Abstract
We comparatively investigated predictive and reactive grip force behaviour in 12 subjects with basal ganglia dysfunction (six subjects with Parkinson’s disease, six subjects with writer’s cramp), two subjects chronically lacking all tactile and proprioceptive sensory feedback and 16 sex- and age-matched control subjects. Subjects held an instrumented receptacle between the index finger and thumb. A weight was dropped into the receptacle either unexpectedly from the experimenter’s hand with the subject being blindfolded or expectedly from the subject’s opposite hand. This paradigm allowed us to study predictive and reactive modes of grip force control. All patients generated an overshoot in grip force, irrespective of whether the weight was dropped expectedly or unexpectedly. When the weight was dropped from the experimenter’s hand, a reactive grip force response lagged behind the load perturbation at impact in patients with basal ganglia dysfunction and healthy controls. When the weight was dropped expectedly from the subject’s opposite hand, patients with basal ganglia dysfunction and healthy subjects started to increase grip force prior to the release of the weight, indicating a predictive mode of control. We interpret these data to support the notion that the motor dysfunction in basal ganglia disorders is associated with deficits of sensorimotor integration. Both deafferented subjects did not show a reactive mode of force control when the weight was dropped unexpectedly, underlining the importance of sensory feedback to initiate reactive force responses. Also in the predictive mode, grip force processing was severely impaired in deafferented subjects. Thus, at least intermittent sensory information is necessary to establish and update predictive modes of grasping force control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When we handle objects in daily life predictive and reactive modes of grip force control are easily exploited depending on the manipulative intend and the autonomy of the objects we interact with (Flanagan and Johansson 2002; Nowak 2004). Accurate sensorimotor integration is necessary for both predictive and reactive control of grasping forces. For example, when we drop a weight from one hand into a receptacle held with the other hand, a predictive increase in grip force occurs prior to the increase in load due to impact (Johansson and Westling 1988; Nowak and Hermsdörfer 2004). In this situation the finger forces necessary to ensure a stable grasp are processed in a predictive mode to counteract the expected perturbation due to impact well before it takes place. Such predictive control strategies established in case perturbations are self-generated (Johansson and Westling 1988; Nowak and Hermsdörfer 2004). In contrast, when an experimenter unexpectedly drops a weight into the receptacle held by a blindfolded subject, the increase in grip force lags some 100 ms behind the increase in load due to impact (Johansson and Westling 1988), suggesting long-loop reactive force responses initiated by peripheral sensory feedback and transferred, at least in part, via the cerebral cortex (Jenner and Stephens 1982).
In recent years it has been shown that a variety of movement disorders are associated with abnormalities of isometric finger force control (Fellows et al. 2001; Fellows and Schwarz 1998; Nowak and Hermsdörfer 2005; Odergren et al. 1996; Serrien et al. 2000). In disorders of the basal ganglia, such as Parkinson’s disease (Fellows and Schwarz 1998; Wenzelburger et al. 2002) and writer’s cramp (Nowak et al. 2005; Odergren et al. 1996; Serrien et al. 2000), this abnormality commonly consists of the use of exaggerated grip force levels in relation to the loads. In healthy subjects an inefficient elevation of grip forces is known to occur when the cutaneous afferents of the fingers and lower arm are subjected to local anaesthesia (Nowak et al. 2001; Witney et al. 2004). Also in subjects with peripheral sensory neuropathy elevated grip force levels have been observed (Hermsdörfer et al. 2004; Nowak et al. 2004a).
Given the vital role of cutaneous afferents for accurate grip force scaling (Witney et al. 2004), the abnormally high levels seen in Parkinson’s disease and writer’s cramp have been attributed to peripheral sensory deficits or, alternatively, to inappropriate selection of accurate force levels by the supplementary motor area as a result of misleading afferent information from the grasping fingers relayed by deficient basal ganglia circuits (Abbruzzese and Berardelli 2003; Fellows and Schwarz 1998). Although laboratory studies have documented deficits of two point discrimination and kinaesthesia in Parkinson’s disease (Jobst et al. 1997; Schneider et al. 1987; Zia et al. 2000) and writer’s cramp (Bara-Jimenez et al. 2000; Sanger et al. 2002), the processing of sensory information at the peripheral level as tested by somatosensory evoked potentials is usually normal in Parkinson’s disease (Abbruzzese et al. 1997; Mauguière et al. 1993) and writer’s cramp (Abbruzzese et al. 2001; Tinazzi et al. 2000). Based on these findings the overflow of grasping forces in basal ganglia disorders is most likely to result from a deficit of sensorimotor integration within the central nervous system (Abbruzzese and Berardelli 2003; Berardelli et al. 2001).
The present study was designed to comparatively investigate how predictive and reactive finger force control is affected by basal ganglia dysfunction and peripheral neuropathy. We used the weight-catching task to assess the rules of predictive and reactive finger force adjustments to expected and unexpected load perturbations in subjects with Parkinson’s disease, writer’s cramp and complete chronic peripheral deafferentation due to large fibre sensory polyneuropathy.
Methods
Subjects
All participants gave their informed consent. The study was conducted in accordance with the Declaration of Helsinki and the methods were approved by the local ethics committee. All participants were naive to the specific purpose of the experiments.
Subjects with Parkinson’s disease
The study involved six patients (aged 42–62 years, mean age 55±8.5 years; two female, four males) with advanced-stage Parkinson’s disease. Written informed consent was obtained from all patients. Six age- and sex-matched healthy subjects served as a control group (aged 41–61 years, mean age 54±7 years; two female, four males). Patients were tested in the morning after a 12-h overnight withdrawal of dopaminergic drugs. Patients were videotaped in the off-drug and on-drug states and the motor score of the Unified Parkinson’s disease rating scale (UPDRS, items 18–31) was rated (Fahn and Elton 1987). None of the patients showed dyskinesia, regardless of whether off- or on-drug.
The levodopa equivalent daily dose (LEDD) was calculated as described previously (Krack et al. 1998). Sense of position and light touch was rated not disturbed/disturbed according to the subjects’ perception of passive movements of the distal joint of the index and the touch with a swab. Clinical details of subjects with Parkinson’s disease are summarised in Table 1.
Subjects with writer’s cramp
Six patients with writer’s cramp (aged 31–55 years, mean age 41±9 years; six men). Clinical data on the patients are summarised in Table 1. Six healthy subjects (aged 30–50 years; mean age 39±7 years; six men) served as a control group. All dystonic patients were able to relax their muscles completely and no dystonic movements or contractions were observed at rest. The diagnosis of writer’s cramp was based on characteristic clinical features: difficulties in writing caused by abnormal muscle contractions or abnormal posturing, with preserved muscle strength. Task specificity was rated according to the classification of Sheehy and Marsden (1982).
Deafferented subjects
Two chronically deafferented subjects participated in the experiments. G.L., a 54-year-old woman, suffered a permanent and specific loss of the large sensory myelinated fibres in all four limbs following two episodes of sensory polyneuropathy that affected her whole body below the V2 cranial nerve division. The illness resulted in a complete loss of the senses of touch, vibration, pressure and kinaesthesia in the neck, trunk, and upper and lower limbs, but temperature and pain sensation were preserved (Fleury et al. 1995; Nowak et al. 2004a; Simoneau et al. 1999). G.L. has no sensation or control of the head, neck or limb position and motion with eyes closed. These clinical observations were documented to be stable over the past two decades [a detailed clinical description of G.L. has been provided by Forget and Lamarre (1987)].
I.W., a 49-year-old male, suffered a complete and permanent loss of large sensory fibres after an episode of sensory polyneuropathy about 30 years ago, leaving him from the neck down without movement sense or position sense, cutaneous touch, proprioceptive or cutaneous reflexes, but with spared nociceptive and thermoceptive afferents. I.W. has some residual sense of muscular fatigue. These clinical observations were documented to be stable over the past three decades. A full description of I.W.’s clinical syndrome can be found in Cole (1995) and Cole and Sedgwick (1992). Four healthy control subjects (aged 48–56 years, mean age 52±4 years; two female, four males) served as control group.
Apparatus
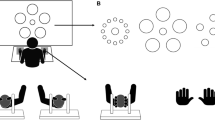
Subjects grasped a cylindrical and cordless instrumented object. A receptacle was fixed at the base of the object. The weight of the configuration was 0.45 kg. The object and the configuration of the hand and fingers used to grasp it are illustrated in Fig. 1. Grip surfaces were of polysterol plastic in all trials performed. The object incorporated a force sensor for grip force registration and a linear acceleration sensor for registration of kinematic acceleration along the axis of gravity (Fig. 1). The force sensor registered grip forces up to 50 N (accuracy ±0.0125 N). The linear acceleration sensor measured linear acceleration within a range of ±50 m/s2. Positive acceleration was directed upward. The centre of mass of the apparatus was exactly vertically below and halfway between the points at which the fingers contacted its surfaces. Recorded grip force and acceleration data were analog-to-digital converted with a sampling rate of 100 Hz and stored within the object. Data were transferred to a personal computer for analysis following each experimental setting with a single subject.
The instrumented object was held between the tips of the thumb and index finger of the dominant hand. The elbow of the arm rested on the supporting thigh to keep the object’s position constant. A 200 g ball was either dropped from the opposite hand or unexpectedly from the experimenter’s hand into a receptacle attached to the instrumented object. The object incorporated a force sensor to register grip force (GF) and a linear acceleration (ACC) sensor to register the perturbation when the ball was dropped into the receptacle. The object’s grip surfaces were oriented in parallel to the axis of gravity
Procedure
Prior to the experiments subjects washed their hands with water and soap and carefully dried them. The room temperature was kept at a constant level throughout the experiments. Subjects were seated in a stable chair and grasped the object between the index and thumb of the dominant hand with the elbow resting on the supporting thigh. This posture ensured that the position of the object was kept constant throughout the experiments. Subjects were instructed to hold the object stationary and to prevent it from slipping. In the experimenter-release condition, subjects were asked to keep their eyes closed for the entire experiment. The experimenter dropped a 200 g ball unexpectedly into the receptacle from a height of 30 cm indicated by a mark (Fig. 1). The ball was fabricated from a deformable non-elastic material. In a second experiment, subjects themselves dropped the 200 g ball into the receptacle with their eyes open. Ten such trials with inter-trial intervals of 5 s were performed for the experimenter- and self-release conditions, respectively. One half of subjects within each group started with the experimenter-release condition, the other half with the self-release condition. At the end of the experiments the minimum grip force necessary to prevent the object from slipping (slip force) was assessed for the configuration with the ball: subjects were asked to hold the object stationary and to slowly separate the thumb and index finger until the object dropped from the grasp. The slip point was defined as the first detectable change in acceleration and the slip force was determined at this time point.
Data analysis and statistics
The time of peak load perturbation was indirectly assessed by determining the minimum of the acceleration signal. When controlling this measure with a load sensor applied between the grip object and the receptacle, peak load indeed occurred synchronously with the minimum in acceleration. We focused our analysis on the grip force measures. For all trials in the experimenter- and self-release conditions the following parameters were analysed: The grip force applied to hold the object prior to weight release (GFHold) was calculated from a 0.5-s period of stationary holding defined to start 1 s prior to the time of minimum acceleration due to impact. Grip forces at the time of minimum acceleration (GFACCMin) and maximum grip forces following impact (GFMax) were obtained. In Fig. 2, the measures used for data analysis are indicated within the grip force and acceleration traces of single weight-catching responses of a healthy subject (male, 31 years) in the experimenter- and self-release conditions.
Grip force and acceleration profiles obtained from single weight-catching trials in the a experimenter- and b self-release conditions of a healthy subject (male 31 years). The boxes indicate the 0.5-s intervals from which the grip force applied to hold the object stationary was averaged (static grip force). The dotted vertical lines indicate the time of impact as signalled from minimum acceleration and the time of peak grip force. Grey circles mark minimum acceleration and peak grip force. It is evident that grip force started to rise prior to the perturbation at impact in the self-release condition
For each subject the average grip force profile during a 2-s interval defined to start 1 s prior to the time of the minimum in acceleration was calculated from the last seven of ten trials performed in the experimenter- and self-release conditions. To compare reactive and predictive grip force adjustments of patients and controls in the two experimental conditions, a correlation analysis between the average grip force signals obtained from the 2-s intervals was performed. Repeated measures analysis of variance (ANOVA) were performed to compare the performance in between patients with Parkinson’s disease, writer’s cramp and healthy controls with “disorder” (Parkinson’s disease, writer’s cramp) and “condition” (self- and experimenter-release conditions) as main factors. T-tests were used for pair-wise comparisons. A P value of 0.05 was considered statistically significant. Due to the limited number of patients, the performance of deafferented subjects is described only qualitatively.
Results
The average slip forces obtained from the slipping experiments were similar (P≥0.2 for all comparisons) for healthy subjects (4.2±0.4 N), subjects with Parkinson’s disease (4.4±0.3 N) and writer’s cramp (4.6±0.5). Thus, the friction between the skin of the grasping fingers and the object surface was similar for healthy subjects and both patient groups and should not be responsible for any differences of grip force scaling in between the groups. Unfortunately, the average slip force was not obtained for the deafferented subjects G.L. and I.W.
Figure 2 illustrates the grip force traces of a healthy subject performing the weight-catching task in the experimenter- and self-release conditions. In the experimenter-release condition, the subject’s grip force started to rise after the time of minimum acceleration signalling weight impact. In the self-release condition, the subject’s grip force started to increase prior to the time of minimum acceleration due to impact. In addition, it appears as if the reactive grip force output generated in the experimenter-release condition is more pronounced than that produced in the self-release condition.
Performance of subjects with Parkinson’s disease
Figure 3a summarises average grip force traces for each subject with Parkinson’s disease and the corresponding sex- and age-matched healthy control subject under both experimental conditions. It is evident that all subjects with Parkinson’s disease perform in a similar way as healthy controls. However, patients increased grip force somewhat earlier than healthy subjects in the self-release condition. To test the hypothesis that the grip force adjustments were similar for patients and healthy controls in the experimenter- and self-release conditions a correlation analysis between average grip force traces of individual patients and their matched control subjects was performed. Indeed, there was a strong correlation between the grip force signals of patients and controls in the experimenter- (median r 2=0.89) and self-release conditions (median r 2=0.91).
a Average grip force traces for each subject with Parkinson’s disease and healthy control subject obtained from a 2-s period starting 1 s prior to the time of minimum acceleration induced by impact. Grip force signals are shown for the self- and experimenter-release conditions. Dotted vertical lines indicate the time of minimum downward acceleration signalling impact. b Average group data and standard deviations of grip force established during a 0.5-s period of stationary holding the receptacle (GFHold), grip force at the time of minimum acceleration (GFACCMin) due to impact and peak grip force (GFMax) are illustrated for weight-catching trials of subjects with Parkinson’s disease and healthy controls in both experimental conditions. Statistical differences are indicated (**P<0.01; ns not significant)
Figure 3b illustrates average group data of each grip force parameter chosen for data analysis. Statistical differences in between these measures as assessed by pairwise T-tests are indicated. It appears as if all subjects with Parkinson’s disease produce higher grip forces than healthy controls, especially in the self-release condition. This observation was statistically confirmed. ANOVA revealed a significant effect of “disorder” on all the grip force measures obtained for data analysis (GFHold: F 1,5=49.5; P<0.001; GFACCMin: F 1,5=30.6; P<0.01; GFMax: F 1,5=37.7; P<0.01). The grip force produced when holding the receptacle prior to weight release (GFHold) seems to be of similar magnitude in the experimenter- and self-release conditions within the group of patients and healthy controls. Indeed, there was no significant effect of “condition” on this measure and no significant interaction “disorder” × “condition”.
In contrast, the grip force level at the time of minimum acceleration seems to be higher in the self-release when compared to the experimenter-release condition for both patients and controls, suggesting that participants started to increase grip force well before weight release in the self-release condition. This was statistically affirmed: there was a significant effect of “condition” (F 1,5=17.1; P<0.01) on this measure, but no significant interaction “disorder” × “condition”. The peak grip forces seem to be more pronounced and variable in the experimenter-release condition for healthy subjects, but of similar magnitude in both conditions for patients. Statistically, neither the factor “condition” nor the interaction “disorder” × “condition” reached significance.
Performance of subjects with writer’s cramp
Figure 4a summarises average grip force traces for each patient with writer’s cramp and matched control subject in the self- and experimenter-release conditions. Patients with writer’s cramp performed similar to healthy controls. A correlation analysis between the average grip force traces obtained from the trials of corresponding patients and healthy control subjects was performed. We found a strong correlation between grip force signals of patients and controls in the self- (median r 2=0.89) and experimenter-release conditions (median r 2=0.89).
a Average grip force traces for each subject with writer’s cramp and healthy control subject obtained from a 2-s period starting 1 s prior to the time of minimum acceleration induced by impact. Grip force signals are shown for the self- and experimenter-release conditions. Dotted vertical lines indicate the time of minimum downward acceleration signalling impact. b Average group data and standard deviations of grip force established during a 0.5-s period of stationary holding the receptacle (GFHold), grip force at the time of minimum acceleration (GFACCMin) due to impact and peak grip force (GFMax) are illustrated for weight-catching trials of subjects with Parkinson’s disease and healthy controls in both experimental conditions. Statistical differences are indicated (**P<0.01; ns not significant)
Figure 4b illustrates average group data for the different grip force measures. Patients generated higher grip forces than healthy controls at least in the self-release condition. Indeed, we found a significant main effect of “disorder” on baseline grip force (GFHold) (F 1,5=17.9; P<0.01) and on grip force at the time of minimum acceleration (GFACCMin) (F 1,5=37.7; P<0.001), but not on peak grip forces (GFMax). The grip force when holding the object stationary seems to be of similar magnitude in the self- and experimenter-release conditions within each group. Indeed, there was no significant effect of “condition” or the interaction “disorder” × “condition” on this measure.
For healthy subjects, the grip force level at the time of minimum acceleration appears to be higher in the self-release condition than in the experimenter-release condition. However, patients produced similar, but more variable force levels at the time of weight impact, regardless of the experimental condition. There was no significant effect of “condition” on this measure, but the interaction “disorder” × “condition” was significant (F 1,5=48.3; P<0.001), implicating that the force output was most pronounced for patients in the self-release condition (P<0.001). It seems as if healthy subjects generated higher peak grip forces (GFMax) in the experimenter-release condition, whereas patients produced peak grip forces of similar magnitudes in the experimenter- and self-release conditions. There was a significant effect of “condition” on the peak grip forces (F 1,5=18.4; P<0.01). The significant interaction “disorder” × “condition” (F 1,5=12; P<0.02) implies that peak grip forces were most pronounced for healthy subjects in the experimenter-release condition (P<0.05).
Comparison between Parkinson’s disease and writer’s cramp
When comparing Figs. 3a and 4a it is evident that subjects with Parkinson’s disease and writer’s cramp performed in a similar manner. There was a strong correlation between the average grip force signals of individual subjects with Parkinson’s disease and writer’s cramp in the self- (median r 2=0.89) and experimenter-release conditions (median r 2=0.90). When comparing the average grip force data we found no significant effect of “disorder” or “condition” on the stationary grip force (GFHold) applied against the receptacle prior to impact, on the grip force produced at the time of minimum acceleration (GFACCMin) and on peak grip force (GFMax). The interactive effect “disorder” × “condition” was not significant for stationary grip forces and peak grip forces, but significant for grip force at the time of minimum acceleration (F 1,5=30.1; P<0.01). The latter suggests that the grip force magnitude at the time of minimum downward acceleration was most pronounced for trials in the self-release condition performed by subjects with Parkinson’s disease.
Performance of deafferented subjects
Figure 5a summarises the performance of both deafferented subjects G.L. and I.W. during five trials performed in the self- and experimenter-release condition. It is evident that G.L. generated significantly greater and more variable grip forces than I.W., regardless of the experimental condition. First, consider the performance of G.L.: G.L. constantly increases her grip force output well before the time of minimum downward acceleration signalling impact in the self-release condition. In this aspect her force control appears to be predictive and similar to that observed for healthy controls and patients with Parkinson’s disease and writer’s cramp. However, in the experimenter-release condition her grip force output appears to be highly variable and irregular. Now consider the performance of I.W.: Interestingly, it appears as if I.W. generates only a slight increase in grip force prior to impact in the self-release condition, but definitely no grip force reaction in the experimenter-release condition. The small bulb evident in the grip force traces obtained from trials in the experimenter-release condition also appears in the trials performed by healthy subjects and patients with Parkinson’s disease and writer’s cramp and represents a technical artefact generated by the force sensor at the time of impact due to small tilts of the receptacle.
a Grip force traces obtained from five trials performed by both deafferented subjects. Grip force data were obtained from a 2-s period starting 1 s prior to the time of minimum acceleration induced by impact. Grip force signals are shown for the self- and experimenter-release conditions. Note the different scaling for the force panels of G.L. and I.W. b Average data and standard deviations of grip force established during a 0.5-s period of stationary holding the receptacle (GFHold), grip force at the time of minimum acceleration (GFACCMin) due to impact and peak grip force (GFMax) are illustrated for weight-catching trials of both deafferented subjects in both experimental conditions. Note the different scaling for the force panels of G.L. and I.W. Statistical differences are indicated (*P<0.05; **P<0.01; ns not significant)
There was only a moderate correlation between the average grip force traces of G.L. and I.W. in the self- (average r 2=0.65) and experimenter-release conditions (average r 2=0.66). The correlation between the grip force traces of both deafferented subjects with the sex- and age-matched control subjects was considerably less reliable in the experimenter-release condition (G.L.: average r 2=0.67; I.W.: average r 2=0.68), but stronger for G.L. than for I.W. in the self-release condition (G.L.: average r 2=0.90; I.W.: average r 2=0.70). G.L. seems to generate higher grip forces than I.W. The grip force output of both G.L. and I.W. appears to be higher than that of their matched control subjects.
Figure 5b illustrates average grip force measures obtained from trials in the self- and experimenter-release conditions for both deafferented subjects. For G.L. the grip force when holding the receptacle stationary was higher than grip force at the time of impact for trials in the self-release condition, but similar for trials in the experimenter-release condition. This finding gives support to the notion that G.L. increased grip force before impact in the self-release condition, but there was no force reaction in the experimenter-release condition. For I.W. there were no significant differences between grip forces produced when holding the receptacle prior to impact and at the time of impact, regardless of the experimental condition. Thus, I.W. did not adjust his grip forces in a predictive or reactive manner according to the demands of the task.
Discussion
The weight-catching task allows the separate investigation of both predictive and reactive control processes during grasping (Johansson and Westling 1988). Accurate execution of predictive and reactive grasping movements crucially depends on both intact peripheral sensory feedback (Hermsdörfer et al. 2004; Johansson and Westling 1988; Nowak et al. 2001; Witney et al. 2004) and accurate projection and integration of afferent sensory information to cortical sensorimotor areas (Hermsdörfer et al. 2004). Sensorimotor integration is the process whereby sensory feedback is integrated within the central nervous system and used to plan and execute motor programs. It is sensorimotor integration that is thought to be abnormal in basal ganglia disorders, such as Parkinson’s disease and writer’s cramp (Abbruzzese and Berardelli 2003). The investigation of deafferented subjects shades some light onto the issue how somatosensory feedback contributes to the processing of both predictive and reactive grip force adjustments.
Performance of subjects with Parkinson’s disease and writer’s cramp
Dysfunction of the basal ganglia output nuclei, substantia nigra pars reticulata and internal segment of the globus pallidum results in functional changes in the complex network of subcortical and cortical sensorimotor structures causing both hypokinetic (Parkinson’s disease) and hyperkinetic movement disorders (focal hand dystonia) (Berardelli et al. 2001; Wichmann and DeLong 1996). The processing of somatosensory information at a peripheral level is usually normal in Parkinson’s disease and focal hand dystonia (Abbruzzese et al. 1997, 2001; Mauguière et al. 1993; Tinazzi et al. 2000). Consequently, the frequently reported abnormalities of sensory processing in basal ganglia disorders (Bara-Jimenez et al. 2000; Jobst et al. 1997; Sanger et al. 2002; Schneider et al. 1987; Zia et al. 2000) are thought to result from impaired sensorimotor integration (Abbruzzese and Berardelli 2003).
Earlier studies demonstrated abnormal control of grip forces in subjects with Parkinson’s disease (Fellows and Schwarz 1998; Nowak and Hermsdörfer 2005) and writer’s cramp (Nowak and Hermsdörfer 2005; Nowak et al. 2005; Odergren et al. 1996; Serrien et al. 2000) repetitively lifting an object between the index finger and thumb. Subjects with Parkinson’s disease and writer’s cramp squeezed the object more forcefully than necessary, but were able to adjust the force output more accurately to the mechanical object properties through the following set of lifts (Fellows and Schwarz 1998; Nowak et al. 2005; Odergren et al. 1996). These data suggest that patients with Parkinson’s disease and writer’s cramp are able to use peripheral sensory information from the grasping digits to assist the scaling of grip force, but that the accuracy of sensorimotor processing is impaired.
Our subjects with Parkinson’s disease and writer’s cramp also generated higher grip forces than normal in both experimental conditions. The force overshoot was similar in both patient groups. Nevertheless, our subjects with basal ganglia dysfunction were able to generate both predictive and reactive grip force adjustments, depending on whether the weight was dropped by themselves or unexpectedly by the experimenter. Given the fact that the force excess does not generally impair the programming and execution of predictive and reactive control strategies, we hypothesise that the selection of inappropriate force levels may result from an abnormal gating of sensory information at the level of the basal ganglia. However, excessive grip forces have been observed in a variety of neurological disorders affecting both sensory and motor structures (Fellows et al. 2001; Fellows and Schwarz 1998; Hermsdörfer et al. 2004; Nowak et al. 2001, 2002, 2004a; Odergren et al. 1996; Rost et al. 2005; Serrien et al. 2000). Consequently, the force excess may also be interpreted to reflect a strategy to ensure a stable grasp when the sensory or motor systems work suboptimally.
Performance of deafferented subjects
Both deafferented subjects were chronically deprived of all tactile or proprioceptive sensations (Fleury et al. 1995; Nowak et al. 2004a; Simoneau et al. 1999). Consequently, they would not have been able to use sensory information from the grasping fingers to judge the mechanical properties of the object at hand and the consequences of the load perturbation at impact necessary for accurate programming of predictive and reactive motor commands. Given the vital role of intact cutaneous, muscle and joint receptors from the grasping fingers for efficient grip force scaling (Hermsdörfer et al. 2004; Nowak et al. 2001; Witney et al. 2004), it is not surprising that both subjects exhibited excessive grip forces. We expected the deficit of force adjustments to be more pronounced in the experimenter-release condition based on the well-known observation that the initiation of reactive force responses strongly depends on intact sensory feedback (Flanagan and Johansson 2002; Johansson and Westling 1988; Nowak 2004; Witney et al. 2004). Indeed, both subjects did not reveal any reactive grip force response following the load perturbation when the weight was dropped unexpectedly from the experimenter’s hand.
In the self-release condition, the predictive increase in grip force prior to weight impact is initiated at a cortical level and thus seems to be less dependent on intact sensory feedback. Indeed, we found an early increase of grasping forces prior to weight impact when G.L. released the weight herself. This observation supports our hypothesis that, in principal, predictive force programming can take place even in the absence of any sensory feedback. However, G.L.’s grip force programming in the self-release condition appears to be less accurate compared to the performance of healthy controls. We recently observed that G.L. also modulates grip force with the load fluctuations induced by voluntary arm movements with a hand-held object (Nowak et al. 2004a). In healthy subjects, grip force is modulated in parallel with movement-induced load fluctuations during arm movements with a grasped object (Nowak 2004). In G.L., the grip force profile was not modulated in parallel with the changes in load, suggesting a severe decalibration of predictive grip force planning (Nowak et al. 2004a). In conjunction with the present set of data, these findings imply that predictive force control requires at least intermittent sensory feedback to signal the effectiveness of the descending motor commands.
Surprisingly, the performance of I.W. at some points clearly differed from that of G.L. when dropping the weight expectedly from the opposite hand. I.W. generated none or only a slight increase in grip force prior to impact. Thus, it appears as if both deafferented subjects, despite very similar loss of sensory feedback, had developed different strategies to counteract the expected perturbation at impact. I.W. also generated grip forces that were higher than those used by healthy subjects, but smaller than those produced by G.L. Why both deafferented subjects performed so differently cannot be answered based on the current study design. However, it is noteworthy that I.W., different to G.L., has been found to be a sophisticated neurological observer with immense skills of introspection and self-interpretation (Cole and Sedgwick 1992; Cole 1995). In addition, I.W. has retained some residual sense of muscular fatigue or effort that may have allowed him to reduce the overall force output.
Internal models for sensorimotor integration
When our body interacts with the environment, such as grasping and transporting objects, prediction of the consequences of our own motor commands requires a system that can simulate the dynamic behaviour of the body and environment. Such a system has been termed internal forward model as it captures the causal relationship between actions and their consequences (Flanagan and Johansson 2002; Wolpert and Flanagan 2001; Wolpert et al. 1998). Predictive grip force behaviour is suggested to be based on the use of such models (Flanagan and Johansson 2002). This type of control is based on the comparison of actual sensory signals and the predicted sensory outcome of a voluntary action, an internal sensory signal (referred to as corollary discharge). Predicted sensory outcomes are generated in conjunction with a copy of the descending motor command (referred to as efference copy) (Flanagan and Johansson 2002). A mismatch between the predicted and the actual sensory outcome triggers force corrections along with an updating of the relevant internal models. As we interact with objects which have their own intrinsic dynamics, such models are not fixed entities, but are learned and updated by manipulative experience.
Theoretical considerations (Stein and Glickstein 1992; Wolpert et al. 1998), imaging data (Hermsdörfer et al. 2005; Kawato et al. 2003), lesion studies (Fellows et al. 2001; Nowak et al. 2002, 2004b) and single cell recordings (Dugas and Smith 1992; Monzee and Smith 2004) suggest that the cerebellum is functionally best suited to establish and incorporate internal forward models. Recently, it has been demonstrated that when subjects with cerebellar degeneration performed the weight-catching task under discussion here, they were unable to process grip force in advance of the expected perturbation when the weight was dropped from the opposite hand (Nowak et al. 2004b). On the other hand, subjects with cerebellar degeneration exhibited a clear reactive mode of force control when the weight was dropped unexpectedly from the experimenter’s hand. These data suggest that cerebellar circuits are involved in a predictive, but less in a reactive mode of force control (Nowak et al. 2004b; Nowak and Hermsdörfer 2005). The present study extends these data demonstrating that the basal ganglia are not directly involved in setting up internal forward models, but rather deal with the accurate processing and integration of sensory information necessary to program predictive and reactive force adjustments.
Conclusion
Both predictive and reactive modes of force control were preserved in subjects with Parkinson’s disease and writer’s cramp. However, subjects with basal ganglia dysfunction employed elevated force levels, suggesting that the dysfunction rather consists of defective central processing or gating of sensory input via the basal ganglia. The reactive mode of force control was severely impaired in both deafferented subjects, underlining the importance of sensory feedback to initiate reactive force responses. Also in the predictive mode, grip force processing was deficient in deafferented subjects, suggesting that at least intermittent sensory information is necessary to establish and update predictive modes of force control.
References
Abbruzzese G, Berardelli A (2003) Sensorimotor integration in movement disorders. Mov Disord 18:231–240
Abbruzzese G, Marchese R, Trompetto C (1997) Sensory and motor evoked potentials in multiple system atrophy: a comparative study with Parkinson’s disease. Mov Disord 12:315–321
Abbruzzese G, Marchese R, Buccolieri A, Gasparetto B, Trompetto C (2001) Abnormalities of sensorimotor integration in focal dystonia. A transcranial magnetic stimulation study. Brain 124:537–545
Bara-Jimenez W, Shelton P, Hallett M (2000) Spatial discrimination is abnormal in focal hand dystonia. Neurology 55:1869–1873
Berardelli A, Rothwell JC, Thompson PD, Hallett M (2001) Pathophysiology of bradykinesia in Parkinson’s disease. Brain 124:2131–2146
Cole JD (1995) Pride and a daily marathon. MIT Press, Boston
Cole JD, Sedgwick EM (1992) The perception of force and of movement in a man without large myelinated sensory afferents below the neck. J Physiol 449:503–515
Dugas C, Smith AM (1992) Responses of cerebellar Purkinje cells to slip of a hand-held object. J Neurophysiol 67:483–495
Fahn S, Elton R (1987) Unified Parkinson’s disease rating scale. In: Fahn S, Marsden C, Calne D, Goldstein M (eds) Recent developments in Parkinson’s disease. MacMillan Health Care Information, Florham Park, pp 153–163
Fellows SJ, Schwarz M, Noth J (1998) Precision grip in Parkinson’s disease. Brain 121:1771–1784
Fellows SJ, Ernst J, Schwarz M, Töpper R, Noth J (2001) Precision grip in cerebellar disorders in man. Clin Neurophysiol 112:1793–1802
Flanagan JR, Johansson RS (2002) Hand movements. In: Ramshandran VS (ed) Encyclopedia of the human brain, vol 2. Academic, San Diego, pp 399–414
Fleury M, Bard C, Teasdale N, Paillard J, Cole J, Lajoie Y, Lamarre Y (1995) Weight judgment. The discrimination capacity of a deafferented subject. Brain 118:1149–1156
Forget R, Lamarre Y (1987) Rapid elbow flexion in the absence of proprioceptive and cutaneous feedback. Hum Neurobiol 6:27–37
Hermsdörfer J, Hagl E, Nowak DA (2004) Deficits of anticipatory grip force control after damage to peripheral and central sensorimotor systems. Hum Mov Sci 23:643–662
Hermsdörfer J, Nowak DA, Lee A, Rost K, Timmann D, Mühlau M, Boecker H (2005) The representation of predictive force control and internal forward models: evidence from lesion studies and brain imaging. Cogn Process 6:48–58
Jenner JR, Stephens JA (1982) Cutaneous reflex responses and their central nervous pathways studied in man. J Physiol (London) 333:405–419
Jobst EE, Melnick ME, Byl NN, Dowling GA, Aminhoff MJ (1997) Sensory perception in Parkinson’s disease. Arch Neurol 54:450–454
Johansson RS, Westling G (1988) Programmed and triggered actions to rapid load changes during precision grip. Exp Brain Res 71:72–86
Kawato M, Kuroda T, Imamizu H, Nakano E, Miyauchi S, Yoshioka T (2003) Internal forward models in the cerebellum: fMRI study on grip force and load force coupling. Prog Brain Res 142:171–188
Krack P, Pollak P, Limousin P, Hoffmann D, Xie J, Benazzouz A (1998) Subthalamic nucleus or internal pallidal stimulation in young onset Parkinson’s disease. Brain 121:451–457
Mauguière F, Broussolle E, Isnard J (1993) Apomorphine-induced relief of the akinetic-rigid syndrome and early median nerve somatosensory evoked potentials (SEP’s) in Parkinson’s disease. Electroencephalogr Clin Neurophysiol 88:243–254
Monzee J, Smith AM (2004) Responses of cerebellar interpositus neurons to predictable perturbations applied to an object held in a precision grip. J Neurophysiol 91:1230–1239
Nowak DA (2004) Different modes of grip force control: voluntary and externally guided arm movements with a hand-held load. Clin Neurophysiol 115:839–848
Nowak DA, Hermsdörfer J (2004) Predictability influences finger force control when catching a free-falling object. Exp Brain Res 154:411–416
Nowak DA, Hermsdörfer J (2005) Grip force behavior during object manipulation in neurological disorders: toward an objective evaluation of manual performance deficits. Mov Disord 20:11–25
Nowak DA, Hermsdörfer J, Glasauer S, Philipp J, Meyer L, Mai N (2001) The effects of digital anaesthesia on predictive grip force adjustments during vertical movements of a grasped object. Eur J Neurosci 14:756–762
Nowak DA, Hermsdörfer J, Marquardt C, Fuchs HH (2002) Grip and load force coupling during discrete vertical movements in cerebellar atrophy. Exp Brain Res 145:28–39
Nowak DA, Glasauer S, Hermsdörfer J (2004a) How predictive is grip force control in the complete absence of somatosensory feedback? Brain 127:182–192
Nowak DA, Hermsdörfer J, Rost K, Timmann D, Topka H (2004b) Predictive and reactive finger force control during catching in cerebellar degeneration. Cerebellum 3:227–235
Nowak DA, Rosenkranz K, Topka H, Rothwell J (2005) Disturbances of grip force behaviour in focal hand dystonia: evidence for a generalised impairment of sensory-motor integration? J Neurol Neurosurg Psychiatr 76:953–959
Odergren T, Iwasaki N, Borg J, Forssberg H (1996) Impaired sensory-motor integration during grasping in writer’s cramp. Brain 119:569–583
Rost K, Nowak DA, Timmann D, Hermsdörfer J (2005) Preserved and impaired aspects of predictive grip force control in cerebellar patients. Clin Neurophysiol 116:1405–1414
Sanger TD, Pascual-Leone A, Tarsy D, Schaug G (2002) Nonlinear sensory cortex response to simultaneous tactile stimuli in writer’s cramp. Mov Disord 17:105–111
Schneider JS, Diammond SG, Markham CH (1987) Parkinson’s disease: sensory and motor problems in arms and hands. Neurology 37:951–956
Serrien D, Burgunder JM, Wiesendanger M (2000) Disturbed sensorimotor processing during control of precision grip in patients with writer’s cramp. Mov Disord 15:965–972
Sheehy MP, Marsden CD (1982) Writer’s cramp—a focal dystonia. Brain 105:461–480
Simoneau M, Paillard J, Bard C, Teasdale N, Martin O, Fleury M et al (1999) Role of the feedforward command and reafferent information in the coordination of a passing prehension task. Exp Brain Res 128:236–242
Stein JF, Glickstein M (1992) Role of the cerebellum in visual guidance of movement. Physiol Rev 72:967–1017
Tinazzi M, Priori A, Bertolasi L, Frasson E, Mauguière F, Fiaschi A (2000) Abnormal central integration of dual somatosensory input in dystonia. Evidence for sensory overflow. Brain 123:42–50
Wenzelburger R, Zhang BR, Pohle S, Klebe S, Lorenz D, Herzog J, Wilms H, Deuschl G, Krack P (2002) Force overflow and levodopa-induced dyskinesias in Parkinson’s disease. Brain 125:871–879
Wichmann T, DeLong MR (1996) Functional and pathophysiological models of the basal ganglia. Curr Opin Neurobiol 6:751–758
Witney AG, Wing A, Thonnard JL, Smith AM (2004) The cutaneous contribution to adaptive precision grip. Trends Neurosci 27:637–643
Wolpert DM, Flanagan JR (2001) Motor prediction. Curr Biol 11:R729–R732
Wolpert DM, Miall RC, Kawato M (1998) Internal models in the cerebellum. Trends Cogn Sci 2:338–347
Zia S, Cody F, O’Boyle D (2000) Joint position sense is impaired by Parkinson’s disease. Ann Neurol 47:218–228
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nowak, D.A., Hermsdörfer, J. Predictive and reactive control of grasping forces: on the role of the basal ganglia and sensory feedback. Exp Brain Res 173, 650–660 (2006). https://doi.org/10.1007/s00221-006-0409-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-006-0409-7