Abstract
Previously we demonstrated the presence of a behavioral phase transition between reactive and predictive eye tracking of alternating targets. Prior studies of repetitive movements have proposed that an “internal clock” is the neural mechanism by which interval timing is achieved. In the present report we tested whether predictive oculomotor (saccade) tracking is based on an internal time reference (clock) by examining the effect of transient perturbations to the periodic pacing stimulus. These perturbations consisted of altering the timing of the stimulus (abruptly increasing or decreasing the inter-stimulus interval) or extinguishing the targets altogether. Although reactive tracking (at low pacing rates) was greatly affected by these timing perturbations, once predictive tracking was established subjects continued to time their eye movement responses at the pre-existing rate despite the perturbation. As expected from certain clock models, inter-stimulus intervals for predictive tracking followed Weber’s law and the scalar property (timing variability increases in proportion to interval duration), but this was not true for reactive tracking. In addition, the perturbation results show that subjects can establish an internal representation of target pacing (the internal clock) in as little as two eye-movement intervals, which suggests that this mechanism is relevant for real-world situations. These findings are consistent with the presence of an internal clock for the generation of these predictive movements, and demonstrate that the neural mechanism responsible for this behavior is temporally accurate and flexible.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Humans are remarkably good at producing repetitive rhythmic behaviors such as tapping (e.g., Stevens 1886; Dunlap 1910; Hary and Moore 1985; Engström et al. 1996; Engbert et al. 2002; Miyake et al. 2004). This motor activity typically involves synchronization with an external pacing stimulus (Hary and Moore 1985, 1987; Roberts et al. 2000; Repp 2001; Ding et al. 2002). The ability to accomplish such synchronization to a high degree of timing accuracy suggests the presence of central predictive mechanisms to overcome inherent neural processing delays. That such activity can persist after the cessation of the stimulus (Harrington et al. 1998; Roberts et al. 2000; Jantzen et al. 2004), and can be invoked even in the absence of an external stimulus (self-paced movements: Rao et al. 1997; Semjen et al. 2000), indicates that these behaviors are mediated by an internal timing reference or “clock.” The idea of such an internal clock is not new in neurophysiological studies of timing and synchronization (Stevens 1886; Treisman 1963). (Although the biological relevance of its role in the generation of predictive saccadic eye movements has not been explored in the same manner as we do here.) Many studies have demonstrated characteristics of timed behaviors that lead to the conclusion that a neural clock is controlling the response (see Ivry 1996 and Schöner 2002 for some examples).
There are two prevailing models for such a clock: pacemaker-counter, and interval timer. A pacemaker-counter model consists of a discrete pacemaker or timer, and a counter, which counts the appropriate number of fundamental periods generated by the pacemaker. This system can produce intervals over a wide range of durations by changing the count threshold. It can also allow a single central pacemaker to control different movements through separate counters. The interval-timer model simply generates an interval of whatever duration is required. This allows for timing flexibility since it is not tied to a preferred pacemaker frequency (Ivry 1996).
The specific form of the model is not crucial to interpreting the results that we present here. Nevertheless, depending on the pacemaker frequency, the pacemaker-counter model might be expected to yield motor timings with discrete increments. Given a fundamental rate of approximately 50 Hz (Treisman 1963; Treisman et al. 1990), or a period of 20 ms, we might expect latencies or intervals to be discretized with this basic unit, but we do not, and this lends support to the interval-timer model. One consequence of either model formulation is Weber’s law (timing variability increases as the duration of the timed interval increases) and the scalar property (distributions of response time overlap when normalized by the mean interval) (Gibbon 1977; Gibbon et al. 1997; Schöner 2002). These characteristics can help to distinguish between clock and non-clock models of predictive behavior, and one of the key results in the present study is that sequences of predictive but not reactive saccades exhibit these traits.
The neural structures responsible for motor event timing (and also perceptual timing) appear to be predominantly distributed between the cerebellum and the basal ganglia (Ivry 1996; Dreher and Grafman 2002; Ivry and Spencer 2004; Buhusi and Meck 2005), with the cerebral cortex concerned with attention and memory (Meck 1996). The cerebellum seems to be most involved in automatic timing: encoding shorter intervals (less than 1 s, Clarke et al. 1996) and discrete-event (discontinuous) timing. Evidence for cerebellar involvement includes the fact that lesions of the cerebellar cortex disrupt the precise timing of eyeblink conditioning (Perrett et al. 1993), and cerebellar lesions in humans impair the ability to make discrete timed movements such as tapping (Spencer et al. 2003). Imaging studies confirm that the cerebellum is involved in these processes (e.g., Dreher and Grafman 2002). The basal ganglia, on the other hand, are implicated in continuous-event, cognitively controlled timing: encoding longer intervals that require attention possibly by using a clock-counter mechanism (Meck 1996). Evidence for their involvement in timing includes the finding of an increase in variability during tapping in patients with Parkinson’s disease (O’Boyle et al. 1996).
A motor-timing model due to Wing and Kristofferson (1973a, b) makes use of a clock mechanism such as one of these, and is one of the most well studied. It distinguishes between central clock variance and motor delays, and assumes that clock intervals and motor delays are independent from trial to trial. The model results in negative dependence between successive intervals, with no correlation beyond that. This clearly is not true for sequences of predictive saccades which show extensive long-term correlations (Shelhamer 2005). This and other results suggest that the model is not true for repetitive saccades (Collins et al. 1998).
Although such a clock concept is well established in some areas of motor control (Meck and Benson 2002; Ivry and Richardson 2002), the operation of a clock in the generation of predictive saccadic eye movements has not been demonstrated. It is our intent here to present experimental evidence for such a clock, to discuss some of the features that make the “saccade clock” unique, and to argue for its biological relevance.
Previous studies of normal human subjects making eye movements to alternating targets (Stark et al. 1962; Ross and Ross 1987; Zambarbieri et al. 1987; Shelhamer and Joiner 2003; Isotalo et al. 2005) have demonstrated that there are distinct pacing frequency ranges that promote high-latency reactive (0.2–0.4 Hz) or low-latency predictive (0.8–1.0 Hz) behaviors. Our earlier experiments (Shelhamer and Joiner 2003) reported a behavioral “phase transition” as subjects tracked alternating targets as the pacing frequency monotonically increased and then decreased. When subjects tracked the targets at a low pacing frequency (0.2 and 0.3 Hz) they made reactive eye movements. As pacing frequency monotonically increased, subjects made an abrupt transition at a critical frequency (near 0.7 Hz) to a predictive response and continued this behavior at the higher pacing frequencies (0.9 and 1.0 Hz). When pacing then monotonically decreased in frequency, a phase transition from predictive to reactive behavior occurred, but at a lower critical frequency (near 0.5 Hz). In other words, once predictive tracking was established, it tended to remain in operation even at stimulus frequencies that previously evoked reactive behavior.
In addition, we showed that the latencies of consecutive predictive saccades are correlated while those of reactive saccades are independent. This supports our interpretation that in the phase-transition experiment subjects switch between reactive stimulus-based saccades (uncorrelated from trial to trial) and predictive saccades that are internally initiated and timed (strong correlations between trials). A subsequent study (Shelhamer 2005) also showed that sequences of predictive saccades are correlated over a span of approximately 2 s, suggesting that the internal timing reference could be established within this time frame.
Our hypothesis that the saccade tracking system makes transitions in to and out of a clock-based predictive mode is also supported by experiments demonstrating that different cortical structures are involved in each type of movement. Reactive visually guided saccades are dependent upon the parietal eye fields (PEF) (Pierrot-Deseilligny et al. 1991) whereas internally triggered saccades (predictive, memory, and antisaccades) involve the frontal eye fields (FEF), dorsolateral prefrontal cortex (DLPFC), and supplementary eye fields (SEF) (Pierrot-Deseilligny et al. 2003, 2004; Schlag-Rey et al. 1997). Based on these data, the transition from reactive behavior to predictive and vice versa might involve a shift in activity from neural systems supporting sensory guided behavior to those supporting internally generated predictive behavior (Simó et al. 2005).
In this study we sought to determine: (1) if predictive tracking of targets persists despite abrupt changes to target timing, as a consequence of these movements being based on an internal clock, (2) the minimum number of stimulus-response intervals required to establish this internal clock, (3) if the conditional probability of making consecutive predictive saccades at a given pacing rate supports our contention that they continue in the face of stimulus perturbations, (4) if there is an increase in timing variability as pacing period increases as expected from a clock, and (5) if distributions of inter-saccade intervals at different predictive pacing frequencies overlap when normalized by the mean interval, also consistent with a neural clock. Subjects tracked targets at initial pacing frequencies used in our previous experiment (0.2–1.0 Hz). The pacing was then abruptly changed to a frequency that mainly promoted reactive saccades (0.2 Hz), predictive saccades (1.0 Hz), or the targets were turned off completely. We find that changes in the stimulus pacing rate between frequencies that promote the same behavior (for example a perturbation from 0.3 Hz to 0.2 Hz) have little effect on subjects’ ability to alter the timing of their movements. This is also true when the stimulus pacing rate abruptly changes from a reactive pacing frequency (0.2 Hz) to a predictive pacing frequency (1.0 Hz). However, the opposite perturbation (predictive pacing frequency to reactive pacing frequency) has a different effect: subjects continue tracking at the predictive pacing frequency even though target pacing has changed to one that normally promotes reactive behavior. The variance of the inter-saccade interval distributions at these predictive frequencies increased with interval length, and these distributions overlapped when normalized by the mean, consistent with an internal clock process (Gibbon 1977; Gibbon et al. 1997; Schöner 2002). When the targets alternated at these predictive frequencies and then were abruptly turned off, subjects continued to make saccades at a rate similar to that prior to the perturbation. We also found that the probability of making consecutive predictive saccades in a sequence is higher than that expected by chance and may explain why this behavior persists through the perturbations. Finally, using an abbreviated version of the perturbation experiment, we found that the internal timing reference can be achieved in as little as two stimulus intervals. Based on these findings, we propose that predictive tracking of alternating targets is based on an internal timing reference or clock, and that this neural mechanism is responsible for the stability of such tracking and for the hysteresis noted in our earlier experiments.
Methods
General
The eye movements of nine subjects were recorded while they performed one of three main saccade tasks. Informed consent, according to the local institutional review board, was obtained from each participant. Data were acquired on a PC-compatible Pentium 166-MHz computer running real-time experiment control software developed in-house. Horizontal movements of the eyes were recorded with a Series 1000 Binocular Infrared Recording System (Microguide), sampled at 1,000 Hz. The system was calibrated prior to data acquisition by having subjects fixate targets at known locations. Subjects were seated in a stationary chair, and the head was fixed with a chin rest.
We report on the performance of nine subjects while they performed three main eye-movement tasks. Subjects A–E performed task 1 (perturbation), subjects B, E, and G-I performed task 2 (continuation), subjects A–D and H performed task 3 (latency distribution), and subjects A–C, E, and F performed an abbreviated version of task 1 (task 4, brief perturbation); only subject B had prior knowledge of the goals of the study. The first task was designed to examine if subjects could alter the timing of their eye movements after an abrupt increase or decrease in the inter-stimulus interval (ISI) of the alternating targets. The second task sought to determine if subjects would continue predictive tracking after the visual stimulus was extinguished. The third task was designed to determine the probability of switching between reactive and predictive tracking within a sequence of saccades at a given pacing frequency. In all tasks, subjects followed target jumps between two LEDs located 15° on either side of the vertical midline in a dark room. In each case, subjects were asked to follow the targets and were given no explicit instructions as to timing or accuracy; they were told simply to “look at the targets.” To ensure that the subject was alert during each experiment, 1-min breaks were given between each block of trials and the order of the blocks was randomized. (“Trial” in this report refers to a single target jump; that is, the stimulus for a single saccade. A “cycle” is two consecutive trials.)
Based on previous experiments (Stark et al. 1962; Ross and Ross 1987; Zambarbieri et al. 1987; Shelhamer and Joiner 2003; Isotalo et al. 2005), there appear to be separate pacing frequency ranges that promote reactive (0.2–0.4 Hz) and predictive (0.8–1.0 Hz) saccades. Between these two ranges is a phase transition range in which both behaviors are present. We based our selection of predictive-inducing and reactive-inducing pacing frequencies on these previous results.
Task 1: Perturbation
In the first task, targets alternated at one pacing frequency and then abruptly changed to another. Targets alternated at each frequency for a random number of trials (8, 9, or 10 cycles). There were nine pacing frequencies (0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, and 1.0 Hz) and therefore 72 possible perturbations, which were randomly divided into four blocks of equal duration (approximately 7 min). “Perturbation” in this case refers to the unexpected change from one pacing frequency to another, for example 0.3 to 1.0 Hz. In order to decrease the duration of the experiment, perturbations were overlapped. For example, the two perturbations 0.3 Hz → 1.0 Hz and 1.0 Hz → 0.6 Hz were presented in the following order: 0.3 → 1.0 → 0.6 Hz. In this task the ISI is half the period of the pacing frequency (e.g., 0.3 Hz pacing has an ISI of 1,667 ms). Thus the above perturbation sequence can be represented as abrupt changes in the ISI: 1,667 → 500 → 833 ms. The order of these changes was random; after any perturbation the pacing frequency could increase or decrease.
Task 2: Continuation
In the second task, targets alternated at pacing frequencies in the predictive range (0.6, 0.8, and 1.0 Hz) and in the reactive range (0.2 and 0.3 Hz) for a random number of trials (4, 5, or 6 cycles) and then were extinguished. Subjects were told to “stop moving your eyes once the targets are extinguished.” The experiment was divided into four blocks: two containing three sets of trials (one set at each predictive pacing frequency) and two containing two sets of trials (one set at each reactive pacing frequency). Within each block, both the order of the pacing frequencies and the number of cycles were chosen randomly. The duration of the dark interval (targets extinguished) was the period of one cycle at the chosen pacing frequency (for example, 1,000 ms for 1.0 Hz). Following the dark interval, a central fixation light was illuminated for 25 s before the start of the next trial.
Task 3: Latency distribution
The purpose of the third task was to form probability distributions of saccade latency at a reactive, a predictive, and a phase-transition pacing frequency (0.2, 1.0, and 0.6 Hz, respectively). We formed these distributions by requesting subjects to track targets at each of these frequencies for approximately 200 trials. To ensure that subjects were attentive we collected data in 3-min blocks for each pacing frequency.
Task 4: Brief perturbation
The fourth task was identical to Task 1, except that there was a fixed order in the perturbation of the pacing frequency: targets alternated at 0.2 Hz for 8–10 cycles, then at 1.0 Hz for 1 cycle, and finished at 0.2 Hz for 10 cycles. The perturbation sequence can also be represented as abrupt changes in the ISI: 2,500 → 500 → 2,500 ms. This task was performed in order to determine if the internal clock can be established with the smallest amount of stimulus exposure (1 cycle at 1.0 Hz pacing is 1 s of exposure).
Analysis
Analysis of eye-tracking data was done off-line. First, eye velocity was calculated using a four-point digital differentiator based upon a least-squares derivative algorithm (Savitzky and Golay 1964). This is an efficient iterative method of fitting a third-order polynomial to each data point and the preceding and following two values, then finding the derivative of the fitted polynomial, and introduces less noise than conventional differentiators. Saccade onset was found using a velocity threshold (> 60°/s). Saccade latency was determined by comparing the onsets of the primary saccade and the target in each trial; inter-saccade interval was the time between each primary saccade.
In the distribution experiment the latency data at the three pacing frequencies were used to determine the conditional probabilities of remaining within or switching between tracking modes in the reactive, predictive, and transition ranges (0.2, 1.0, and 0.6 Hz, respectively). A predictive saccade threshold was defined for each subject by the latency value that effectively separated the latency distributions at low (0.2 Hz) and high (1.0 Hz) pacing frequencies. (This latency value was selected so as to divide the two latency distributions into approximately equal portions. For example, a possible threshold would be the latency value that was ≤ 80% of the latency values found for 0.2 Hz pacing and ≥ 80% of the latency values found for 1.0 Hz pacing.) For each subject, saccades occurring with a latency less than or equal to this threshold (ranging from −15 to + 75 ms) were categorized as predictive. To calculate the conditional probability of remaining within the predictive state at a given pacing frequency we determined the number of times a predictive response occurred at that pacing frequency, M, and the number of times a predictive saccade was followed by another predictive saccade, m. Thus, the conditional probability of saccade i + 1 being predictive (S i+1 = P) if saccade i is predictive (S i = P) is:
were N is the total number of saccades. A similar analysis was used to find the P(S i+1 = R | S i = R), where R represents a reactive response. We are interested in these probabilities as the number of saccades subsequent to the current one is increased (i + 2, i + 3,...). As a numerical control, “surrogate” data were created by randomizing the latency values and carrying out the same analysis for 300 iterations. The mean and standard deviation for these probabilities were found in order to determine if the probabilities calculated from the original data set were purely due to chance (similar to a binomial distribution).
Results
All subjects were easily able to make a transition from tracking at a reactive pacing frequency (0.3 and 0.4 Hz) to the reactive frequency of 0.2 Hz. An example of this is shown in Fig. 1a for subject B. To the left of the dashed black line is the region for which the target pacing (black line) is 0.3 Hz. When stimulus pacing frequency abruptly changes to 0.2 Hz (right of the dashed line), the subject’s pacing (gray line) flawlessly changes to the new pacing frequency. This was also the case for perturbation between pacing frequencies within the predictive range: in Fig. 1b, the same subject is able to change smoothly from pacing in the predictive range (0.9 Hz, left of the dashed line) to another predictive frequency (1.0 Hz, right of the dashed line).
Examples showing saccade tracking during intra-state perturbations for subject B. a Abrupt perturbation from a pacing frequency that promotes reactive behavior (0.3 Hz, left of the dashed black line) to one that also encourages reactive behavior (0.2 Hz, right of the dashed line). b Abrupt perturbation from a pacing frequency that promotes predictive behavior (0.9 Hz, left of the dashed line) to one that also encourages predictive behavior (1.0 Hz, right of the dashed line)
These results were the same for the perturbation from a reactive to a predictive pacing frequency. As displayed in Fig. 2a, the subject is able to make a transition from pacing at a reactive frequency (0.2 Hz, left of the dashed line) to tracking at a predictive frequency (1.0 Hz, right of the dashed line); although there is an initial lag, the inter-saccade intervals immediately have the correct value for the new pacing frequency. This was not the case for the opposite perturbation, predictive to reactive. As shown in Fig. 2b, in this case the subject continues to track at the predictive frequency (1.0 Hz pacing, left of the dashed line) although the target pacing has changed to a reactive frequency (0.2 Hz pacing, right of the dashed line). The subject makes three saccades with intervals corresponding to pacing at 1.0 Hz. First there is a saccade prior to the perturbation (before the dashed line) and therefore before the subject could have any knowledge of the change in pacing frequency. The timings of the next two saccades (the inter-saccade intervals) are 578 and 676 ms. These times are much greater than the latencies of the reactive saccades made after the stimulus changed from 1.0 to 0.2 Hz (The last five saccades in Fig. 2b have latencies of 188, 189, 163, 167, and 180 ms). Therefore, these two saccades, made after the change in stimulus pacing rate, represent a continuation of the preceding timing behavior, and in particular the final saccade is not simply a reactive movement back to the target. This is an important observation in that it demonstrates that once tracking at a predictive rate has been established the subject continues to make eye movements with this timing despite changes in stimulus timing.
Examples showing saccade tracking during inter-state perturbations for subject B. a Abrupt perturbation from a pacing frequency that promotes reactive behavior (0.2 Hz, left of the dashed black line) to one that encourages predictive behavior (1.0 Hz, right of the dashed line). b Abrupt perturbation from a pacing frequency that promotes predictive behavior (1.0 Hz, left of the dashed line) to one that encourages reactive behavior (0.2 Hz, right of the dashed line)
In the main experiment (task 1), we used several pacing frequencies to perturb the reactive and predictive states. A summary of these perturbations is shown for subject C in Figs. 3 and 4. Figure 3 displays the results as the pre-perturbation frequency (0.2–0.9 Hz, white background) abruptly changes to a predictive frequency (1.0 Hz, gray background). The thick black lines show the half-period of the target pacing frequency (for example, 2,500 ms for 0.2 Hz) and the gray circles represent the inter-saccade interval. The ten inter-saccade intervals prior to the perturbation and following the perturbation are indexed as 1–10 and 11–20, respectively. For all pre-perturbation frequencies the subject’s tracking (inter-saccade intervals 11–20) changes to the timing of the new stimulus timing (500 ms intervals) after at most one interval.
Example of tracking behavior as pacing is abruptly changed to the predictive mode (1.0 Hz) for subject C. Each plot displays inter-saccade interval (gray circle) as pacing is abruptly changed from the pre-perturbation frequency (0.2–0.9 Hz, white background) to the predictive pacing frequency (1.0 Hz, gray background). The stimulus interval is displayed as the black line (e.g., 2,500 ms for 0.2 Hz pacing)
Example of tracking behavior as pacing is abruptly changed to the reactive frequency (0.2 Hz) for subject C. Each plot displays inter-saccade interval (gray circle) as pacing is abruptly changed from the pre-perturbation frequency (0.3–1.0 Hz, white background) to the reactive pacing frequency (0.2 Hz, gray background). The stimulus interval is displayed as the black line (e.g., 1,666 ms for 0.3 Hz pacing)
Figure 4 displays the results as the pre-perturbation frequency (0.3–1.0 Hz, white background) abruptly changes to a reactive frequency (0.2 Hz, gray background). For intra-state perturbations within the reactive range (the two upper left plots of Fig. 4) the subject easily made transitions from pacing at one reactive frequency (0.3 and 0.4 Hz, white background) to another reactive frequency (0.2 Hz, gray background). However, with pre-perturbation pacing frequencies in the phase-transition and predictive ranges (other six plots in Fig. 4, 0.5–1.0 Hz, white background), the subject continued to track at or near these pacing frequencies despite the change in stimulus timing.
A summary of the perturbation experiments for all subjects is shown in Fig. 5. Each graph is a plot of the first and second inter-saccade intervals (circles and squares) after the perturbation versus the mean of the five inter-saccade intervals prior to the perturbation. Figure 5a displays the results for the 0.2–0.9 Hz → 1.0 Hz perturbations (ISI perturbations of 556–2,500 → 500 ms). The thick black dashed line is a linear regression fit to the data for all subjects and has a slope of −0.01 (r = −0.05, intercept ≈ 555 ms) indicating that the subjects almost immediately change the timing of their eye movements to the new ISI. Figure 5b displays the results for the 0.3 → 0.2 and 0.4 → 0.2 Hz perturbations (ISI perturbations of 1,667 → 2,500 and 1,250 → 2,500 ms, respectively). The linear regression fit in this case also has a slope near zero, −0.15 (r = −0.22, intercept ≈ 2,500 ms), again showing that the subjects immediately change the timing of their eye movements to the new ISI. Figure 5c displays the results for the 0.5–1.0 Hz → 0.2 Hz perturbations (ISI perturbations of 500–1,000 → 2,500 ms). Unlike the previous results, the linear regression fit in this case has a slope of 0.88 (r = 0.64) indicating that there is a linear relationship between the timing of post-perturbation tracking and the pre-perturbation rate. In other words, subjects continue to time their eye movements at predictive pre-perturbation frequencies (> 0.4 Hz) despite the change in stimulus timing. In addition, there is not a gradual decrease to the new ISI over the first two intervals. If that were the case then one would expect the first intervals (circles) to be grouped below the second intervals (squares) in Fig. 5c. The fact that the symbols are inter-mixed suggests that the subjects continue to make movements at the internalized pre-perturbation rate.
The inter-saccade intervals of the first two saccades made after the perturbation versus the mean inter-saccade interval before perturbation for five subjects. In each panel there is one pair of data points for each subject (the first interval is represented by circles, the second by squares). a Perturbations from 0.2 to 0.9 → 1.0 Hz (ISI perturbations of 556–2,500 → 500 ms), b perturbations from 0.3 and 0.4 → 0.2 Hz (ISI perturbations of 1,250 and 1,667 → 2,500 ms) and c perturbations from 0.5 to 1.0 → 0.2 Hz (ISI perturbations of 500–1,000 → 2,500 ms)
To determine further if the saccades generated after a predictive-to-reactive perturbation are driven by an internal clock we next examined tracking behavior after extinguishing the LED targets. Subjects tracked targets at pacing frequencies that promote predictive behavior (0.6, 0.8, and 1.0 Hz). (Though 0.6 Hz is in the phase transition range and usually promotes a mix of predictive and reactive tracking.) After a steady state had been reached (4–6 cycles) the targets were extinguished for one cycle. The results for subject E are shown in Fig. 6 for all three frequencies. The point where the LED trace (black line) ends is the time at which the targets are extinguished. For each pacing frequency, the subject makes a saccade just prior to the extinguishing of the LEDs, revealing that he or she is anticipating the target jump. Also, for each pacing frequency the subject makes one saccade after the targets are extinguished. This extra saccade is made without a visual stimulus and therefore must be internally triggered. The time interval between these two saccades (802, 669, and 565 ms, for each frequency) scales proportionally with the pacing frequency and closely matches the half-period of the corresponding pacing frequencies (833, 625, and 500 ms). These results were not seen for the reactive mode (pacing at 0.2 and 0.3 Hz, not shown). In this case, subjects did not make an additional movement in darkness after the ISI of 1,667 or 2,500 ms. That is, at these pacing frequencies there were not multiple saccades representing a continuation of movement timing when the stimulus was turned off.
Figure 7 plots the inter-saccade interval after the extinguishing of the targets vs. the mean of the preceding five inter-saccade intervals for five subjects (one subject did not make any extra saccades in one set of trials at 0.6 Hz). As shown in the plot, the intervals between the internally triggered saccades made after the targets are extinguished are highly correlated with the preceding pacing rate. The straight line is a linear regression fit to the data for all subjects and has a slope of 1.04 (r = 0.91), indicating that saccade timing after the targets disappear almost exactly matches the timing of the preceding stimulus, which represents the stability and accuracy of the internal clock.
One question that these results raise is how quickly the internal clock can be established. That is, what is the minimum number of stimulus-response intervals required to establish this internal clock? To address this question we conducted an abbreviated version (task 4) of our perturbation experiment on five subjects. The results for three subjects (B, E, and F) are shown in Fig. 8. Refer first to the left column. All three subjects make reactive saccades to the stimulus at 0.2 Hz pacing (left of the dashed black line). When the pacing abruptly changes to 1.0 Hz (right of the dashed line) there is a delay in the first saccade (172, 191, and 194 ms) and in the second saccade (218, 413, and 313 ms) made at the faster pacing rate. (We chose to perturb the pacing frequency to 1.0 Hz because this pacing rate results in the smallest amount of time—1 s—available to the subject to establish the internal clock.) This is more clearly seen in the detailed plots in the right column. According to latency, it appears that the subjects make long-latency (reactive) saccades to the new 1.0 Hz stimulus pacing. However, when the pacing abruptly changes back to 0.2 Hz (at approximately 7.3 s in each graph), the subjects continue to track at the briefly perturbed 1.0 Hz rate for two more saccades. (This was not the case for subjects A and C. After tracking the stimulus at 1.0 Hz for two saccades, both subjects immediately went back to tracking at 0.2 Hz.) The intervals between these two continuation saccades after the return to 0.2 Hz pacing (447, 407, 336 and 325, 575, 501 ms) are much smaller than the intervals before the first perturbation (approximately 2,500 ms). Thus, even though saccade latency suggests that the subjects are still in the reactive mode during the transient perturbation to 1.0 Hz, the intervals between the continuation saccades show that the behavior has indeed switched to an internally predictive mode. Based on this result we believe that, for some subjects in some cases, the internal clock can be established in as few as two inter-saccade intervals (three saccades). The observation that the inter-saccade intervals made after the brief exposure fluctuate around an ISI of 500 ms (325–575 ms) suggests that the internal representation of stimulus timing may become more precise with more movement-timing information (additional inter-movement intervals), as in Task 1.
Example from experiment 3 showing saccade tracking during a brief inter-state perturbation for subjects B, E, and F. Left column abrupt transition from a pacing frequency that promotes reactive behavior (0.2 Hz, left of the dashed black line) to one that encourages predictive behavior (1.0 Hz, right of the dashed line) immediately followed by a return to 0.2 Hz pacing. Right column the same data as above, between 6 and 9 s
We were also interested in trial-to-trial fluctuations of behavior while tracking in each pacing frequency range (reactive, predictive, and phase transition), and whether or not the trend of these fluctuations would correspond with the above results. Specifically we wished to determine the probability of remaining within the reactive or predictive tracking mode once that mode had been entered. Figure 9 shows the probability of remaining within a given tracking mode as a function of the number of trials into the future during the distribution experiment for 0.2, 0.6, and 1.0 Hz pacing (left, middle and right columns) for subjects F and H (top and bottom rows, respectively). For each plot the probability of remaining within the reactive or predictive mode is represented by the black and gray dashed lines, respectively. The solid black line with error bars is from the surrogate control (random data), for reactive saccades in the left column and for predictive saccades in the middle and right columns. As is evident in these plots, reactive behavior dominates at 0.2 Hz pacing, and there is a non-zero probability of sequences of reactive-only saccades even as long as 35 trials. However, these results can simply be attributed to a random arrangement of the number of reactive saccades made by the subject in a given sequence: the surrogate data result overlaps that from the experimental data and signifies that even long sequences of reactive saccades can be attributed to chance arrangements of the finite number of trials. For pacing at 0.6 and 1.0 Hz, predictive behavior is dominant, and sequences of predictive-only saccades of 15–30 trials are possible. Unlike the case for reactive saccades, these results cannot be attributed solely to the number of predictive saccades made at these pacing frequencies: the probabilities of occurrence of each sequence length are greater than chance (note the separation between the experimental and surrogate results) and demonstrate that once the subject begins predicting the movement of the target, tracking behavior tends to stay in this mode. This finding that the sequence of saccades following a given predictive saccade is highly likely also to be predictive agrees with the results of the perturbation experiments and supports our hypothesis of an internal clock.
Conditional probability plots of remaining within the reactive (black line) or predictive (gray line) mode for Subjects F and H (top and bottom row, respectively) at 0.2, 0.6, and 1.0 Hz pacing. The black line with error bars represents the surrogate control for the reactive mode in the left column and for the predictive mode in the middle and right columns
Clock mechanisms exhibit two properties that we see in predictive, but not reactive, saccades. The first is Weber’s law: the variability of estimating the passage of time is proportional to the interval being estimated (Gibbon 1977; Gibbon et al. 1997). This relationship can be modeled as an integrate-to-threshold operation (Treisman 1963; Gibbon et al. 1997; Meck and Benson 2002), where an event (saccade) is generated when the threshold is reached. Noise in the integrated signal leads to variance in the time to reach threshold, and this variance increases as the integration time (inter-saccade interval) increases (Schöner 2002). This relationship is readily apparent in our data. Figure 10 shows the variance, averaged across subjects, of saccadic tracking at those frequencies that lead to pure reactive saccades (left side of graph) and those that lead to pure predictive saccades (right side of graph) during the perturbation task. (Variance for all subjects was normalized by dividing by the variance at 0.2 Hz. This normalized the variability within subjects and allowed a comparison between pacing frequencies.) In the predictive range, variance of the inter-saccade intervals does indeed increase with increasing inter-stimulus interval (decreasing frequency). In the reactive range, where a clock is not in operation, the variance is relatively constant across the range of inter-stimulus intervals.
Normalized variance of inter-saccade interval. Inter-saccade interval variance, averaged across subjects, is plotted for reactive (ISI of 2,500, 1,667, and 1,250 ms) and predictive (ISI of 714, 633, 556, and 500 ms) pacing frequencies. For each subject, the variance at each ISI is normalized by that subject’s inter-saccade interval variance at 0.2 Hz (ISI of 2,500 ms). Thus, the ordinate values reflect the relative magnitude of the inter-saccade interval variance across subjects at each respective ISI
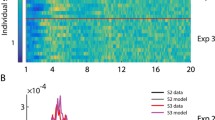
The second property of clock timing is a stronger form of Weber’s law, the scalar property: in an interval-timing task, response-time distributions will be identical when scaled by the mean of each distribution (the standard deviation of the interval estimate is proportional to the interval). Thus, inter-saccade interval distributions from two different predictive ISIs should overlap when each is normalized by its mean. Figure 11 plots the latency and interval distributions for subjects B, C, and D (bottom, middle, and top rows, respectively) from the distribution experiment. The first three columns are histograms (20 ms bins) of saccade latency at each pacing frequency (0.2, 0.6, and 1.0 Hz). The thick gray dashed line marks a latency of 150 ms. As evident in the figure, all subjects made mostly reactive saccades at 0.2 Hz and predictive saccades at 1.0 Hz. (The latency distributions are to the right of the 150 ms marker for 0.2 Hz and to the left for 1.0 Hz.) Similar to 1.0 Hz pacing, subjects C and D (top and middle rows) made mostly predictive saccades at 0.6 Hz. However, subject B displayed bi-modal behavior at this pacing frequency; the black arrows mark the two peaks, a reactive and predictive component, in the latency distribution.
The first three columns are histograms (20 ms bins) of saccade latency for subjects B, C, and D (bottom, middle, and top rows, respectively) at each pacing frequency (0.2, 0.6, and 1.0 Hz) during the distribution experiment. The thick gray dashed line marks a latency of 150 ms. The black arrows in the second column point out the bi-modal distribution of saccade latency for subject B at 0.6 Hz pacing. The fourth column displays the probability distributions of inter-saccade intervals at 0.2, 0.6, and 1.0 Hz pacing (black dashed, gray solid, and black solid traces, respectively). The last column displays the normalized inter-saccade interval distributions: the distributions in the fourth column divided by the mean inter-saccade interval. The black arrows in the fifth column point out the resulting bi-modal normalized inter-saccade interval distribution for subject B at 0.6 Hz pacing. Predictive tracking distributions from each subject can be almost completely superimposed, demonstrating the scalar property of clock models
The fourth and fifth columns of Fig. 11 are probability distributions of inter-saccade intervals for each subject at 0.2, 0.6, and 1.0 Hz (black dashed, gray solid, and black solid traces, respectively). As evident in the fourth column, the inter-saccade interval distributions center on the inter-stimulus interval for the respective pacing frequency (2,500, 833, and 500 ms for 0.2, 0.6, and 1.0 Hz). The last column of Fig. 11 displays the normalized inter-saccade interval distributions (each distribution presented in the fourth column divided by the corresponding mean interval). In this case the distributions of inter-saccade intervals at 0.6 and 1.0 Hz for subjects C and D are almost identical and can be superimposed, as predicted by the scalar property. This was not true for the distributions for inter-saccade intervals at 0.2 Hz; in this case, the variability in inter-saccade intervals is due to variability in reaction time (the passage of time is not internally estimated) and thus the scalar property does not apply. Interestingly, the normalized inter-saccade distribution at 0.6 Hz for subject B lies between the distributions for 0.2 and 1.0 Hz (marked by the black arrow). This is a result of the bimodal behavior this subject demonstrated at this pacing frequency as previously noted.
Discussion
In this study we have shown that predictive tracking behavior is based on an internal timing reference or neural clock. Subjects could abruptly change their saccade pacing between two reactive frequencies (0.2 and 0.3 Hz) or two predictive frequencies (0.9 and 1.0 Hz) when the stimulus dictated. This was also the case when target pacing changed from a reactive frequency to a predictive frequency (0.2–1.0 Hz for example). However, this was not true for the opposite perturbation, predictive to reactive pacing. In this case tracking at the predictive rate persisted for at least three saccades (two inter-saccade intervals) despite a change in pacing stimulus to a frequency that normally promotes reactive behavior. This was also true when the stimulus was abruptly extinguished. In related experiments we also showed that once predictive tracking was established the sequence of saccades following any given predictive saccade was highly likely to also be predictive. This confirms and extends our earlier study (Shelhamer and Joiner 2003) in which we found an abrupt transition between reactive and predictive tracking, but did not demonstrate that the latter was based on an internal timing mechanism.
Alternatives to an internal clock
One could argue that the continuation of predictive tracking despite the change in target pacing is simply the result of the difficulty in ceasing quick rhythmic movements. If this was the complete reason then, for example, all pacing at frequencies greater than 0.2 Hz should persist when the stimulus pacing changed to 0.2 Hz. However, most subjects had no difficulty in making such high-to-low intra-state transitions within the reactive mode: 0.3 → 0.2 and 0.4 → 0.2 Hz (Figs. 1a, 4, 5b). Intra-state transitions within the predictive mode were also made without difficulty: 0.8 → 1.0 and 0.9 → 1.0 Hz (Figs. 1b, 3, 5a). Although in this case the post-perturbation frequency was higher, there was not a large pacing error due to continued tracking at 0.8 or 0.9 Hz; correct pacing at 1.0 Hz was achieved immediately.
It is true that quick rhythmic movements are difficult to stop making once the rhythm has been established. Nevertheless, we have shown that the frequency at which this difficulty began in our experiment, 0.5 Hz (Fig. 5c), was also the pacing frequency at which our subjects began to predict the target motion. In addition, when the pre-perturbation frequency was greater than or equal to 0.5 Hz, the timing of the saccades made after the change in pacing frequency was always close to that of the pre-perturbation frequency. This is shown in the eye movement data displayed in Figs. 2b, 6, and 8 as well as the inter-saccade intervals in Figs. 4, 5c, and 7. If our subjects simply made an eye movement back to the lit target upon making a post-perturbation saccade without a corresponding target step, the interval of this response would be at most the latency of a reactive saccade: 150–200 ms (Leigh and Zee 1999). Instead, our subjects timed their saccades as if the targets had indeed alternated (Figs. 2b, 6, 8), thus demonstrating an internal predictive timing process. Therefore, we propose that the predictive behavior demonstrated in these examples is a consequence of an internal clock that dictates the timing of these movements and consequently makes them difficult to stop in the face of a temporal perturbation.
Another potential argument against our conclusions may be that the eye movement timing at the lowest pacing rates (ISIs of 2,500 and 1,667 ms) is the result of an internal clock as well. Similar to the fast pacing rates, the timing of these movements (inter-saccade intervals) closely matches the interval of the pacing stimulus (see Figs. 1a, 3). Two considerations do not support this alternative explanation. First, the timing of these movements is affected by the perturbations to the stimulus: abrupt changes in stimulus timing alter the reactive saccade timing behavior (Figs. 2a, 3). This implies that the accurate timing of these reactive movements is a consequence of stimulus timing and not due to an internal clock. In fact it is only in the case of responses that begin before each stimulus—predictive saccades—that a clock is necessary at all. Second, it is only for predictive and not reactive saccades that there is an increase in timing variability as the pacing interval increases (frequency decreases), as would be expected from an internal clock mechanism (Fig. 10).
One might ask if there is any alternative to an internal clock for the generation of repetitive predictive movements: are our results simply a tautology? We think that this is not the case. Clearly there are mechanisms, based on the expectation of imminent target motion, for the generation of individual predictive or anticipatory responses. The observation that longer intervals have more variability (Fig. 10) would be true for any process that accumulates error over time; the longer the accumulation period the more variability. It is perhaps a matter of semantics to ask whether a periodic series of such predictions constitutes a clock. Our results suggest that there is more to it than that. Inter-trial correlations (Shelhamer and Joiner 2003; Shelhamer 2005), and the greater-than-chance probability of making repeated predictive movements once prediction has been established (Fig. 9), both show that sequences of predictive saccades are not just strings of otherwise isolated predictive movements, but rather represent programmed sequences as would be manifest by a clock. In addition, the fact that inter-saccade intervals at predictive pacing frequencies (inter-stimulus intervals of 500 and 833 ms) demonstrate the scalar property (Fig. 11) suggests that the predictive mechanisms at these pacing rates share an underlying scale-invariant error distribution for time estimation (Gibbon et al. 1997).
Relation to previous behavioral studies
Some of the results that we present here for saccades are similar or identical to those found previously for predictive smooth pursuit and finger tapping. Nevertheless, there are differences between the saccadic movements presented here and other motor systems that justify carrying out a set of dedicated oculomotor studies. For example, similar to the results presented in Figs. 2b, 4, 5c, 6, and 7, previous studies of predictive smooth pursuit eye movements have also shown that once steady state has been established these movements persist with the same predictive timing when the inter-stimulus interval is unexpectedly increased or the stimulus fails to appear (Barnes and Asselman 1991a, b; Barnes and Grealy 1992; Kao and Morrow 1994; Barnes et al. 2005). More recent studies of pursuit (Barnes and Marsden 2002; Medina et al. 2005) have also shown an increase in timing variance with increasing inter-stimulus interval, similar to Fig. 10. Despite these behavioral similarities, there are important differences between the two movements. First, pursuit is normally a steady-state response depending on continuous visual feedback, while saccades are generally open loop (Leigh and Zee 1999). As a result, pursuit movements under any circumstance are difficult to sustain in the absence of a moving target (Heywood and Churcher 1971), whereas predictive saccades can persist for many movements post-stimulus (see Fig. 7, Shelhamer 2005). These differences are important to consider when studying the internal timekeeping process. As demonstrated here, the timing precision and flexibility of the internal clock are readily observed in the timing between two or more internally driven movements. However, due to the limitations mentioned above, the timing between two internally driven pursuit movements in sequence cannot easily be studied. In addition, due to the rapid nature of a saccadic eye movement, it is experimentally easier to examine the long-term timing relationships for saccades (Figs. 9, 11) than for pursuit.
Though timing has traditionally been studied with repetitive tapping movements (Stevens 1886; Dunlap 1910), there are important behavioral, mechanical, and cortical processing differences between these movements and saccadic eye movements which justify these oculomotor studies. First, a behavioral phase transition in tapping behavior (reactive to predictive) is seen when the rate of an auditory pacing stimulus increases (inter-stimulus interval decreases). However, the transition frequency when the experiment is conducted with pacing frequencies in random order (0.20–0.28 Hz in Mätes et al. 1994; 0.14–0.28 in Miyake et al. 2004) is lower than if the pacing frequency changes monotonically (0.50–0.75 Hz in Engström et al. 1996). This is not the case for repetitive saccades, where similar results are found for both randomized and continuous presentations (transition frequency between 0.50 and 0.75 Hz in Shelhamer and Joiner 2003). This suggests that the transition point represents an important aspect of the dynamics of each system and is not merely an experimental artifact. Second, each finger tap is a combination of bidirectional movements (an up motion and a down motion) while a single saccade is inherently unidirectional. Thus, in the repetitive tapping case, it is possible to choose cognitively a synchronization strategy that corresponds to either component of the motion, complicating the paradigm and the resulting analysis. Third, sensory reafference in the case of tapping is of a different modality than the cue used to induce the tapping, and this reafference has been shown to decrease tapping variability (Drewing et al. 2002). Fourth, saccades to alternating targets involve changes of target representation between visual and cortical hemifields (Leigh and Zee 1999), while the tapping of a single finger does not. As a result, repetitive tapping produces mainly unilateral cortical activation (Rao et al. 1997) while repetitive saccades generally produce bilateral activation (Simó et al. 2005). Fourth, the fact that there are qualitatively similar behaviors in the different motor systems shows that effector dynamics (mass, inertia) are effectively internally compensated for in generating these motions, and in particular that the motor system does not always depend on effector mass in order to establish rhythmic motion (the mass of the oculomotor plant—the eyeball—is negligible relative to other dynamic factors; Robinson 1964). This last point is not at all obvious, as one can imagine cases in which it might be easier to synchronize movements with an external pacing stimulus if one can take advantage of effector mass to produce a harmonic oscillator (e.g., Milsum 1966; Todd et al. 2002).
A prior study of repetitive saccadic eye movements (Collins et al. 1998) has shown that movement timing at pacing frequencies that we consider to be at least partly predictive (inter-stimulus-intervals of 496, 752, and 1,000 ms) can persist when an auditory pacing stimulus is turned off, and that increasing the inter-stimulus interval leads to an increase in inter-saccade interval variability. However, these results were used to test the applicability of the Wing and Kristofferson motor-timing model (1973a, b) for repetitive saccades, and only indirectly imply that these movements are the result of an internal clock. In the present report we have shown that in the transition and predictive pacing ranges (0.6 and 1.0 Hz), predictive saccades are highly likely to be followed by predictive saccades; the conditional probability of a predictive saccade being followed by a predictive saccade is greater than that expected by chance, and this enhanced probability can persist for many saccades into the future (Fig. 9). This analysis supports our findings that predictive tracking persists when the pacing frequency changes or the targets are extinguished; the probability of generating many predictive saccades in a row (> 2) remains high at 0.6 and 1.0 Hz pacing and explains why this behavior continues despite changes in stimulus timing.
In our previous formulation (Shelhamer 2005) we reached the conclusion that, when a predictive saccade is made, the system starts to program the next one as soon as timing error from the current one is available. This feed-forward programming is manifest as an internal clock, in the sense that the neural command of the future saccade is initiated before the future stimulus actually occurs. Based on the finding that predictive saccades are correlated over a span of approximately 2 s, two or more equally timed movements within 2 s should be adequate to establish the internal clock. We tested this hypothesis in task 4 by determining if subjects could establish the internal clock with the least possible amount of timing information. That is, we only gave the subjects two movement intervals at the fastest predictive pacing frequency (1.0 Hz) and therefore the shortest amount of exposure within the 2-s window. We demonstrated that three of five subjects could establish an internal timing reference after such a brief reactive-to-predictive perturbation, even though their initial saccade latencies suggested a reactive response (Fig. 8). The other two subjects (A and C) did not show the same result: after reacting with faster-paced saccades to the brief perturbation to 1.0 Hz pacing, these subjects quickly reverted back to tracking at 0.2 Hz when the stimulus did so. However, in the main perturbation experiment (task 1) these two subjects continued to make saccades at the predictive rate once that mode had been established (Fig. 5c), consistent with other subjects. We believe that the difference in behavior between tasks 1 and 4 for these two subjects reflects that these subjects required more exposure (more movement intervals) to establish the internal clock.
We believe that the ease with which our subjects could begin predicting the stimulus is a result of the brain’s preference to predict. Previous studies (Kowler and Steinman 1979) suggest that there is a predictive component even when tracking targets that alternate at low pacing frequencies. Similarly for pursuit, some amount of predictive (anticipatory) eye movement is present even when stimulus parameters are randomized and, presumably, non-predictable (Heinen et al. 2005). However, in these conditions there may not be sufficient information within an appropriate time period for the brain to develop the timekeeping process necessary to begin successfully predicting the stimulus. Recent studies of smooth pursuit eye movements (Jarrett and Barnes 2005; Medina et al. 2005) have shown that when the direction of the moving target is changed at a fixed interval into the movement, with repeated exposure subjects learn to change their response at the required time. In addition, when catch trials are given (a trial in which the target direction is not changed) subjects time their responses based on the learned response. In these studies, the range of times into the movement at which the target changes direction (500–1,000 ms) is the same as the inter-stimulus intervals of the predictive pacing frequencies we report here. As stated by others (Medina et al. 2005), we argue that repeated movements timed at these intervals promote motor learning by an internal representation of time and that this information can be used to time a variety of movements.
Biological relevance
One might question the biological relevance of a neural mechanism to generate prolonged rhythmic behavior. While it might not reflect more natural stimuli, our stimulus allows for analysis by standard mathematical approaches. In addition, the fact that the predictive clock for saccades can be brought into operation within just a few target movements shows that the clock has relevance for tracking under conditions that might arise more naturally, and that the prolonged stimuli that we use are not strictly necessary to evoke predictive behavior.
The results here suggest that in the saccadic system, once predictive behavior is established this tracking mode persists even when the pacing stimulus is no longer present. The importance of the basal ganglia and cerebellum in predictive behavior (Dreher and Grafman 2002; Gagnon et al. 2002; Harrington and Haaland 1999; Ivry and Keele 1989) suggests that these structures are involved in this continued behavior. Similar to previous imagining studies (Rao et al. 1997, 2001; Simó et al. 2005) the paradigms presented in this report may assist in defining the respective roles of cortical and subcortical structures in the internal representation of time (Meck and Benson 2002).
References
Barnes GR, Asselman PT (1991a) The assessment of predictive effects in smooth eye movement control. Acta Otolaryngol 481:343–347
Barnes GR, Asselman PT (1991b) The mechanism of prediction in human smooth pursuit eye movements. J Physiol 439:439–461
Barnes GR, Grealy MA (1992) Predictive mechanisms of head-eye coordination and vestibulo-ocular reflex suppression in humans. J Vestib Res 2:193–212
Barnes GR, Marsden JF (2002) Anticipatory control of hand and eye movements in humans during oculo-manual tracking. J Physiol 539:317–330
Barnes GR, Collins CJ, Arnold LR (2005) Predicting the duration of ocular pursuit in humans. Exp Brain Res 160:10–21
Buhusi CV, Meck WH (2005) What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci 6:755–765
Clarke S, Ivry R, Grinband J, Roberts S, Shimizu N (1996) Exploring the domain of the cerebellar timing system. In: Pastor MA, Artieda J (eds) Time, internal clocks, and movement. Elsevier, New York
Collins CJ, Jahanshahi M, Barnes GR (1998) Timing variability of repetitive saccadic eye movements. Exp Brain Res 120:325–334
Ding M, Chen Y, Kelso JA (2002) Statistical analysis of timing errors. Brain Cogn 48:98–106
Dreher J-C, Grafman J (2002) The roles of the cerebellum and basal ganglia in timing and error prediction. Eur J Neurosci 16:1609–1619
Drewing K, Hennings M, Aschersleben G (2002) The contribution of tactile reafference to temporal regularity during bimanual finger tapping. Psychol Res 66:60–70
Dunlap K (1910) Reactions to rhythmic stimuli, with attempt to synchronize. Psych Rev 17:399–416
Engbert R, Krampe RT, Kurths J, Kliegl R (2002) Synchronizing movements with the metronome: nonlinear error correction and unstable periodic orbits. Brain Cogn 48:107–116
Engström DA, Kelso JAS, Holroyd T (1996) Reaction-anticipation transitions in human perception-action patterns. Hum Mov Science 15:809–832
Gagnon D, O’Driscoll AO, Petrides M, Pike GB (2002) The effect of spatial and temporal information on saccade and neural activity in oculomotor structures. Brain 125:123–139
Gibbon J (1977) Scalar expectancy theory and Weber’s law in animal timing. Psychol Rev 84:279–325
Gibbon J, Malapani C, Dale CL, Gallistel CR (1997) Toward a neurobiology of temporal cognition: advances and challenges. Curr Opin Neurobiol 7:170–184
Hary D, Moore GP (1985) Temporal tracking and synchronization strategies. Hum Neurobiol 4:73–79
Hary D, Moore GP (1987) Synchronizing human movement with an external clock source. Biol Cybern 56:305–311
Harrington DL, Haaland KY (1999) Neural underpinnings of temporal processing: a review of focal lesion, pharmacological, and functional imaging research. Rev Neurosci 10:91–116
Harrington DL, Haaland KY, Hermanowicz N (1998) Temporal processing in the basal ganglia. Neuropsychology 12:3–12
Heinen SJ, Badler JB, Ting W (2005) Timing and velocity randomization similarly affect anticipatory pursuit. J Vis 5:493–503
Heywood S, Churcher J (1971) Eye movements and the afterimage. I. Tracking the afterimage. Vision Res 11:1163–1168
Isotalo E, Lasker AG, Zee DS (2005) Cognitive influences on predictive saccadic tracking. Exp Brain Res 165:461–469
Ivry R (1996) The representation of temporal information in perception and motor control. Curr Opin Neurobiol 6:851–857
Ivry RB, Keele SW (1989) Timing functions of the cerebellum. J Cogn Neurosci 1:136–152
Ivry RB, Richardson TC (2002) Temporal control and coordination: the multiple timer model. Brain Cogn 48:117–132
Ivry RB, Spencer RM (2004) The neural representation of time. Curr Opin Neurobiol 14:225–232
Jantzen KJ, Steinberg FL, Kelso JA (2004) Brain networks underlying human timing behavior are influenced by prior context. Proc Natl Acad Sci USA 101:6815–6820
Jarrett C, Barnes G (2005) The use of non-motion-based cues to pre-programme the timing of predictive velocity reversal in human smooth pursuit. Exp Brain Res 164:423–430
Kao GW, Morrow MJ (1994) The relationship of anticipatory smooth eye movement to smooth pursuit initiation. Vision Res 34:3027–3036
Kowler E, Steinman RM (1979) The effect of expectations on slow oculomotor control. I. Periodic target steps. Vision Res 19:619–632
Leigh RJ, Zee DS (1999) The neurology of eye movements, 3rd edn. FA Davis Company, Philadelphia
Mätes J, Radil T, Muller U, Poppel E (1994) Temporal integration in sensorimotor synchronization. J Cogn Neurosci 6:332–340
Meck WH (1996) Neuropharmacology of timing and time perception. Cogn Brain Res 3:227–242
Meck WH, Benson AM (2002) Dissecting the brain’s internal clock: how frontal-striatal circuitry keeps time and shifts attention. Brain Cogn 48:195–211
Medina JF, Carey MR, Lisberger SG (2005) The representation of time for motor learning. Neuron 45:157–167
Milsum JH (1966) Biological control systems analysis. McGraw-Hill, New York
Miyake Y, Onishi Y, Poppel E (2004) Two types of anticipation in synchronization tapping. Acta Neurobiol Exp 64:415–426
O’Boyle DJ, Freeman JS, Cody FWJ (1996) The accuracy and precision of timing of self-paced, repetitive movements in subjects with Parkinson’s disease. Brain 119:51–70
Perrett SP, Ruiz BP, Mauk MD (1993) Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eyelid responses. J Neurosci 13:1708–1718
Pierrot-Deseilligny C, Milea D, Muri RM (2004) Eye movement control by the cerebral cortex. Curr Opin Neurol 17:17–25
Pierrot-Deseilligny C, Muri RM, Ploner CJ, Gaymard B, Demeret S, Rivaud-Pechoux S (2003) Decisional role of the dorsolateral prefrontal cortex in ocular motor behaviour. Brain 126:1460–1473
Pierrot-Deseilligny C, Rivaud S, Gaymard B, Agid Y (1991) Cortical control of reflexive visually guided saccades. Brain 114:1473–1485
Rao SM, Harrington DL, Haaland KY, Bobholz JA, Cox RW, Binder JR (1997) Distributed neural systems underlying the timing of movements. J Neurosci 17:5528–5535
Rao SM, Mayer AR, Harrington DL (2001) The evolution of brain activation during temporal processing. Nat Neurosci 4:317–323
Repp BH (2001) Processes underlying adaptation to tempo changes in sensorimotor synchronization. Hum Mov Sci 20:277–312
Roberts S, Eykholt R, Thaut MH (2000) Analysis of correlations and search for evidence of deterministic chaos in rhythmic motor control by the human brain. Phys Rev E 62:2597–2607
Robinson DA (1964) The mechanics of human saccadic eye movement. J Physiol 174:245–264
Ross SM, Ross LE (1987) Children’s and adults’ predictive saccades to square wave targets. Vision Res 27:2177–2180
Savitzky A, Golay MJE (1964) Smoothing and differentiation of data by simplified least squares procedures. Anal Chem 38:1627–1639
Schlag-Rey M, Amador N, Sanchez H, Schlag J (1997) Antisaccade performance predicted by neuronal activity in the supplementary eye field. Nature 390:398–401
Schöner G (2002) Timing, clocks, and dynamical systems. Brain Cogn 48:31–51
Semjen A, Schulze HH, Vorberg D (2000) Timing precision in continuation and synchronization tapping. Psychol Res 63:137–147
Shelhamer M (2005) Sequences of predictive saccades are correlated over a span of ∼2 s and produce a fractal time series. J Neurophysiol 93:2002–2011
Shelhamer M, Joiner WM (2003) Saccades exhibit abrupt transition between reactive and predictive, predictive saccade sequence have long-term correlations. J Neurophysiol 90:2763–2769
Simó LS, Krisky CM, Sweeney JA (2005) Functional neuroanatomy of anticipatory behavior: dissociation between sensory-driven and memory-driven systems. Cereb Cortex 15:1982–1991
Spencer RMC, Zelaznik HN, Diedrichsen J, Ivry RB (2003) Disrupted timing of discontinuous movements by cerebellar lesions. Science 300:1437–1439
Stark L, Vossius G, Young LR (1962) Predictive control of eye tracking movements. IRE Trans Hum Factors Electron 3:52–57
Stevens LT (1886) On the time-sense. Mind 11:393–404
Todd NP, Lee CS, O’Boyle DJ (2002) A sensorimotor theory of temporal tracking and beat induction. Psychol Res 66:26–39
Treisman M (1963) Temporal discrimination and the indifference interval: implications for a model of the ‘internal clock.’ Psychol Monogr 77:1–31
Treisman M, Faulkner A, Naish PLN, Brogan D (1990) The internal clock: evidence for a temporal oscillator underlying time perception and some estimates of its characteristic frequency. Perception 19:705–743
Wing AM, Kristofferson AB (1973a) Response delays and the timing of discrete motor responses. Percep Psychophys 14:5–12
Wing AM, Kristofferson AB (1973b) The timing of interresponse intervals. Percep Psychophys 13:455–460
Zambarbieri D, Schmid R, Ventre J (1987) Saccadic eye movements to predictable visual and auditory targets. In: O’Regan JK, Lévy-Schoen A (eds) Eye movements: from physiology to cognition. Elsevier, New York, pp 131–140
Acknowledgments
Supported by NIH grants T32-MH20069 and EY015193. The authors would like to thank Dale Roberts, Adrian G. Lasker, Andrew Zorn, and Faisal Karmali for technical assistance and help with experimental design, and Michael Tadross, Drs. Jing Tian, Mark Walker, and Maurice Smith for helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Joiner, W.M., Shelhamer, M. An internal clock generates repetitive predictive saccades. Exp Brain Res 175, 305–320 (2006). https://doi.org/10.1007/s00221-006-0554-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-006-0554-z