spatial thresholds at which muscles begin to be active, transferring the stretch reflex and other posture-stabilizing mechanisms to the posture on the tilted surface. In particular, when stepping from a horizontal surface to stand on the ascending ramp, the CNS increased the spatial threshold (R) for activation of ankle extensors. In this case, the stretch reflex was not attenuated but was re-addressed to a new posture to preserve body orientation and stable balance.
These explanations equally refer to suppression of the stretch reflex of ankle extensors to lengthening elicited by tilting of the support surface from the horizontal to the ascending position (Nashner 1976; Horak and Nashner 1986; Horak et al. 1989; Schieppati and Nardone 1995). Usually this observation is considered to result from a decrease in gain, i.e., sensitivity of the stretch reflex to muscle lengthening. However, this explanation is not specific enough to explain how body balance is transferred to the final posture during standing on the tilted surface. Instead of gain, the system shifted the referent ankle position such that the stretch reflex was re-addressed to the final posture to provide stable balance in that posture. Although indirectly, this suggestion has been confirmed by analysing the H-reflex in this task: initially attenuated, the reflex was fully restored at the final body posture (Miranda et al. 2014).
Referent control helps explain the difference in the tonic EMG levels as well as other biomechanical characteristics of standing on different support surfaces (Fig. 2c). At the neutral position of the ankles, passive torque was approximately zero and it increased with increasing dorsiflexion associated with passive stretching of ankle extensors. By changing parameter R, the nervous system shifted the characteristic of active torque and thus the equilibrium point (filled circles on horizontal dashed line in Fig. 2c) in the interaction between body segments and external torque and thus actively changed the body posture at which balance and stability was regained. Thereby, the net ankle torque and thus the body orientation were preserved.
Ankle extensor EMG depends on the difference between the actual (Q) and threshold angle (R). This distance decreases (thick horizontal bars on the abscissa in Fig. 2c) with the transition between standing surfaces. This explains the monotonic decrease in GL and SOL activity across conditions in our study (see also Mezzarane and Kohn 2007; Sasagawa et al. 2009). Thereby, the necessary modulation of reflexes (including the stretch reflex) from limb mechanoreceptors was caused by increasing the activation threshold (R D, R, R A; Fig. 2c), and balance and stability were re-addressed to respective body postures.
Self-initiated forward leaning
If RO is the control variable for orientation to gravity, it would need to be leaned forward to initiate forward body leaning (Fig. 3a). As RO advances, it would approach AO causing EMG activity in ankle extensors (GL, SOL) to decrease. The unbalanced gravitational torque would start to lean the body forward, re-stretching and thus reactivating ankle extensors. The EMG levels and active torques of these muscles would increase until active and passive torques begin to balance the increased gravitational torque at a new AO. Thus, the EMG and kinematic features during leaning can be caused by referent control. Thereby a monotonic, ramp-and-hold pattern shift in the RO was sufficient to elicit a non-monotonic EMG pattern in ankle extensors. The expected pattern in ankle extensors was seen in most trials while the antagonist, TA could be co-activated with ankle extensors from trial to trial. These variations of EMG patterns could be elicited by additional shifts in activation thresholds from those defined by the RO and by creating a coactivation zone at the final body position to enhance stability (Fig. 3b, c).
Local changes in the activation thresholds of ankle joint muscles in Task 1 could be mediated by corticospinal (CS) pathways, whereas the required body orientation was likely maintained by tonic descending influences from the vestibular and visual systems. In contrast, in Task 2, the vestibular system, in cooperation with the somatosensory and visual systems, might be involved in active control of the body orientation, which is consistent with results of studies of the influence of different descending systems on body posture in humans and animals (Maurer et al. 2000; Peterka 2002; Beloozerova et al. 2003; Fitzpatrick and Day 2004; Deliagina et al. 2006, 2014). However, based on the notion of referent control, Figs. 2c and 3b, c suggests new aspects of postural regulation. One can assume that in Tasks 1 and 2, the CS system was mainly responsible for the referent (threshold) control of body configuration, whereas the vestibular system was mainly responsible for the referent control of body orientation. Both systems can accomplish these functions by shifting the threshold positions of body segments at which muscles begin to be activated (Feldman and Orlovsky 1972; Raptis et al. 2010; Sangani et al. 2011; Ilmane et al. 2013).
Referent control can be performed in an open-loop manner in the following way. Given a motor goal, the CNS likely decides which form(s) of threshold control can be used to reach the goal. In the case of body tilting, the natural form is RO shifts. Based on all available sensory information and the task demand, the CNS could begin to shift the RO at a certain rate, i.e., independently of sensory feedback until current sensory feedback indicates that the required tilting posture will be reached. If not, the system could prolong, shorten or initiate new RO shifts, e.g., to elicit a step to regain balance if the emerging body leaning threatened to exceed the limits of body stability. In other words, open-loop control of the RO implies transient (“piecewise”) independence of sensory feedback, as in eye saccades during which vision is blocked (Matin 1974). Also note that RO shifts in Task 2 would be initiated in advance and cease before the end of actual body motion. This is suggested based on the finding of a similar timing of referent shifts in rapid arm movements (Ghafouri and Feldman 2001). The rationale for such shifts is that the final body position (attractor point) is virtually set before the offset of the actual movement, and therefore, the CNS can start correcting or initiating new RO shifts without waiting for the end of the actual body motion. In other motor actions, such timing of referent control might also be used to generate rapid movement sequences, e.g., in piano playing and speech production.
The piecewise open-loop nature of referent (threshold) control can be important for the stability of posture and movement (Feldman 2016). To lean the body forward, the nervous system monotonically increases the activation threshold of ankle extensors to a certain final value to elicit forward body leaning without provoking a step. If the shift were done in closed-loop fashion, based on continuous afferent feedback, threshold shifts would be prolonged such that the body would eventually be forced to make a step to prevent a fall. Thus, physically and physiologically, threshold shifts based on continuous afferent feedback would be destabilizing.
A piecewise open-loop control strategy could be used during rhythmic tilting of the support surface in quadrupeds (Beloozerova et al. 2003; Deliagina et al. 2006, 2014). Although referent shifts may be triggered by afferent signals (e.g., from foot pressure receptors) evoked in response to tilting, once triggered, referent shifts could be accomplished in an open-loop piecewise way, at a rate that is periodically adjusted to pursue the motion of the support surface (cf. Deliagina et al. 2014).
Swaying
Although the RO concept was applied to the explanation of EMG patterns at a single, ankle joint in Tasks 1 and 2, the difference between the AO and RO is a major factor in the control of all muscles of the body, regardless of their biomechanical differences. This aspect of referent control was studied in Task 3, and is illustrated by the finding of EMG minima of multiple muscles across the body during voluntary back and forth swaying around the ankle joints. We hypothesized that swaying results from appropriate rhythmical changes in the RO. Throughout most of the movement, the location of AO would be different from RO but at some phase(s) of the rhythmical motion, RO and AO may transiently overlap, resulting in EMG minima in the activity of multiple muscles across the body. We found minima when subjects leaned forward as well as when they leaned backward, implying that the body RO shifted in a task-specific way. Minima did not occur in every sway cycle, and this was expected since subjects did not always sway in the same manner each time: both RO and AO could vary from cycle to cycle. In addition, the same RO and AO orientation could be associated with somewhat different body configurations. Muscle co-activation is also a factor restricting the occurrence of EMG minima.
If rhythmical changes were elicited by rhythmical changes in the RO, one can suggest that after an initial period, the RO and AO oscillated with some delay between them (Fig. 4b). The deflection of AO from RO resulted in muscle activation and torques responsible for body acceleration approximately in anti-phase with body displacement, which is characteristic of many rhythmic oscillations.
More minima were found when subjects swayed on an ascending/horizontal ramp versus a descending ramp. This is likely because subjects had greater difficulty performing the sways on the descending ramp due to stability restrictions. Subjects may also have been more fearful of falling when swaying in this position. This may have resulted in greater variability of sway cycles, thereby reducing the probability of overlap between RO and AO responsible for EMG minima.
Note that during quiet standing before the onset of swaying, the RO was somewhat behind the AO and the difference between these orientations was responsible for a small tonic activation of extensors (see above). All subjects started swaying by leaning the body forward via the forward motion of RO, resulting in an initial global minimum when RO crossed AO (line 0 in Fig. 4a, b). Such a pre-swaying EMG minimum was observed in most subjects. Taken together, our results support the notion that the RO plays an important role in the control of posture and movement. This new finding of minima in the activity of multiple muscles across the body at two different body configurations during sway is a critical test of the theory: The minima show that spatial activation thresholds for multiple muscles are shifted during swaying. If the minima were not observed, the RO hypothesis would have to be rejected or modified.
Empirical (non-computational) nature of referent control
How does the system find the values of control variables to meet the motor goal, for example, the desired final joint angle in a single-joint task or final body orientation in the forward body leaning task. This question is referred to as the mapping problem. It is usually assumed that the nervous system computes the requisite values of motor commands in advance and specifies them based on hypothetical internal models of the system interacting with the environment (e.g., Wolpert and Kawato 1998). In contrast, the requisite values of referent control variables are set empirically, i.e., without any computations and internal models. Specifically, to establish a desired joint angle (Fig. 1b) or desired degree of body leaning (Fig. 1c), the system starts changing the appropriate referent parameter (R or RO, respectively) from an initial value, until available afferent feedback (vision, sound, kinesthesis, etc.) indicate that the goal is reached. If necessary, the system can enhance stability and/or movement speed by adding co-facilitation of agonist and agonist MNs (i.e., a C command).
The understanding of the non-computational nature of actions in the framework of referent control can be facilitated by considering referent parameters as pre-existing neural control tools. To clarify, consider driving a car. To move the car in the desired direction, we use an appropriate tool—the steering wheel. We turn it until the car begins to move in the desired direction. The degree of wheel rotation to accomplish the task is not a matter of the driver’s concern—the required wheel rotation is not computed but is set empirically by turning the wheel and observing the resultant car motion direction. Similarly, to accelerate the car, the driver gradually pushes on the gas pedal, another tool. Different forms of referent control can also be considered as neural tools for a non-computational solution to the mapping problem. In this aspect, referent control is principally different from engineering, computational control schemes (see also Tomita et al. 2016).
Fundamental role of sensory feedback in the origin of referent control
In the threshold control framework, sensory feedback is something more than feedback in the traditional, engineering sense. Figure 6a explains that only in the presence of length-dependent proprioceptive feedback, electrochemical control signals descending to α-MNs from the brain acquire the dimensionality of muscle length. Specifically, consider the α-MN that is the first in the order of recruitment during muscle stretching. Before recruitment, the membrane potential of this MN will increase with increasing muscle length (x) until the electric threshold (Vth) is reached at some muscle length (λ i). Independent central changes in the membrane potential (∆V, vertical arrow) will result in a shift in the threshold length to λ (horizontal arrow). In the supra-threshold range, the MN and subsequently recruited MNs will generate activity (A) depending on the difference between the actual and threshold muscle length: A = f(x − λ).
Possible physiological origin of the referent orientation and its central regulation. a, b MN receives length-dependent afferent signals such that its membrane potential increases when the muscle is stretched from length x 1 (lower diagonal line). At the threshold length, λ i , the MN is recruited. The recruitment threshold can be shifted to λ (horizontal arrow) via central inputs (ΔV, vertical arrow). After the shift in the spatial threshold, the MN remains silent at muscle length x 1 but is recruited at length (x 2). c With respective modifications, the scheme in a can be applied to other neurons. If a neuron receives sensory inputs depending on body orientation (AO) then the membrane potential reaches electrical threshold at a certain spatial threshold defining the referent body orientation (RO). Independent change in the membrane potential shifts the RO. In the supra-threshold area, neuronal firing depends on the difference between AO and RO (formula at the bottom of the c panel). Shifts in RO can be pre-determined in a feedforward way based on all available sensory information (vestibular, visual and somatosensory). Once this is done, the RO is shifted in an open-loop way until sensory feedback indicates that shifts should be ceased or modified, in particular, to prevent an error in reaching the desired AO. d Similarly, one can describe the origin of the RC and basic properties of RC neurons. e In the proposed hierarchical scheme, the output signals of a higher level play the role of central inputs for neurons of the subordinated level and thus modify their spatial thresholds and, eventually, the spatial threshold of MNs. It is assumed that the neural projections are established such that the changes in MN thresholds tend to minimize the difference between AO and RO. Symbols f, g, h in panels c–e are functions of the variables in brackets
Note that λ is not the muscle length itself but a parameter, the central regulation of which is essential for pre-determining the spatial range in which MNs function. Any central input to MNs, even subthreshold for muscle activation, changes λ, thus shifting the muscle force–length characteristic (Fig. 1a) and setting a new equilibrium (attractor) point of the system prior to the offset of emerging actions (Ghafouri and Feldman 2001). This illustrates the feed-forward nature of referent control, allowing the nervous system, for example, to prepare reflex reactions to future perturbations while preserving the background EMG activity (Sangani et al. 2011).
It is also important that without length-dependent proprioceptive feedback, parameter λ does not exist, a pathological condition observed in deafferented subjects who cannot stand or walk in the absence of vision and who lose their sense of body schema (Cole and Paillard 1995).
Sensory feedback penetrates all levels of referent control but this feedback is specific for each level. The existence of the RO and its central regulation would be impossible in the absence of vestibular, visual and probably proprioceptive feedback that transmit information about the actual body orientation to relevant, presumably vestibulo-spinal neurons (Fig. 6c). With respective modifications, the scheme in Fig. 1a, is applicable to other neurons mediating RO and RC controls. In particular, the membrane potential of such neurons will increase with increasing afferent feedback about AO until their electric threshold is reached. The AO at which the recruitment threshold is reached is the RO. Independent central changes in the membrane potential will result in a shift in the RO. In the supra-threshold range, neurons will generate activity depending on the deflection of AO from RO.
The question of how the output signal of RO neurons influences MNs can be answered in the following way (Fig. 6e). The output signal, g (AO-RO), is transmitted as a central signal to RC neurons, and therefore, changes the RC. Through the existing projections (direct, or indirect, via interneurons and γ-MNs), the output signal, h (AC-RC) of RC neurons is transmitted as a central signal to α-MNs. For the latter, the transmitted signals signify changes in λ that are independent of proprioceptive feedback to α-MNs (but dependent on sensory feedback at the RO and RC levels). It is assumed that descending projections to α-MNs predominantly manifest a reciprocal pattern such that the activation thresholds are decreased for muscles that counteract the difference between AO and RO and increased for antagonist muscles. The difference (AO − RO) affects only part (λ) of the total threshold (λ*) of α-MNs. The other part remains dependent on continuous proprioceptive information about velocity of muscle contraction and intermuscular reflexes (Fig. 1a). The hierarchical neuromuscular circuitry acts to minimize the difference between AO and RO until the activity of muscles becomes sufficient to balance gravitational and, if present, other external torques.
In the context of referent control of body orientation, the RO is the leading control parameter. However, the scheme in Fig. 6 can be considered in a broader sense. One can assume that depending on the task demands the brain can access different levels of the control hierarchy. In a single joint task (Fig. 1b), the system can initiate a local change (∆R) in the referent position of the respective single joint. In this case, ∆R is a control parameter. At a higher level of this hierarchy, the system may focus on the entire body configuration resulting from changes in the referent configuration (RC) of the body, e.g., in ballet dancing. In this case, control parameter is ∆RC. Locomotion (single step, waking, running, crawling, jumping forward, etc.) can be controlled by translating body balance in the environment via shifts of the referent location of the body. Figure 6 is just a preliminary draft of the hierarchical organization of referent control that needs to be made more specific in appropriate experiments.
Alternative, computational and hybrid theories of motor control
Another theory (computational theory) that has been proposed to explain how the brain controls motor actions, suggests that the brain directly pre-programs and specifies motor commands to muscles (EMG patterns or forces) that guide body segments according to the desired trajectories. These commands are pre-computed by neural emulators of mechanical and physiological laws (Hollerbach 1982; Ghez et al. 1991; Wolpert and Kawato 1998; Kawato 1999; Todorov and Jordan 2002) that depend on the inversion of the causality inherent in these laws (inverse internal dynamic models). The computational theory is used in robotics and its applicability to biological systems is motivated by correlations of brain activity with kinematic and kinetic variables (e.g., Imamizu et al. 1998; Sussillo et al. 2015).
Another similar approach is the hybrid theories in which empirical, threshold control is combined with computational, at least partial pre-programming of motor commands, for example, to modulate movement speed.
The existence of the referent control and alternative theories creates uncertainty in the understanding of basic principles of motor control, necessitating a resolution of the controversy. Consider first if computational and hybrid approaches are consistent with basic physiological properties of MNs and muscles. We also consider if they are consistent with general, physical principles stemming from the dynamic systems theory. Finally, although the present study was not designed to test the alternative theories, we will evaluate the consistency of our results with such alternative theories.
In robotics, motor commands are torques generated by electrical motors moving robotic arms. Based on equations of motion and desired movement trajectories, these torques can be pre-computed. Computations per se yield symbolic values of variables. With the help of an interface, the symbolic torque values are converted into physical, electric currents to execute the desired motor action. This is done using the known electro-mechanical characteristics of motors. The computed currents are generated to move robotic arms. In contrast, even if we accept that the requisite values of motor commands (EMG patterns or muscle forces) can be pre-computed using hypothetical internal models, the nervous system would be unable to physically produce the computed commands. Specifically, suppose the system determined that a MN should generate, say, 15 spikes/s. These spikes characterize the desired output of the MN. Like in robotics, the computed output signals should be converted into input, synaptic currents to activate MNs and muscles. Mathematically, however, the input/output relationships of MNs are irreversible: it is impossible to determine which synaptic inputs should be activated to generate the computed output of the MN. One can only assume that the output spikes of the MN could be elicited by some supra-threshold synaptic inputs to the MN, but even this is not assured: MNs can transiently generate spikes in the absence of synaptic inputs (Hultborn et al. 2004; Heckman et al. 2008). In other words, conversion of computed motor commands into physical actions is impossible because of fundamental non-linear properties of MNs—the existence of the electrical thresholds for recruitment and intrinsic ability of MNs to generate rhythmic spikes due to plateau potentials after recruitment. Note that the problem of conversion of computed symbolic motor commands into physical actions does not exist in the referent control theory since it deals with physiological parameters that can be changed to elicit motor commands without any computations (see above).
There is another way to illustrate that neural control levels cannot and do not pre-determine motor commands. Figure 6a shows that in the absence of muscle stretching, central signal ∆V delivered to MNs at muscle length x 2 elicits muscle activation (right vertical arrow). This activation is conditional: the same central signal at a shorter muscle length (x 1) does not produce muscle activation. Consistent with this prediction are experimental findings that the same tonic influences of different descending systems that elicit MN recruitment at a certain muscle length become sub-threshold at a shorter muscle length (Feldman and Orlovsky 1972). This analysis shows that an adequate measure of independent central influences on MNs is the shift in the muscle activation thresholds, thus pre-determining the spatial boundaries within which EMG patterns can emerge due to the interaction of muscles with external forces. In this way, central control signals only indirectly influence but do not pre-determine EMG patterns. This shows, once again, that computational and hybrid theories of motor control are physiologically unfeasible.
Note that threshold (referent) control is self-sufficient for modulating kinetic and kinematic characteristics such that hybrid control schemes are not only unrealistic but would also be unnecessary. For example, movement speed is modulated by changing the rate of shifts of the R command and additionally by the C command resulting from co-facilitation of agonist and antagonist MNs. This command is also produced not by direct activation of MNs but by shifts in their spatial thresholds from those specified by the R command (for more details see Pilon et al. 2007).
As mentioned above, regular observations of correlations of the activity of different brain areas, including the motor cortex, with motor commands (EMG signals) are considered as evidence of the computational nature of motor control, even though correlations do not necessarily imply causality. There are several studies showing that CS influences and EMG activity levels can be de-correlated to reveal that the CS system sets the threshold position at which MNs begin to be recruited without pre-determining motor commands produced by MNs. Specifically, the EMG levels are different at different actively established wrist positions in humans. By compensating passive muscle forces with a torque motor, EMG levels were equalized at two wrist positions. The hypothesis of direct pre-programming of EMG levels by the CS system would predict that in this situation CS influences should be similar at these positions. In contrast, active motion requires the transfer of balance from one position to another, such that according to threshold control, CS influences should change to shift the balance. A robust finding in the experiments using transcranial magnetic stimulation was that the CS influences were substantially different at these wrist positions, thus rejecting the hypothesis of pre-programming of motor commands (Raptis et al. 2010).
Another way to demonstrate de-correlation between CS output and EMG patterns is using the unloading reflex. CS influences were not changed when joint position changed involuntarily due to unloading of pre-loaded wrist muscles (the unloading reflex) despite substantial changes in EMG activity with the transition from the initial to the final post-unloading position (Ilmane et al. 2013). Previous experiments have shown that not only the CS but the vestibulo-spinal and other descending and spinal systems also sets spatial thresholds for muscle activation, without pre-determining EMG levels (Matthews 1959; Feldman and Orlovsky 1972).
Computational and hybrid motor control approaches are not only inconsistent with basic physiology of MNs but also with the dynamical principle (see the “Introduction”) expressed as the classic posture–movement problem. As initially recognised by von Holst (von Holst and Mittelstaedt 1950/1973; von Holst 1954), muscles and reflexes are responsible for posture-stabilizing mechanisms producing position- and velocity-dependent responses that resist deviations from the initial posture. Deflections from the initial posture elicited by centrally pre-programmed motor commands to muscles would evoke resistance of these mechanisms, preventing the execution of the central program. The assumption by von Holst that reflexes are suppressed during voluntary actions is inconsistent with existing data on the behaviour of the stretch and other reflexes (Turpin et al. 2016). Thus, the idea of central pre-programming of EMG and kinematic patterns does not solve the posture–movement problem since, according to the dynamic principle, being related to muscle forces, EMG signals are not responsible for shifts in balance, and attempts to drive the system to a new posture by these signals would be met with resistance from reflexes driving the system back to its initial posture. This prediction conflicts with everyday observations that in any posture-to-posture movement there is no tendency to reverse the motion after its end.
As argued above, the notion of pre-programming of motor commands is incompatible with parametric, referent control. Therefore, the finding of two different body postures at which transient EMG minima in multiple muscles occur signifies changes in parameters (muscle activation thresholds) underlying rhythmical shifts in body balance during swaying. This finding thus supports the threshold control theory but not alternative approaches.
The EMG patterns during leaning the body forward are fully consistent with laws of mechanics: EMG activity and torques of ankle extensors initially decrease to let the body move forward and then increase to decelerate and stop motion. The consistency of EMG patterns with mechanical laws does not necessarily mean that these patterns are pre-programmed: these patterns are an emergent property of referent control. Attempts to explain these patterns by assuming that they are pre-programmed run into problems that have been described above as well as into new problems. The leaning starts from an initially stabilized posture during quiet standing, such that in the case of occasional deviations from the posture, the neuromuscular mechanisms generate restoring position- and velocity-dependent forces, characteristic of the stretch reflex of ankle muscles (e.g., Gottlieb and Agarwal 1979). Von Holst (1954) suggested that stretch reflex resistance to motion is suppressed with a copy of the pre-programmed motor commands (efference copy, EC). In the case of the body leaning forward, the extensor EMG activity is di-phasic, initially decreasing and then increasing (Fig. 3a). The EC of such an EMG pattern can suppress the stretch reflex in one phase but release it in the other phase, conflicting with the original proposal on the function of EC. Suppose that the system somehow overcame this problem and eventually leaned the body while balancing the increased gravitational torque. Here, another problem is encountered: deprived of position- and velocity-dependent feedback provided by the stretch reflex, the final body posture will be unstable, i.e., random deviations will drive the body away from the final posture. For example, if the body is occasionally deflected forward from the final position, the increased gravitational torque will amplify the forward leaning to a fall. In addition, as explained above theories of direct pre-programming of kinematic and kinetic variables, including hybrid theories fail to explain how computed torques are converted into physical ones to elicit a motor action. Therefore, the assumption that EMG patterns are pre-programmed is associated with several unsolvable problems.
Dynamic models of postural control
The predictions tested in the present study were derived qualitatively, from known central and reflex mechanisms integrated in the referent control theory. The advantage of the theory is the possibility of verification without computer-based models. Although such models may reproduce some aspects of motor behaviour, their significance is often limited: this result is achieved by fitting experimental curves based on adjusting coefficients, the values of which are unknown. Most models of postural control consider the body as an inverted pendulum (Winter et al. 1998; Micheau et al. 2003; Peterka 2000; Welch and Ting 2009). In itself such a pendulum has one unstable equilibrium position aligned with the direction of gravity. In contrast, by analysing motion of the centre of mass, Zatsiorsky and Duarte (2000) found that the body equilibrium position during quiet standing is not unique but discretely changes over time. This means that muscle and reflex properties providing stable balance in each position have to be shifted or re-addressed through neural mechanisms to the subsequent positions. Thus, without a control structure, the model of the inverted pendulum is insufficient to describe dynamic postural stability.
Several models emphasised the role of reflexes (afferent feedback) in postural control (Peterka 2000; Welch and Ting 2009) but only one model (Micheau et al. 2003) explicitly indicates physiological variables—shifts in spatial thresholds of reflexes—underlying changes in equilibrium body postures during quiet standing. Such a model explains how the system can shift body balance and how EMG patterns emerge in the task of leaning the body forward. The same model can be advanced by considering the multi-joint and multi-muscle structure of the body controlled by the RC and RO variables, as has been done in the model of multi-muscle control of sit-to-stand motion (Feldman et al. 2007).
Further implications
Referent control offers a specific interpretation of the notion of reference frames (RFs) in the production of posture and movement (cf. Colby 1998; Hadders-Algra and Carlberg 2008). Referent control implies that neural RFs are spatial and that RC and RO represent the origins of such RFs for body configuration and orientation in the gravitational field, respectively. Unlike traditional approaches, these frames are not fixed: motor actions are caused by shifting the origins of RFs (Feldman and Levin 1995). Referent control suggests that the system only pre-determines the spatial RF within which a motor action emerges due to mechanical and neural interactions of neuromuscular elements between themselves and with the environment. This point was illustrated for standing on different support surfaces and intentional discrete leaning or swaying (Figs. 2, 3, 4, 5). In other words, the nervous system pre-determines the spatial boundaries for motor actions and changes these boundaries if the emergent motor action is expected to be in error.
Many studies emphasize the role of anticipatory postural adjustments (APAs) in the control of stability of posture and movement (Belen’kiĭ et al. 1967; Cordo and Nashner 1982; Horak and Macpherson 1996). Usually, while analysing APAs, researchers focus on EMG signals associated with them. As illustrated in Fig. 6a, the RO and other referent variables start changing due to sub-threshold modifications of input signals and the intrinsic state of respective neurons, i.e., in advance of changes in EMG patterns and kinematics. EMG patterns associated with APAs are thus a consequential, emerging aspect of the subthreshold control processes associated with APAs. An important role of the subthreshold aspect of APAs has been illustrated in studies showing that the CS system can set muscle activation thresholds long before the onset of perturbation to pre-determine an appropriate response to it (Sangani et al. 2011; Zhang et al. 2017). In the present study, we emphasised that the subthreshold nature of referent control is essential for postural adjustment in preparation to stand on a tilted surface.
Conclusions
In this study, the concept of RO was supported and used to explain how posture and movement are controlled empirically, i.e., without computations. Referent control allows the system to transfer, in a feedforward way, balance and stability to the posture to which movement is directed. These aspects of referent control may further be tested in future studies.
Theories suggesting that the nervous system controls motor actions based on complete or partial pre-programming of motor commands computed with the help of hypothetical internal models are incompatible with a set of dynamical and physiological principles, rejected in previous studies, and do not explain the present results.
References
Andronov AA, Vitt AA, Khiken SE (1966) Theory of oscillators. Pergamon Press, Oxford
Asatryan DG, Feldman AG (1965) Functional tuning of the nervous system with control of movements or maintenance of a steady posture: I. Mechanographic analysis of the work of the joint on execution of a postural task. Biophysics 10:925–935
Belen’kiĭ VE, Gurfinkel’ VS, Pal’tsev EI (1967) Control elements of voluntary movements. Biofizika 12:135–41
Beloozerova IN, Zelenin PV, Popova LB, Orlovsky GN, Grillner S, Deliagina TG (2003) Postural control in the rabbit maintaining balance on the tilting platform. J Neurophysiol 90:3783–3793
Colby CL (1998) Action-oriented spatial reference frames in cortex. Neuron 20(1):15–24
Cole J, Paillard J (1995) Living without touch and information about body position and movement. Studies on deafferented subjects. In: Bermudez J, Marcel A, Iylan N (eds) The body and the self. MIT Press, Cambridge, pp 245–266
Cordo PJ, Nashner LM (1982) Properties of postural adjustments associated with rapid arm movements. J Neurophysiol 47:287–302
Deliagina TG, Orlovsky GN, Zelenin PV, Beloozerova IN (2006) Neural bases of postural control. Physiology (Bethesda) 21:216–225
Deliagina TG, Beloozerova IN, Orlovsky GN, Zelenin PV (2014) Contribution of supraspinal systems to generation of automatic postural responses. Front Integr Neurosci 28:76
Feldman AG (2015) Referent control of action and perception. Springer, New York
Feldman AG (2016) The relationship between postural and movement stability. Adv Exp Med Biol 957:105–120
Feldman AG, Levin MF (1995) The origin and use of positional frames of reference in motor control. Behav Brain Sci 18:723–806
Feldman AG, Orlovsky GN (1972) The influence of different descending systems on the tonic stretch reflex in the cat. Exp Neurol 37:481–494
Feldman AG, Goussev V, Sangole A, Levin MF (2007) Threshold position control and the principle of minimal interaction in motor actions. Prog Brain Res 165:267–281
Fitzpatrick R, Day B (2004) Probing the human vestibular system with galvanic stimulation. J Appl Physiol 96:2301–2316
Ghafouri M, Feldman AG (2001) The timing of control signals underlying fast point-to-point arm movements. Exp Brain Res 137:411–423
Ghez C, Hening W, Gordon J (1991) Organization of voluntary movement. Curr Opin Neurobiol 1:664–671
Gottlieb GL, Agarwal GC (1979) Response to sudden torques about ankle in man: myotatic reflex. J Mot Behav 11(2):123–133
Hadders-Algra M, Carlberg EB (2008) Postural control: a key issue in developmental disorders. Mac Keith Press, London
Heckman CJ, Hyngstrom AS, Johnson MD (2008) Active properties of motoneurone dendrites: diffuse descending neuromodulation, focused local inhibition. J Physiol 586:1225–1231
Hislop HJ, Montgomery J (1995) Daniels and Worthingham’s muscle testing: techniques of manual examination. WB Saunders, Philadelphia
Hollerbach JM (1982) Computers, brains, and the control of movement. Trends Neurosci 5:189–192
Horak FB, Macpherson JM (1996) Postural orientation and equilibrium. In: Rowell LB, Shepherd JT (eds) Handbook of physiology 1. Exercise: regulation and integration of multiple systems. Oxford University Press, New York, pp 255–292
Horak FB, Nashner LM (1986) Central programming of postural movements: adaptation to altered support surface configurations. J Neurophysiol 55:1369–1381
Horak FB, Diener HC, Nashner LM (1989) Influence of central set on human postural responses. J Neurophysiol 62:841–853
Hultborn H, Brownstone RB, Toth TI, Gossard JP (2004) Key mechanisms for setting the input-output gain across the motoneuron pool. Prog Brain Res 143:77–95
Ilmane N, Sangani S, Feldman AG (2013) Corticospinal control strategies underlying voluntary and involuntary wrist movements. Behav Brain Res 236:350–358
Imamizu H, Uno Y, Kawato M (1998) Adaptive internal model of intrinsic kinematics involved in learning an aiming task. J Exp Psychol Hum Percept Perform 24:812–829
Kawato M (1999) Internal models for motor control and trajectory planning. Curr Opin Neurobiol 9(6):718–727
Matin E (1974) Saccadic suppression: a review and an analysis. Psychol Bull 81:899–917
Matthews PBC (1959) A study of certain factors influencing the stretch reflex of the decerebrated cat. J Physiol 147:547–564
Mattos DJ, Latash ML, Park E, Kuhl J, Scholz JP (2011) Unpredictable elbow joint perturbation during reaching results in multijoint motor equivalence. J Neurophysiol 106:1424–1436
Mattos D, Schöner G, Zatsiorsky VM, Latash ML (2015) Task-specific stability of abundant systems: structure of variance and motor equivalence. Neurosci 310:600–615
Maurer C, Mergner T, Bolha B, Hlavacka F (2000) Vestibular, visual, and somatosensory contributions to human control of upright stance. Neurosci Lett 281:99–102
Mezzarane RA, Kohn AF (2007) Control of upright stance over inclined surfaces. Exp Brain Res 180:377–388
Micheau P, Kron A, Bourassa P (2003) Evaluation of the lambda model for human postural control. Biol Cybern 89:227–236
Miranda Z, Pham A, Barthélemy D (2014) Presynaptic control of balance in healthy subjects. ISPGR World Congress. Abstract no P3-K-50, Vancouver, p 71
Nashner LM (1976) Adapting reflexes controlling the human posture. Exp Brain Res 26:59–72
Perez MA, Rothwell JC (2015) Distinct influence of hand posture on cortical activity during human grasping. J Neurosci 35:4882–4889
Peterka RJ (2000) Postural control model interpretation of stabilogram diffusion analysis. Biol Cybern 82:335–343
Peterka RJ (2002) Sensorimotor integration in human postural control. J Neurophysiol 88:1097–1118
Pilon J-F, De Serres SJ, Feldman AG (2007) Threshold position control of arm movement with anticipatory increase in grip force. Exp Brain Res 181(1):49–67
Raptis H, Burtet L, Forget R, Feldman AG (2010) Control of wrist position and muscle relaxation by shifting spatial frames of reference for motoneuronal recruitment: possible involvement of corticospinal pathways. J Physiol 588:1551–1570
Sangani SG, Raptis HA, Feldman AG (2011) Subthreshold corticospinal control of anticipatory actions in humans. Behav Brain Res 224:145–154
Sasagawa S, Ushiyama J, Masani K, Kouzaki M, Kanehisa H (2009) Balance control under different passive contributions of the ankle extensors: quiet standing on inclined surfaces. Exp Brain Res 196:537–544
Schieppati M, Nardone A (1995) Time course of ‘set’-related changes in muscle responses to stance perturbation in humans. J Physiol 487:787–796
St-Onge N, Feldman AG (2004) Referent configuration of the body: a global factor in the control of multiple skeletal muscles. Exp Brain Res 155:291–300
Sussillo D, Churchland MM, Kaufman MT, Shenoy KV (2015) A neural network that finds a naturalistic solution for the production of muscle activity. Nat Neurosci 18:1025–1033
Todorov E, Jordan MI (2002) Optimal feedback control as a theory of motor coordination. Nat Neurosci 5:1226–1235
Tomita Y, Feldman AG, Levin MF (2016) Referent control and motor equivalence of reaching from standing. J Neurophysiol. https://doi.org/10.1152/jn.00292.2016
Turpin NA, Levin MF, Feldman AG (2016) Implicit learning and generalization of stretch response modulation in humans. J Neurophysiol 115:3186–3194
von Holst H (1954) Relations between the central nervous system and the peripheral organs. Br J Anim Behav 2:89–94
von Holst E, Mittelstaedt H (1950/1973) Das reafferenzprinzip. Naturwissenschaften 20:464–476 (English translation. Martin R (1973) The reafference principle. In: the behavioral physiology of animals and man. The collected papers of Erich von Holst. University of Miami Press, Florida)
Welch TD, Ting LH (2009) A feedback model explains the differential scaling of human postural responses to perturbation acceleration and velocity. J Neurophysiol 101:3294–3309
Winter DA (2009) Biomechanics and motor control of human movement. John Wiley, New Jersey
Winter DA, Patla AE, Prince F, Ishac M, Gielo-Perczak K (1998) Stiffness control of balance in quiet standing. J Neurophysiol 80:1211–1221
Wolpert DM, Kawato M (1998) Multiple paired forward and inverse models for motor control. Neural Netw 11:1317–1329
Zatsiorsky VM, Duarte M (2000) Rambling and trembling in quiet standing. Motor Control 4:185–200
Zhang L, Turpin NA, Feldman AG (2017) Threshold position control of anticipation in humans: a possible role of corticospinal influences. J Physiol 2017 595:5359–5374
Acknowledgements
Study supported by NSERC (AGF). AAM received a doctoral fellowship from Heart and Stroke Foundation of Canada. MFL holds a Canada Research Chair in Motor Recovery and Rehabilitation. Authors wish to thank Dorothy Barthélemy and Anouk Lamontagne for help in data collection as well as the individuals who volunteered for the study.
Author information
Authors and Affiliations
Contributions
The work was performed at the research centres of the Jewish Rehabilitation Hospital and the Institut de readaptation Gingras-Lindsay de Montreal, sites of the Center for Interdisciplinary Research in Rehabilitation, Montreal, Quebec. AGF and MFL conceived of the research design, AAM, SCH, SKS and AGF participated in data collection, AAM and NT performed data analysis. AGF, MFL and AAM interpreted the data and drafted the final version of the manuscript. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Corresponding author
Ethics declarations
Conflict of interest
All authors state that they have no competing interests.
Rights and permissions
About this article
Cite this article
Mullick, A.A., Turpin, N.A., Hsu, SC. et al. Referent control of the orientation of posture and movement in the gravitational field. Exp Brain Res 236, 381–398 (2018). https://doi.org/10.1007/s00221-017-5133-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-017-5133-y