Abstract
Rats with lesions of the entorhinal or parietal cortex were tested in a homing task on a circular platform containing food cups and surrounded by curtains. The animals had to leave a refuge, explore the platform to find a hidden piece of food and carry it back to the refuge. Once the rats were proficient at performing the procedural aspects of the task, they were tested in two successive types of trials in which the food pellet was either always located in the central cup (food at center, “FAC” trials) or placed in a randomly chosen cup (food at random, “FAR” trials). Except in the first FAC trials, all groups displayed similar outward paths in FAC and FAR trials, showing that both types of trials involved equivalent path integration demand. Analysis of the homing accuracy showed that rats with entorhinal cortex or parietal cortex lesions exhibited inaccurate returns to the starting hole, suggesting that these two cortical areas are part of a neural network mediating path integration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Navigation in rodents is assumed to depend on two complementary categories of cues: allothetic and idiothetic cues. Allothetic cues are provided by the environment and include mainly visual, auditory and olfactory cues. Idiothetic cues are derived from the animal’s own movements and encompass internal self motion information provided by the vestibular, proprioceptive, and somatosensory systems, efference copies of motor commands, and external motion-related information such as optic flow. By relying on these several sources of information, rats are able to use a variety of navigational strategies to locate a goal in their environment. Allothetic cues themselves underlie different forms of spatial navigation such as beacon homing, i.e., moving toward a directly perceived goal, or piloting, i.e., moving toward a hidden goal based on configurations of distant cues (Morris 1981; Gallistel 1990).
When allothetic cues are absent or irrelevant, rats are able to rely on the sole idiothetic cues to display navigational abilities. In particular, it is proposed that vestibular signals generated by angular and linear accelerations during movements are measured and integrated, allowing rodents to establish and continuously update a homing vector that estimates the direction and distance of the starting place (Potegal 1987). This strategy is referred to as path integration (Barlow 1964; Etienne 1980; Mittelstaedt and Mittelstaedt 1980; Gallistel 1990; Benhamou 1997; Biegler 2000 for a review). Thus, by path integration, a rodent is able to return back directly to its starting place after a circuitous outward journey. The efficiency of this navigational system is, however, somewhat limited due to rapid accumulation of errors involving both distance and direction as the animal proceeds. As a consequence, path integration can underlie accurate navigation for short-distance and relatively simple trips only. Elimination of errors requires recurrent resetting of the path integrator, which can be achieved by returning back to the starting place or by using environmental cues.
The notion that the vestibular system contributes to spatial navigation and more specifically to path integration is not recent (Barlow 1964; Potegal 1982). However, the anatomical and functional organization underlying the processing of vestibular signals in the brain as well as the neural mechanisms mediating path integration still remain poorly understood. An increasing number of electrophysiological and behavioral studies in the rat suggest that the hippocampus plays a crucial role in vestibular processing and in path integration. For instance, unit recordings have demonstrated that place cell activity can be controlled by vestibular input (Sharp et al. 1995; Wiener et al. 1995) and by movement-related information (Gothard et al. 1996). Lesion studies have shown that lesions of the fimbria-fornix or hippocampus produce deficits in path integration tasks (Whishaw and Jarrard 1996; Whishaw 1998; Whishaw and Maaswinkel 1998; Golob and Taube 1999; Maaswinkel et al. 1999; Whishaw and Gorny 1999). However, there is some evidence that extra-hippocampal regions are also involved in path integration. First, vestibular nuclei do not send projections directly to the hippocampal formation, but rather to specific sub-cortical (thalamic) and cortical areas (Fukushima 1997; Smith 1997; Nishiike et al. 2000). Second, selective lesions of cortical regions such as parietal or retrosplenial cortices result in deficits in tasks requiring the use of movement-related information (Save and Moghaddam 1996; Cooper et al. 2001) and path integration (Save et al. 2001). Third, hippocampal lesions do not systematically impair path integration performance (Alyan and McNaughton 1999). One plausible interpretation taking into account these results is that vestibular information and more generally movement-related information reaches, via the thalamus, some neocortical area such as the parietal cortex and is then transmitted to the hippocampus (Smith 1997). According to this hypothesis, it is expected that the entorhinal cortex contributes to such processing since it has a key function in conveying cortical information to the hippocampus. Indeed, the entorhinal cortex receives projections from many cortical areas including the parietal and retrosplenial cortices (Burwell and Amaral 1998), two structures that are suspected to be involved in path integration, and sends major projection to the hippocampus via the perforant path.
The purpose of the present study was, therefore, to examine the idea that path integration results from the involvement of a cortico-hippocampal network including the parietal cortex and the entorhinal cortex. In a previous study, we compared the effects of dorsal hippocampal and parietal lesions on performance in a homing task (Save et al. 2001). Rats had to leave their nest and explore a large circular board to find a hidden piece of food. Once they had found it, they carried it back in straight line to the nest. Because rats could not rely on external cues, it was assumed that the only available strategy to return to the nest was to use movement-related information and therefore path integration. We found that both groups of rats were impaired in this task, thus supporting the hypothesis that cortico-hippocampal transmission is necessary for path integration performance. In the present study, we examined and compared the effects of entorhinal cortex and parietal cortex lesions on path integration in a behavioral situation similar to that used by Save et al. (2001).
Method
Subjects
Subjects were 28 male Long-Evans black-hooded rats (Elevage Janvier, Le Genest-St-Isle, France), weighing between 300 and 324 g before surgery. Upon arrival, they were housed by groups of two with food and water ad libitum and placed in a naturally lit room. After surgery, they were housed in individual cages (40 cm long × 26 cm wide × 16 cm high) to be submitted, following recovery, to a food deprivation schedule. They had free access to water throughout the experiment. All procedures complied with guidelines from international (“Principles of laboratory animal care”, NIH publication no. 86–23, revised 1985) and national institutions (Council directive no. 87848 of the Direction des Services Vétérinaires de la Santé et de la Protection Animale; permission to E.S. no. 13–24). The work was approved by the local ethics committee.
Surgery
Rats were deeply anesthetized by injection of sodium pentobarbital (40 mg/kg i.p., Sanofi Santé Animal, Libourne, France) preceded by atropine sulfate (0.25 mg/kg, i.p.). Additional injections of ketamine (50 mg/kg i.p., Imalgène, Merial, France) were occasionally made to maintain appropriate anesthesia throughout surgery. The rats were placed in a Kopf stereotaxic apparatus (Kopf instruments, Tujunga, CA, USA). A midline incision of the scalp was made and the skin and muscles were carefully retracted to expose the skull. For bilateral entorhinal cortex lesions, holes were drilled above the target regions. Lesions were made by passing a radio-frequency current at the tip of an electrode (70°C for 15 s; RFG 4, Radionics, Burlington, MA, USA) lowered in the brain at the following coordinates relative to bregma: AP: −6.8 mm, L: ±4.3 mm and ±5.4 mm; and AP: −8 mm, L: ±5 mm (Zilles 1985). For each lesion point, the electrode was lowered very slowly until the tip reached the floor of the brain (calvarium) and then raised 1 mm. This position was taken as the dorso-ventral coordinate of the lesion. Due to the posterior curvature of the calvarium, the dorso-ventral coordinate was different for each lesion point, thus allowing damage of the entorhinal cortex along all its extent. Sham-operated rats were treated the same way as lesioned rats except that no current was passed through the electrode. For bilateral parietal cortex lesions, a window was opened in the skull at the following coordinates relative to bregma: AP: −2 to −6 mm; L: ±1.5 to ±5.5 mm. Lesions were made by thermocoagulation of the exposed cortical area. The tip (1 mm2) of a calibrated soldering iron (temperature: 120°C) was applied directly on the dura (0.5 s) at different points (2 or 3) of the cortical surface until the whole area was damaged. Sterile vaseline was placed in the openings. The rats were sutured and as postoperative treatment, received an injection of antibiotic (Terramycine, 60 mg/kg, i.m.). They were then placed back in their home cages for recovery. The sham-operated rats for parietal cortical lesions were anesthetized, had their skin and muscles cut and were sutured. Training began 10 days after surgery. Eight rats received parietal lesions (PAR), nine rats received entorhinal lesions (ENTO), five rats received sham surgery for parietal lesions (SHAM-PAR), and six rats received sham surgery for entorhinal lesions (SHAM-ENTO).
Histology
At the completion of the experiment, lesioned and sham-operated rats received a lethal dose of sodium pentobarbital and were transcardially perfused with a 10% formalin solution. The brains were removed and stored in a 4% formalin solution. Later, coronal and horizontal 40-μm thick sections were made. Every fifth section was mounted and stained with cresyl violet. The slides were observed under the microscope to determine the lesions extent.
Apparatus
The apparatus is similar to that used in our previous study (Save et al. 2001). It consisted of an elevated circular wooden platform (1.90 m in diameter, 85 cm above the ground) located in the middle of a 4×4 m experimental room. The white-painted surface was plastic-impregnated so that it could be easily cleaned during the experiment. At the periphery of the platform, were located eight equidistant circular holes (11.5 cm in diameter, 75 cm from the center of the platform). Beneath each of these holes, a cylindrical box made in gray polyvinyl chloride (19 cm in diameter, 14.5 cm high) could be fixed. Each box, filled with sawdust, served as a transportation box from the room containing the rat’s home cage to the experimental room and as a refuge during a given trial. During transportation, an opaque cover was placed on the box, therefore preventing the animal from seeing the environment. In addition, the box was slowly rotated in order to disorient the animal relative to the room frame of reference. When the box was inserted beneath a hole, the rat could easily climb onto the platform. To neutralize olfactory cues that may emanate from the refuge box during trials, the seven other boxes, filled with soiled sawdust, were inserted beneath the other holes. The odors were therefore homogeneously distributed around the platform. A thin wire mesh, not visible to the animal from a distance because recessed relative to the platform surface, covered these incorrect boxes and prevented the rat from entering them. Because the starting hole was different on each trial, each box served as a transportation and refuge cage several times.
On the central part of the platform, 17 gray cylindrical cups (4 cm in diameter, 3 cm deep) were homogeneously distributed with one cup located at the center of the platform. Each cup could contain a food pellet (cylindrical: diameter 1.5 cm, length 1.5–2.0 cm) which could not be seen by the animal.
The apparatus was surrounded by a circular opaque white curtain that prevented rats from using visual room cues and was brightly illuminated by a toroidal 24W-neon, positioned above the platform. A camera located above the center of the platform and connected to a VCR allowed to videotape the trials. A radio tuned to an FM station was located near the camera and provided >70 dB background noise aimed at masking possible directional auditory cues. The VCR and monitor were located in an adjacent room. One experimenter stayed in this adjacent room while the other was performing the various manipulations necessary for testing the animals.
Behavioral procedure
After recovery from surgery, rats were placed on a food deprivation schedule: they received a minimal quantity of 5 g food pellets (Safe, Villemoisson-sur-Orge, France) until they reached 80% of their expected weight. Their weight loss was monitored daily. The rats were trained in the homing task and then tested in two successive types of trials, either with food always at center (FAC) or with food at random (FAR). After training and testing each day, the rats received additional pellets in their home cage. Throughout the study, the rats received water ad libitum.
Training
The aim was to train the animals in the basic aspects of the path integration task, i.e., get out of the refuge, climb on the platform, take a food pellet in the mouth and return to the refuge. Each cup contained one cylindrical food pellet whose size was appropriate to allow the rat to carry it in its mouth to the refuge. The animals received one trial a day for 20 days. We set a learning criterion to three successful trials in a row (pick up a pellet and return to the refuge). However, to be sure that the animals exhibited reliable homing behavior, they received additional trials up to 20 days. A typical trial started when the rat climbed onto the platform and ended when it returned to the refuge with a food pellet. Once in the refuge, the animal could eat the pellet before being carried back in its home cage. When the rat returned to an incorrect hole, it was allowed to self-correct until it found the correct one. The starting hole was pseudo-randomly chosen for each trial and the whole platform was carefully cleaned between trials to eliminate the possibility that the animal could rely on room cues and on local olfactory traces, respectively, to locate the correct hole. Training was completed when the rats exhibited consistent homing behavior, that is, they exited rapidly the refuge, they searched for a food pellet and carried it back to their cage.
Path integration testing
Testing was carried out in two successive types of trials. In the first type, the food pellet was always located in the central cup (food at center, “FAC” trials) while in the second type, the food pellet was placed in a different cup at each trial (food at random, “FAR” trials). The rats received two FAC trials a day for 10 days and one FAR trial a day for 17 days (corresponding to the 17 cups). In the two types of trial, the starting hole was pseudo-randomly chosen for each trial. In addition, because all rats were tested in a row for a given starting hole during FAC trials, the inter-trial interval for an animal within a day was approximately 3 h. An interesting observation is that, during FAC trials, rats did not exhibit straight trajectories toward the food cup, although the center of the circular platform was the only place that could be identified on the basis of external information (i.e., geometric information provided by the apparatus). We observed that, after the rat went out the hole, it usually explored the platform and the cups before getting the food pellet. Thus, that rats performed long and circuitous paths suggests an interaction between exploratory activity and goal-directed behavior.
The FAC and FAR situations are, in essence, similar to those used by Save et al. (2001). In the present experiment, one of our main objectives was to analyze the path integration capabilities of lesioned- and sham-operated rats. In particular, it is classically assumed that the efficacy of path integration is limited due to rapid accumulation of errors involving distance and direction. If this is true, we should observe that the inaccuracy or return paths decreases as the complexity of outward paths increases. To examine this hypothesis, we have tested rats in 20 FAC trials and 17 FAR trials, to obtain a large number of outward paths differing in distance and complexity. Correlation between outward path characteristics and path integration performance was performed.
Data analysis
All path integration testing trials were recorded on videotapes. For off-line analysis, we used an image analyzer (Image PC, Grenoble, France) connected to the video recorder to transform the trajectories into ASCII files. Each file consisted of a series of pairs of integer numbers (from 0 to 255) which represented the x-y position coordinates of the rat. Sampling was done at 12.5 samples per second (i.e., each 80 ms). The files were then stored in a computer for further analysis. Custom-built programs were used so as to extract the raw data for each trial and calculate several trajectory parameters: (1) the total length of outward paths (i.e., the distance covered by the rat until it found the food pellet), and (2) a complexity index of outward paths which was calculated as the total sum of changes in direction at each 10-cm step of the path. First, a tortuosity angle was calculated. Long straight curves decrease tortuosity while frequent and abrupt changes of direction increase tortuosity. The average deflection (or tortuosity) was calculated as an average angle of a vector change from one point to another. The points were defined by the trajectory x-y coordinates at a tortuosity step distance (10 cm) from the previous point, starting with the trajectory start point. Because the complexity of a trajectory must also take into account the distance, we calculated a complexity index equal to (distance × tortuosity)/10.
In addition, path integration performance was assessed by measuring the angular deviation from the correct hole and the number of errors (incorrect holes visited during returns). The number of errors is assumed to reflect the disorganization of the path integration system. When incidentally returning to an incorrect hole, an animal may not reset its path integration vector, hence allowing it to reach the correct hole at the second choice. In contrast, a rat with path integration impairment not only would return to an incorrect hole but also would be subsequently prone to visit holes at random or perform a stereotypic pattern of visits to correct its initial choice. Quantitative analyses were completed by a qualitative examination of the outward and return paths.
Group comparisons were made using analyses of variance and post-hoc (Newman-Keuls) tests. For comparing the angular deviation between groups, we considered the magnitude of deviation (i.e., absolute value) but not the sign (i.e., whether it was right or left relative to the starting hole). Thus, angular deviations ranged from 0 to 180° and were analyzed by linear statistics. In addition, we analyzed the distribution of the angular deviations for each group using circular statistics (Batschelet 1981).
Results
Histology
Entorhinal cortex lesions
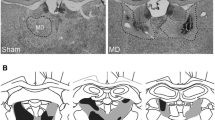
Figure 1 shows horizontal sections of a brain with reconstruction of the smallest and largest lesion extent in rats with entorhinal cortex lesions levels (adapted from Paxinos and Watson 1986). All rats sustained extensive bilateral lesions of the entorhinal cortex. The subiculum, presubiculum and the hippocampus were totally spared in all rats. Lesions slightly encroached upon the parasubiculum, medially at the most ventral level of the lesion, and the perirhinal cortex, laterally, at the most dorsal level of the lesion.
Horizontal sections showing maximal (light shading) and minimal (dark shading) extent of bilateral entorhinal lesions. From Paxinos and Watson (1986), Figs. 93–111. b Photomicrographs of horizontal sections in sham-operated and entorhinal-lesioned rats at two levels relative to bregma
Parietal cortex lesions
The extent of the lesion is shown in Fig. 2. Figure 2a represents a view from above of a rat brain where the lesioned area in all rats have been overlapped. This shows that the lesions were consistently placed from one animal to the other. Figure 2b shows the maximal and minimal extent of parietal lesions at five coronal levels (adapted from Paxinos and Watson 1986). The neocortical area, described as “area 7” by Krieg (1946), Kolb and Walkey (1987), and Reep et al. (1994) and assumed to be an associative area, was damaged. Fiber bundles such as the alveus or the cingulum were totally spared and, most importantly, no damage to the underlying hippocampus was observed in any of the brains.
Top view of a brain with parietal lesions in all eight rats superimposed. b Coronal sections showing maximal (light shading) and minimal (dark shading) extent of parietal lesions. From Paxinos and Watson (1986), Figs. 26–42
Behavior
Training
Rats were trained with all cups containing a food pellet. At the beginning of training, the rats remained in the refuge for several minutes before they climbed onto the platform. Once on the platform, they were often seen to explore the immediate surroundings of the hole, with frequent returns to the refuge. Later, they eventually came to explore the whole platform and take a pellet. Rats then carried back the pellet to a hole. In subsequent trials, they learned to correct an initial incorrect return. Thus, at the end of training, rats from the three groups exhibited reliable food carrying behavior. They exited the refuge rapidly, i.e., within 3 s, explored the platform, took a food pellet and returned to a hole. When the return was incorrect, rats visited other holes until they found the correct one. Analysis of the number of trials to attain the criterion (three successful trials in a row) revealed that there was no difference between groups (mean number of trials to criterion, SHAM: 9.55, ENTO: 12.72, PAR: 12.00; one-way ANOVA: F(2,25)=1.45, P>0.05).
Path integration testing
Several analyses revealed that there was no difference between the two sham groups, SHAM-PAR and SHAM-ENTO, in either distance, path complexity of outward paths or number of errors and angular deviation during returns in the FAC trials (non paired t-test: all ts9<1.7, NS). The two groups were therefore pooled into a single SHAM group (n=11) for all subsequent analyses.
Outward paths
FAC trials
Only the central cup was baited. Figure 3a shows the time-course of the distance run during the outward journey and of path complexity in the three groups during training.
A two-way analysis of variance with repeated measures (Group × Session) revealed a main effect of Group (F(2,25)=6.90; P<0.01), Session (F(9,225)=7.43,P<0.001), and a significant Group × Session interaction (F(18,225)=1.95,P<0.05) for distance. Subsequent post-hoc analyses (Newman-Keuls tests) indicated that the difference between groups could be attributed to the PAR group that run longer distance to reach the food cup than the SHAM group in day 1 (P=0.02). No difference was found for days 2–10 (all P>0.05). A two-way analysis of variance conducted on path complexity revealed no effect of Group (F(2,25)=2.87;P>0.05) and no significant Group × Session interaction (F(18,225)=1.58,P>0.05). There was, however, an effect of session (F(9,225)=8.36,P<0.001). Overall, these results indicate that all groups 1) improved their path efficacy across days—they exhibited shorter and less sinuous paths at the end of training than at the beginning, and 2) exhibited similar outward journeys at the end of training.
FAR trials
One-way analyses of variance conducted on the data averaged over the whole phase revealed no effect of group (distance: F(2,25)=0.65, P>0.05; path complexity: F(2,25)=0.16, P>0.05). This shows that rats from all groups performed similar outward trajectories (Fig. 3b).
Interestingly, in both FAC and FAR trials, the distance run was longer than the minimum distance between the starting hole and the rewarded cup (75 cm for the central cup and 83 cm in average for the other cups), showing that all groups exhibited complex trajectories (one sample t-tests, FAC trials: SHAM: t10=9.1, P<0.0001; PAR: t7=9; P<0.0001; ENTO: t 8 =12.3; P<0.0001; FAR trials: SHAM: t10=8.7, P<0.0001; PAR: t7=7.7; P<0.0001; ENTO: t8=11.9, P<0.0001).
Homing performance
The measures of performance, i.e., angular deviation ranging from 0 to 180° and number of errors, were averaged over trials. We thus compared path integration performance between the three groups for 17 possible food locations including the central food cup (37 trials per rat). Figure 4 shows the angular deviation (a) and number of errors (b) in the three groups. A one-way analysis of variance on angular deviation revealed a main effect of Group (F(2,25)=8.43, P<0.01). Post-hoc analyses (Newman-Keuls test) showed that both ENTO and PAR groups exhibited greater angular deviation than the SHAM group (ENTO vs. SHAM: P=0.002; PAR vs. SHAM: P=0.013). The ENTO group and the PAR group did not differ, however (P>0.05). A one-way analysis of variance on the number of errors revealed also a main effect of Group (F(2,25)=13.22, P<0.001). Post-hoc analyses (Newman-Keuls test) showed that both ENTO and PAR groups exhibited greater number of errors than the SHAM group (ENTO vs. SHAM: P=0.0002; PAR vs. SHAM: P=0.018). In addition, the ENTO group had greater number of errors than the PAR group (P=0.024). Thus, analyses of these two measures, angular deviation and number of errors, converge, to indicate that the ENTO group and the PAR group were less accurate during returns.
Homing accuracy was also assessed by analyzing the distribution of angular deviations (Fig. 5). For each rat, a mean vector length (r) and mean angle (φ) were calculated for the angular deviations averaged across FAC and FAR trials. Randomness of the distribution in each group was examined by using a second-order non-parametric test (Moore’s test, Batschelet 1981, p 212). The results revealed that SHAM rats displayed a directional bias in their return paths (D’=1.71, P<0.001). In contrast, the distribution of angular deviations in both ENTO and PAR rats was not different from a homogenous distribution over 360° (ENTO: D’=0.53, P>0.05; PAR: D’=1.04, P>0.05).
Distribution of angular deviations during homing in the three groups. Each dot represents the deviation angle averaged over FAC and FAR trials for each rat. The dotted line shows the direction of the correct hole. The vector indicates the mean deviation angle (φ) and the vector length (r) represents the concentration of the angles around the mean angle (SHAM: φ=3°, r=0.93; ENTO: φ=21°, r=0.31; PAR: φ=−3°, r=0.51)
Figure 6 shows examples of outward paths and returns for FAC and FAR trials in the three groups.
Correlation between outward path and return accuracy
Because it is assumed that the efficiency of path integration depends on the complexity of outward paths, we also examined the correlation between outward trajectory characteristics, i.e., path complexity, and path integration performance, i.e., angular deviation. The correlation was calculated on the basis of individual means for each group. Surprisingly, no significant correlation was found in the three groups (SHAM (n=11): r=−0.046, P>0.05; PAR (n=8): r=0.02, P>0.05; ENTO (n=9): r=0.11, P>0.05), suggesting that complex outward trajectories did not necessarily result in larger angular deviation than short paths.
Behavioral strategies
Observation of the return strategies revealed that the rats sometimes used an alternative strategy to correct an incorrect choice. They did not turn back to cross the environment toward another hole but followed the periphery of the platform, passing by successive holes until they reached the correct one. We found that ENTO rats adopted this strategy more frequently (38% of FAC and FAR trials) than SHAM rats (14%) and PAR rats (19%) (ENTO vs. SHAM: χ2=65.20, df=1, P<0.001; ENTO vs. PAR: χ2=23.28, df=1, P<0.001). In addition, PAR rats used this strategy more than SHAM rats (χ2=3.97, df=1, P<0.05). This behavior was observed in particular for large angular deviations. It thus cannot be considered as a stereotypy but rather probably reflects some adaptive mechanism. Indeed, it allowed the animals that made a very inaccurate return (>90°) resulting from a path integration impairment to reach more rapidly the correct hole than by using a random search.
Discussion
This study examined the contribution of the entorhinal and parietal cortices to path integration. Rats with either entorhinal cortical or parietal cortical lesions were trained in a homing task: they had to get out of a safe refuge, explore a large platform to find a hidden food pellet and carry it back directly to the refuge. All groups acquired the procedural aspects of the task. In contrast, analysis of the performance revealed that rats with entorhinal cortex lesions or parietal cortex lesions exhibited inaccurate returns. In addition, they visited more holes after they had made an incorrect return compared with control rats. Thus, both measures indicate that entorhinal cortex lesions and parietal cortex lesions affected homing behavior. Our results provide the first evidence that the entorhinal cortex is involved in homing based on path integration. Additionally, they confirm the contribution of the parietal cortex as previously reported in Save et al. (2001).
A number of studies using various laboratory settings have demonstrated that rodents are able to rely on path integration to return to a reference location (Etienne et al. 1996; Benhamou 1997, Whishaw and Tomie 1997; Alyan and McNaughton 1999). In particular, rats have been shown to be proficient in food carrying tasks in which they are motivated to return to a refuge with food by using motion-related information (Whishaw and Tomie 1997; Whishaw and Maaswinkel 1998; Maaswinkel and Whishaw 1999). In the present study, similar proficiency was observed in all groups. Indeed, they exhibited reliable food carrying behavior and rapidly learned to return to a hole. The fact that explicit environmental cues were concealed or made irrelevant supports the assumption that rats exclusively used idiothetic information, and therefore path integration to return to the refuge.
One theoretical property of the path integration system is that errors accumulate as the animal proceeds and eventually lead to inaccurate estimation of the starting point location. In other words, the accuracy of returns is supposed to be a function of the complexity of outward paths. Surprisingly, no correlation between path complexity of outward journey and angular deviation of returns was found in any group. Two hypotheses may account for this result. First, return performance would not be the product of pure path integration mechanism but may result from an interaction between this mechanism and the use of some environmental information. Although it is unlikely that rats could use some specific cue as a source of reliable information indicating the position of the correct hole, the animals’ path integration behavior may be influenced by the mere visual perception and knowledge of the working space as suggested by Whishaw and Brooks (1999). A second, more theoretical account is that the function between the complexity and the accuracy may not be linear. Thus, a correlation would be obtained for values of path complexity much higher that those measured in the present study. Specific studies would have to be conducted to examine these hypotheses. Note that Benhamou (1997) failed likewise to obtain a correlation between path complexity and path integration performance.
In test trials, return accuracy was found to be different across groups, indicating that entorhinal and parietal lesions disrupted path integration mechanisms. Thus, our results suggest that path integration requires activation of a number of brain structures, including the entorhinal and the parietal cortices. This complements other studies showing that the hippocampus plays a key role in path integration (for instance, Whishaw and Maaswinkel 1998; Maaswinkel et al. 1999). Together, these studies provide evidence that the processing of idiothetic information is not a specific attribution of the hippocampus but requires the involvement of several other brain areas. Thus, although the hippocampus receives movement-related information and in particular vestibular and optic flow information (Sharp et al. 1995), path integration computation could be performed, partly or totally, in other structures. This is consistent with data showing that vestibular information is processed upstream (Potegal 1982; Smith 1997). Thus, we subscribe to the idea that the hippocampal function is based on computation performed elsewhere in the brain and that path integration results from the involvement of a functional neural network. Within this network, the entorhinal cortex may play a pivotal role. There are various possible pathways allowing the entorhinal cortex to receive movement-related information and in particular vestibular information. One pathway may involve the projections from the parietal cortex (Nishiike et al. 2000) or from the thalamus (Fukushima 1997). Thus, vestibular information could reach the medial entorhinal cortex directly via projections from the parietal cortex or indirectly via the postrhinal cortex (Burwell and Amaral 1998) or the retrosplenial cortex (Reep et al. 1994). Another pathway may involve the vestibular nuclei, the lateral mammillary nuclei, the anterior thalamic nuclei, and the postsubiculum which, finally, sends projections to the entorhinal cortex (Taube et al. 1996). The functional specificity of these pathways is poorly understood but it indicates that the entorhinal cortex is a site of convergence of vestibular information. Our results show that this structure is involved in path integration, and thus are consistent with these anatomical considerations. They support the idea that the parietal cortex and the entorhinal cortex are part of a neural circuit that conveys and processes movement-related information.
In the present study, rats with entorhinal cortical lesions appeared to be somewhat more affected than rats with parietal cortical lesions since they displayed a larger number of errors. Analysis of the strategies for error correction revealed that rats with entorhinal lesions used a consecutive hole choice strategy to reach the correct hole in higher proportion than rats with parietal cortex lesions. The use of such a strategy is not without reminding the algorithm response pattern sometimes observed in the radial arm maze (Foreman and Ermakova 1998). In this perspective, it is interesting to relate our results to data showing that rats with lesions of the hippocampal formation (fimbria-fornix) adopt such a strategy in a radial arm maze task (Walker and Olton 1979). Should we observe that rats with hippocampal lesions display consecutive hole choice strategy in our task, this would suggest a close interaction between the entorhinal cortex and the hippocampus for path integration. However, this remains to be demonstrated. The consecutive hole choice strategy was much less prominent in rats with parietal cortex lesions, thus suggesting that the parietal cortex brings a distinct contribution than the entorhinal cortex and the hippocampus to path integration. Overall, our results are consistent with the view that the entorhinal cortex is a site of convergence for the output of various cortical areas that are suspected to play a role in path integration such as the parietal cortex (Save et al. 2001) or the retrosplenial cortex (Whishaw et al. 2001), and cooperates with the hippocampus to activate path integration mechanisms.
It is interesting to examine the present results in the light of recent theoretical and electrophysiological work suggesting that one function of the entorhinal cortex is to process movement-related information in relation with environmental cues. More specifically, it has been proposed that such combination may be used to assist the hippocampus in the formation of a spatial map of the environment (Sharp 1999) or to manage the interaction between internal and external frames of reference (Gothard et al. 2001). Our results are compatible with these views and the conjunction of these two sources of information that may occur in the entorhinal cortex is probably a fundamental function that underlies all spatial behaviors. The present results also suggest, in agreement with other data, that elucidating the neural bases of path integration requires taking into account a large network that goes beyond the hippocampal system. Further work should allow us to understand the functional nature of the interaction between the structures that belong to this network.
References
Alyan S, McNaughton BL (1999) Hippocampectomized rats are capable of homing by path integration. Behav Neurosci 113:19–31
Batschelet E (1981) Circular statistics in biology. Academic Press, New York
Barlow JS (1964) Inertial navigation as a basis for animal navigation. J Theor Biol 6:76–117
Benhamou S (1997) Path integration by swimming rats. Anim Behav 54:321–327
Biegler R (2000) Possible uses of path integration in animal navigation. Anim Learn Behav 28:257–277
Burwell RD, Amaral DG (1998) Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J Comp Neurol 398:179–205
Cooper BG, Manka TF, Mizumori SJY (2001) Finding your way in the dark: the retrosplenial cortex contributes to spatial memory and navigation without visual cues. Behav Neurosci 115:1012–1028
Etienne AS (1980) The orientation of the golden hamster to its nest-site after the elimination of various sensory cues. Experientia 36:1048–1050
Etienne A, Maurer R, Séguinot V (1996) Path integration in mammals and its interaction with visual landmarks. J Exp Biol 199:201–209
Foreman N, Ermakova I (1998) The radial arm maze: twenty years on. In: Foreman N, Gillett R (eds) Handbook of spatial research paradigms and methodologies, vol 2, clinical and comparative studies. Psychology Press, Hove (UK), pp 87–143
Fukushima K (1997) Corticovestibular interactions: anatomy, electrophysiology, and functional considerations. Exp Brain Res 117:1–16
Gallistel CR (1990) The organization of learning. MIT Press, Cambridge
Golob EJ, Taube JS (1999) Head direction cells in rats with hippocampal or overlying neocortical lesions: evidence for impaired angular path integration. J Neurosci 19:7198–7211
Gothard KM, Skaggs WE, McNaughton BL (1996) Dynamics of mismatch correction in the hippocampal ensemble code for space: interaction between path integration and environmental cues. J Neurosci 16:8027–8040
Gothard KM, Hoffman KL, Battaglia FP, McNaughton BL (2001) Dentate gyrus and CA1 ensemble activity during spatial reference frame shifts in the presence and absence of visual input. J Neurosci 21:7284–7292
Kolb B, Walkey J (1987) Behavioral and anatomical studies of the posterior parietal cortex in the rat. Behav Brain Res 23:127–145
Krieg WJS (1946) Connections of the cerebral cortex. 1. The albino rat. A. Topography of the cortical areas. J Comp Neurol 84:221–275
Maaswinkel H, Whishaw IQ (1999) Homing with locale, taxon, and dead reckoning strategies by foraging rats: sensory hierarchy in spatial navigation. Behav Brain Res 99:143–152
Maaswinkel H, Jarrard LE, Whishaw IQ (1999) Hippocampectomized rats are impaired in homing by path integration. Hippocampus 9:553–561
Mittelstaedt H, Mittelstaedt ML (1980) Homing by path integration in a mammal. Naturwissenschaften 67:566
Morris RGM (1981) Spatial localization does not require the presence of local cues. Learn Motiv 12:239–260
Nishiike S, Guldin W, Bäurle J (2000) Corticofugal connections between the cerebral cortex and the vestibular nuclei in the rat. J Comp Neurol 420:363–372
Paxinos G, Watson C (1986) The brain in stereotaxic coordinates. Academic Press, New York
Potegal M (1982) Vestibular and neostriatal contributions to spatial orientation. In: Potegal M (ed) Spatial abilities: development and physiological foundations. Academic Press, New York, pp 361–387
Potegal M (1987) The vestibular navigation hypothesis: a progress report. In: Ellen P, Thinus-Blanc C (eds) Cognitive processes and spatial orientation in animals and man II Nato ASI Series. Martinus Nijhoff, Dordrecht, pp 28–34
Reep RL, Chandler HC, King V, Corwin JV (1994) Rat posterior parietal cortex: topography of corticocortical and thalamic connections. Exp Brain Res 100:67–84
Save E, Moghaddam M (1996) Effects of lesions of the associative parietal cortex in the acquisition and use of spatial memory in egocentric and allocentric navigation tasks in the rat. Behav Neurosci 110:74–85
Save E, Guazzelli A, Poucet B (2001) Dissociation of the effects of lesions of the dorsal hippocampus and parietal cortex on path integration in the rat. Behav Neurosci 115:1212–1223
Sharp P (1999) Complementary roles for hippocampal versus subicular/entorhinal place cells in coding space, context and events. Hippocampus 9:432–443
Sharp PE, Blair HT, Etkin D, Tzanetos DB (1995) Influences of vestibular and visual motion information on the spatial firing pattern of hippocampal place cells. J Neurosci 15:173–189
Smith PF (1997) Vestibular-hippocampal interactions. Hippocampus 7:465–471
Taube JS, Goodridge JP, Golob EJ, Dudchenko PA, Stackman RW (1996) Processing the head direction cell signal: a review and commentary. Brain Res Bull 40:477–486
Walker JA, Olton DS (1979) Spatial memory following fimbria-fornix lesions: independent of time for stimulus processing. Physiol Behav 23:11–15
Whishaw IQ (1998) Place learning in hippocampal rats and the path integration hypothesis. Neurosci Biobehav Rev 22:209–220
Whishaw IQ, Brooks BL (1999) Calibrating space: exploration is important for allothetic and idiothetic navigation. Hippocampus 9:659–667
Whishaw IQ, Gorny B (1999) Path integration absent in scent-tracking fimbria-fornix rats: evidence for hippocampal involvement in “sense of direction” and “sense of distance” using self-movement cues. J Neurosci 19:4662–4673
Whishaw IQ, Jarrard LE (1996) Evidence for extrahippocampal involvement in place learning and hippocampal involvement in path integration. Hippocampus 6:513–524
Whishaw IQ, Maaswinkel H (1998) Rats with fimbria-fornix lesions are impaired in path integration; a role for the hippocampus in “sense of direction”. J Neurosci 18:3050–3058
Whishaw IQ, Tomie J-A (1997) Piloting and dead reckoning dissociated by fimbria-fornix lesions in a rat food carrying task. Behav Brain Res 89:87–97
Whishaw IQ, Maaswinkel H, Gonzalez CLR, Kolb B (2001) Deficits in allothetic and idiothetic spatial behavior in rats with posterior cingulate cortex lesions. Behav Brain Res 118:67–76
Wiener SI, Korshunov VA, Garcia R, Berthoz A (1995) Inertial substratal and landmark cue control of hippocampal CA1 place cell activity. Eur J Neurosci 7:2206–2219
Zilles K (1985) The cortex of the rat: a stereotaxic atlas. Springer-Verlag, Berlin
Acknowledgements
This research was supported by a grant from the French Ministry of Education and Research. We thank B. Poucet for stimulating discussion, H. Lucchessi for histological work, and two anonymous referees for their insightful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parron, C., Save, E. Evidence for entorhinal and parietal cortices involvement in path integration in the rat. Exp Brain Res 159, 349–359 (2004). https://doi.org/10.1007/s00221-004-1960-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-004-1960-8