Abstract
Goal-directed behaviors are thought to be supported by a neural circuit encompassing the prefrontal cortex, the dorsomedial striatum, the amygdala, and, as more recently suggested, the limbic thalamus. Since evidence indicates that the various thalamic nuclei contribute to dissociable functions, we directly compared the functional contribution of the mediodorsal thalamus (MD) and of the anterior thalamic nuclei (ATN) in a new task assessing spatial goal-directed behavior in a cross-maze. Rats sustaining lesions of the mediodorsal or the anterior thalamus were trained to associate each of the two goal arms with a distinctive food reward. Unlike control rats, both lesioned groups failed to express a bias for the goal arm corresponding to the non-devalued outcome following devaluation by sensory-specific satiety. In addition, MD rats were slower than the other groups to complete the trials. When tested for spatial working memory using a standard non-matching-to-place procedure in the same apparatus, ATN rats were severely impaired but MD rats performed as well as controls, even when spatial or temporal challenges were introduced. Finally, all groups displayed comparable breaking points in a progressive ratio test, indicating that the slower choice performance of MD rats did not result from motivational factors. Thus, a spatial task requiring the integration of instrumental and Pavlovian contingencies reveals a fundamental deficit of MD rats in adapting their choice according to goal value. By contrast, the deficit associated with anterior thalamic lesions appears to simply reflect the inability to process spatial information.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To survive in a changing environment, organisms have to make responses adapted to the goal pursued. This requires the integration of events prediction and response control capabilities according to Pavlovian and instrumental contingencies, respectively (Balleine et al. 1994; Holmes et al. 2010). In both rodents and primates, including humans, instrumental conditioning can capture many of the basic cognitive mechanisms of goal-directed behavior through tests assessing the subjects’ ability to adapt to change in goal value (Balleine and Dickinson 1998; Dickinson 1985; Tanaka et al. 2008; Valentin et al. 2007).
A number of recent studies in rodents have shed light on the network of cortical and subcortical structures underlying goal-directed actions. Indeed, lesions (or inactivation) of the dorsomedial striatum (Corbit and Janak 2010; Yin et al. 2005), the basolateral nucleus of the amygdala (Balleine et al. 2003; Coutureau et al. 2009; Parkes and Balleine 2013) or the prelimbic region (PLC) of the medial prefrontal cortex (Corbit and Balleine 2003; Killcross and Coutureau 2003; Ostlund and Balleine 2005; Tran-Tu-Yen et al. 2009), render subjects insensitive to outcome devaluation treatments. All the aforementioned brain areas share abundant connections with limbic thalamic nuclei (Gabbott et al. 2005; Groenewegen 1988; Hoover and Vertes 2007) such as the anterior thalamic nuclei (ATN) and mediodorsal (MD) nuclei. While the ATN is known to support spatial navigation (Jankowski et al. 2013; van Groen and Kadish 2002; Warburton et al. 1999, 2001; Wilton et al. 2001; Wolff et al. 2008a, b), the role of MD in goal-directed behavior has been the focus of recent studies in essentially non-spatial, instrumental settings (Mitchell et al. 2007; Ostlund and Balleine 2008; Pickens 2008). Still few studies have directly compared the differential impact of lesion to either the ATN or the MD on goal-directed behavior and none of them involved processing of spatial information (Corbit et al. 2003).
The present study, therefore, aims at providing a more general understanding of the respective contributions of the ATN and the MD on goal-directed behaviors under conditions where subjects must use spatial information to guide behavior. To this end, we directly compare the performance of ATN and MD rats in a new goal-directed spatial task in a cross-maze which requires the use of the representation of goal value to guide spatial behavior (experiment 1), and show that control, but not lesioned rats avoid choosing an arm associated with a food reward when the corresponding food is devalued. We furthermore show that spatial working memory is impaired by ATN but not MD lesions in a reinforced spatial alternation procedure (experiment 2). We finally verify that the lesions do not alter motivation in an instrumental progressive ratio task.
Materials and methods
Animals and housing conditions
Thirty three male Long-Evans rats obtained from Centre d’Elevage Janvier (France) were used (weight 275–325 g at surgery). Rats were initially housed in pairs and accustomed to the laboratory facility for 2 weeks before the beginning of the experiments. The facility was maintained at 21 ± 1 °C with lights on from 7 a.m. to 7 p.m. and rats were tested only during the light portion of the cycle. The experimental protocols received approval # 5012035-A from the Ethics Committee on December 7, 2012, in accordance with current French (council directive 2013-118, February 1, 2013) and international (directive 2010-63, September 22, 2010, European Community) laws and policies regarding animal experiments.
Surgery
Rats were anesthetized with 4 % Isoflurane and placed in a stereotaxic frame with atraumatic ear bars (Kopf, Tujunga, CA) in a flat skull position. Anesthesia was maintained with 1.5–2 % Isoflurane complemented by subcutaneous administration of diazepam (0.2 ml of Valium© per rat) and Carprofen (Norocarp©, 5 mg/kg). Neurotoxic ATN lesions were made using multiple NMDA micro-injections. 20 µg/µl NMDA (Sigma-Aldrich) in artificial cerebrospinal fluid (CMA Microdialysis AB, Solna, Sweden) was pressure injected into the brain through a glass micropipette (outside diameter: around 100 µm) and polyethylene tubing (Picospritzer, General Valve Corporation, Fairfield, NJ, USA). Two lesion sites per side were used: AP −1.4 mm from bregma, laterality ±1.2 mm, ventrality −5.4 mm from dura; and AP −1.5 mm, laterality ±1.5 mm and ventrality −5.3 mm. Each site was injected with 0.12 µl of NMDA. Neurotoxic MD lesions were made in the same fashion, with one lesion site per side at the following coordinates: AP: −3.1; L: ±1.0; DV: −4.9. Each site was injected with 0.18 µl of NMDA. In all cases, the pipette was left in place 3 min after injection before slow retraction. The Sham groups received similar surgery except that the micropipette was lowered only in the cortex and no injection was made (ventrality −2.0 mm). Rats were given at least 10 days of recovery before behavioral testing.
Experiment 1: goal-oriented spatial behavior
Behavioral apparatus
Experiment 1 was performed using a maze located in a room containing numerous distal cues. The maze was used in a T-maze configuration, embedded in a cross-maze raised 75 cm above the floor. The PVC runways were 13 cm wide and painted gray, with 5 cm-high transparent plastic edges. The two start arms were 100 cm long with a guillotine door located 15 cm from each end to create a North (N) and a South (S) start area. The two goal arms were 40 cm long, the end of which included a raised plastic food well (2.5 cm diameter, 1 cm deep). Guillotine doors (13 cm wide by 30 cm high) were used to restrict access to any arm. Diffuse lighting was provided by one overhead light.
Behavioral procedures
Habituation
All rats were initially acclimated to the maze over four 20 min sessions during which they freely explored the maze with various configurations of open or closed doors and with food rewards available on all arms.
Training
During this phase, the rats were trained to associate each arm of the maze with the retrieval of a distinctive food reward. Throughout training, the left goal arm was associated with grain pellets and the right goal arm with sucrose pellets as reward. Training proceeded over 2 days, two sessions each day, with one session taking place in the morning and the other in the afternoon. Each session was composed of ten forced-choice trials with the same goal arm–reward arrangement, the alternate arrangement being used on the other session of the same day. Trial began with the placement of the rat in the start arm. The door was then raised and the rat was allowed to enter one of the arms, the other being kept closed. Each trial ended when the rat had eaten the reward or when 2 min had elapsed. The animal was then returned in the start arm, and the following trial began.
Test following outcome devaluation by sensory-specific satiety
The day following the last training session, the rats were tested after being sated on one of the food rewards. The animals were individually placed in one of eight Perspex cages (42 × 28 × 20 cm) located in a separate room. A glass dish (7.5 cm in diameter) containing food pellets could be fixed on the floor of each cage. During 1 h, the animals could freely consume 20 g of either grain or sucrose pellets (counterbalanced between rats and groups). Immediately after devaluation, the animals were placed in the start arm of the maze, the door was raised and the two goal arms were left open but contained no reward. Each trial ended when the rats entered one of the goal arms, or after 2 min elapsed. The rats were immediately placed in the start arm for a new trial. The test session comprised 5 trials.
Consumption test
To ensure that devaluation was effective and sensory specific, a consumption test was conducted immediately after testing. The animals were transferred to the consumption cages and allowed access to 10 g of each of the two rewards successively (the order of the pre-fed and the non-pre-fed reward being counterbalanced across animals) for 15 min.
Experiment 2: spatial working memory
Behavioral apparatus
Experiment 2 was performed in the same apparatus as in Experiment 1.
Behavioral procedures
Acquisition
Acquisition was conducted over 6 consecutive daily sessions of six trials. Each trial began with a forced “sample” run in one of the two arms. Correct performance on the subsequent test run required the rat to choose the alternate arm from that previously visited during the sample run (i.e., both arms were now accessible).
To ensure that the rats were not simply using an egocentric strategy (alternating body turn) from sample to test runs, a pseudorandom half of the trials used the opposite start area across the “sample” and “test” runs. The other half of the daily trials used the same start area. On the sample run, the rat was placed in the start area, the door raised and the rat allowed to enter the open arm, where it was confined for approximately 10 s while it ate a single food pellet. It was then picked up and returned to the appropriate start area. The door was then raised for the test run and the rat was allowed a free choice between the two maze arms. If the rat chose the previously blocked arm (non-matching alternation) it was rewarded with three food pellets, confined in that arm for about 10 s while it ate the reward and returned to the home cage. If the rat returned to the arm previously visited on the sample run it was confined to that arm for 10 s without reward and returned to the home cage. Each rat experienced a pseudorandom sequence of correct arm choices (left or right), start position for the sample run, and same versus opposite start position for the test run, which varied across rats and sessions.
After 6 days of acquisition, we evaluated the resistance of spatial working memory to temporal interferences. To this end, a 90 s delay was introduced between the sample and the test runs and performance was evaluated under these conditions for two consecutive days (12 trials in total).
Experiment 3: progressive ratio
Behavioral apparatus
Animals were trained in eight identical conditioning chambers (40 cm wide × 30 cm deep × 35 cm high, Imetronic, France), each located inside a sound and light-attenuating wooden chamber (74 × 46 × 50 cm). Each chamber had a ventilation fan producing a background noise of 55 dB and four LEDs on the ceiling for illumination of chamber. Each chamber had two opaque panels on the right and left sides, two clear Perspex walls on the back and front sides and a stainless steel grid floor (rod diameter: 0.5 cm; inter-rod distance: 1.5 cm). In the middle of the left wall, a magazine (6 × 4.5 × 4.5 cm) received food pellets (45 mg, F0165, Bio_Serv, NJ, USA) from a dispenser located outside the operant chamber. Each magazine was equipped with infra-red cells to detect the animal’s visits. A retractable lever (4 × 1 × 2 cm) could be inserted next to each of the magazines. A personal computer connected to the operant chambers via an Imetronic interface and equipped with SKAA_PROG software (Imetronic, France) controlled the equipment and recorded the data.
Behavioral procedures
Magazine training
Initially, all rats were trained for one 30 min session to collect the food pellet rewards which were delivered on a random time 60 s schedule.
Instrumental training
All rats were then trained to press the lever to obtain a reward during four 30 min-long instrumental training sessions. The cage was illuminated and the lever inserted during the duration of the whole session. Different reinforcement schedules were used. The rats first received training for 2 days under a continuous reinforcement, fixed ratio 1 schedule (FR1, i.e., each lever press was rewarded) until they had earned 30 pellets or 30 min had elapsed. During the next two sessions, the animals were shifted to a fixed ratio 5 schedule (FR5, i.e., lever press was rewarded only following 5 consecutive lever presses).
Progressive ratio
Following training under the FR5 schedule, rats were tested for motivation under a progressive ratio (PR) schedule. In this test, the number of lever presses required to earn each successive reward was incremented by steps of 3, starting at 1 (i.e., 1 press for the first reward, 4 presses for the second reward, 7, 10, 13, etc., for the next ones). Each reward was a single food pellet. The breaking point was defined as the last ratio completed before the effort required caused the animal to cease responding.
Histology
Animals received a lethal dose of sodium pentobarbital and were perfused transcardially with 150 ml of saline followed by 400 ml of 10 % formalin. The sections throughout the ATN and the MD regions were collected onto gelatine coated slides and dried before being stained with thionine. Histological analysis of the lesions was performed under the microscope by two experimenter (MW & FA) blind to lesion conditions.
Data analysis
The data were submitted to ANOVAs on StatView® software (SAS Institute Inc.) with Lesion (Sham/ATN/MD) and Devaluation (Devalued/Non-Devalued) as between subject factors and Trial type (Same start/Opposite start), Delay (no Delay/90 s), Session, Trial, or Time as repeated measures when appropriate. The alpha value for rejection of the null hypothesis was 0.05 throughout.
Results
Histology
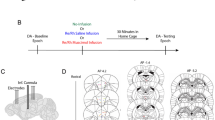
The ATN and MD lesions are shown in Fig. 1. Two ATN and three MD rats had only minimal damage and were discarded from further analysis. The ATN lesions were highly specific, comparable to previous work (Dupire et al. 2013; Marchand et al. 2013; Wolff et al. 2006, 2008a). Damage to other non-target thalamic structures including midline nuclei was generally minimal, with the exception of the inter-anteromedial nucleus, the centromedial and the parataenial nucleus; there was minor damage to the laterodorsal nucleus and negligible damage for paraventricular and posterior paraventricular nuclei, anterior paraventricular nucleus, reuniens nucleus, and rhomboid nuclei. In a few cases, minor damage was apparent in the most rostral portion of the MD. The thin glass micropipette caused no detectable mechanical injury to the fornix and hence the latter does not influence the behavioral profile of ATN rats reported here (see Fig. 1a). The MD lesions were deliberately performed slightly more posterior than usually in more conventional MD studies to avoid any damage to the ATN region. As a result, the most rostral portion of the MD was often spared by the lesion and the ATN was intact in all included MD rats. Substantial damage was, however, apparent at the level of the MD, including the lateral, the central, and the medial segments. Additional damage was apparent at the level of the intralaminar nuclei, more especially the centrolateral and the paracentral nuclei. Modest damage also occurred in the centromedian nuclei while it was negligible in the midline thalamic nuclei. A few cases exhibited minor damage to the ventral blade of the dentate gyrus but these animals behaved as the others from that group. Moreover, two rats (1 in group Sham and 1 in group ATN) had to be euthanized due to serious illness. The final groups were, therefore, as follows: Sham (n = 9); ATN (n = 9); MD (n = 8).
a Representative photomicrographs of the ATN (upper panels) and MD regions (lower panels) in Sham (left) and lesioned rats (right). Anterodorsal (AD), anteroventral (AV), and anteromedial (AM) damage is evident on this example (boundary of the lesion depicted by the dotted line) but the fornix is intact. b Representation of the included largest (gray) and smallest (black) ATN (upper panel) and MD (lower panel) at three different levels of the antero-posterior axis (indicated in mm relative to Bregma)
Experiment 1: goal-oriented spatial behavior
Training
During training, all rats learned to collect the reward at the end of the goal arm as indicated by the decreased latency across the two sessions of training. There was indeed a main effect of Session [F(1,23) = 9.91; P = 0.045] and no significant effect of Lesion [F(2,23) = 2.52; ns] or Session × Lesion interaction [F(2,23) <1; ns] was found.
Test
Figure 2a shows the performance of the rats during the 5 trials, as a proportion of choice between the goal arm associated with the devalued reward and the goal arm associated with the non-devalued reward. As shown, the results are clear for the Sham group since this group biased its choice toward the goal arm associated with the non-devalued reward, therefore demonstrating that satiety-induced devaluation was efficient in affecting choice performance during test. Such an effect was not present in both ATN and MD groups which both displayed a similar choice for the two goal arms, irrespective of devaluation. This description of the data was confirmed by an ANOVA showing a main effect of Lesion [F(2,23) = 6.56; P = 0.006]. In addition, the performance of Sham, but not ATN nor MD rats, was significantly above the 50 % chance level [t(8) = 3.25; P = 0.011, t(8) < 1; n.s. and t(7) < 1; n.s., respectively].
The altered performance in MD and ATN rats was associated with different latencies to reach the goal across the five trials of the test, as shown on Fig. 2b. The latencies increased throughout the test as confirmed by the statistical analysis which showed a main effect of Trial [F(4,23) = 7.01; P < 0.001]. More importantly, however, there was a main effect of Lesion [F(2,23) = 3.67; P = 0.041], but no Trial × Lesion interaction [F(8,23) < 1; ns]. Separate analyses in the lesion groups showed that the MD rats were slower than the ATN rats to reach the goal [P = 0.013]. This distinctive behavior of MD rats is consistent with the notion that they have difficulties in choosing an arm on the basis of a representation of the outcome. However, it could also reflect an effect of the lesion on motivational control, which is assessed directly in Experiment 3.
Consumption test
The result of the consumption test confirmed that the pre-feeding treatment induced a specific satiety in all three groups (Table 1). All animals rejected the sated reward but consumed high quantities of the non-sated reward. The ANOVA shows a significant effect of the Devaluation factor [F(1,23) = 6.97; P = 0.015], but no significant effect of Lesion [F(2,23) <1; ns] nor any significant Lesion × Devaluation interaction [F(2,23) <1; ns]. These results confirm devaluation is effective in all groups and specific for one reward; i.e., groups differentiate correctly the non-devalued from the devalued reward.
Experiment 2: spatial working memory
Acquisition
Figure 3a shows the performance of the different groups during the acquisition phase of reinforced spatial alternation in the cross-maze. While both Sham and MD rats exhibited high levels of performance, ATN rats performed poorly without showing any sign of improvement throughout testing. These observations were confirmed by an ANOVA conducted on the performance with the factors Lesion (Sham, MD, ATN) and Time (3 blocks), indicating an highly significant effect of Lesion [F(2,23) = 22.26, P < 0.001] but not Time [F(2,23) <1; ns] and no interaction [F(2,23) <1; ns]. A post hoc Fisher analysis confirmed that ATN rats performed at lower levels than both Sham and MD rats (Ps < 0.001), which did not differ from each other (P = 0.56).
a Percent of correct trials during the acquisition phase of the spatial working memory task (3 blocks of 2 sessions, 10 trials per session) for Sham (gray), ATN (white), and MD (black) groups. b Percent of correct trials when the starting point during the test run is the same or the opposite than during the sample run. c Percent of correct trials for the two delay conditions. Data are expressed as mean ± SEM
To ensure that the spatial working memory task could not be solved by the sole use of an egocentric strategy, half of the trials began in the opposite start point for the choice run. These trials have previously been shown to be more challenging (Loukavenko et al. 2007). Figure 3b shows the overall performance of the three groups of rats during the acquisition period averaged as a function of trial type (same vs. opposite start). The performance of Sham and MD rats indeed appeared to be slightly decreased when facing the more challenging “opposite start” trials, but ATN rats performed poorly on both types of trial. The ANOVA conducted on these data with the factors Lesion (Sham, MD, ATN) and Trial Type (Same, Opposite) yielded highly significant effect of Lesion [F(2,23) = 21.49, P < 0.001] and Trial type [F(1,23) = 7.44, P = 0.012]. The post hoc Fisher analysis confirmed that ATN rats were impaired relative to both the Sham and MD groups (Ps < 0.001), which did not differ from each other (P = 0.64). Furthermore, the significant Lesion × Trial type interaction [F(2,23) = 5.98, P = 0.008] confirmed decreased performance in Sham [F(1,8) = 16.79, P = 0.003] but not in ATN rats [F(1,8) <1; ns] when facing a spatial challenge, presumably because the performance of the latter group was already severely impaired even for the easier “same start” trials. MD rats exhibited a similar trend than Sham rats at this occasion [F(1,7) = 4.30, P = 0.079].
Delay
To confirm that spatial working memory was severely impaired by ATN but not MD lesions, we then assessed the resistance of such memory to temporal interferences. Figure 3c shows the overall performance of rats with and without a 90 s delay between the sample and the choice runs. As expected, the introduction of a delay produced an overall decrease in the performance of all rats. ATN rats continued to perform poorly even without delay. The ANOVA conducted on the performance with the factors Lesion (Sham, MD, ATN) and Delay (no delay, 90 s) produced a highly significant effect of Lesion [F(2,23) = 14.30, P < 0.001] and Delay [F(1,23) = 4.94, P = 0.036] but no interaction [F(2,23) <1; ns]. Again, post hoc analysis revealed considerably impaired performance in ATN versus both Sham (P < 0.001) and MD (P < 0.001) rats but could not differentiate the Sham and the MD groups (P = 0.98).
Taken together, these results indicate a major impairment in ATN but not MD rats. While ATN rats were near chance level throughout the entire period of testing and irrespective of testing conditions, MD rats performed consistently accurately and could not be distinguished from Sham rats even when facing a spatial challenge or temporal interferences.
Experiment 3: progressive ratio
Instrumental training
The three groups of animals acquired the instrumental response at the same rate, as shown on Fig. 4a. A mixed ANOVA with Session and Lesion as factors showed an effect of Session [F(3,21) = 103.0; P < 0.001], but no effect of Lesion [F(2,21) <1; ns] nor any significant Lesion × Session interaction [F(2,21) = 0.91; ns], indicating that lesions of the MD or the ATN had no effect on the acquisition of the instrumental response.
a Mean number of lever presses during the fixed ratio 1 (FR1) and fixed ratio 5 (FR5) sessions of instrumental conditioning for Sham (gray), ATN (white), and MD (black) groups. b Mean number of lever presses during the progressive ratio (PR) test represented in 10 min bins. c Breaking point reached during the PR test. Data are expressed as mean ± SEM
Progressive ratio
When the requirement to obtain a reward increases, the lever pressing rate decreases as shown on Fig. 4b. This decrease is similar in the three groups as demonstrated by an ANOVA which showed a significant effect of Time [F(5,21) = 67.26; P < 0.001] but no effect of Lesion [F(2,21) <1; ns] or interaction between these two factors [F(2,21) <1; ns].
A similar conclusion is also reached when considering at the breaking point which corresponds to the maximum of lever presses that the animals performed to obtain the reward. As shown on Fig. 4c, this measure showed no effect of Lesion [F(2,21) <1; ns].
Discussion
The present study investigated the involvement of limbic thalamic nuclei in the control of spatial goal-directed behavior. The results demonstrate a clear dissociation between ATN and MD nuclei. Indeed, MD lesions were shown to abolish the sensitivity of spatial response to outcome devaluation without markedly impacting spatial memory, whereas ATN lesions were shown to affect spatial tasks indiscriminately. These results have important implications for our understanding of the neural circuit of goal-oriented behavior, which are discussed below.
The role of thalamic nuclei in goal representation
The results obtained in the Sham-operated group show that following sensory-specific satiety, the animals selectively decreased their entries in the arm which was previously associated with the devalued reward, which is consistent with previous findings (Babb and Crystal 2006; Smith et al. 2012). The associative structure involved in this task is probably the result of a mixture of both Pavlovian and instrumental contingencies. Such a response might reflect a change in approach behavior resulting from the encoding of the Pavlovian relationship between the place and a specific reward (Hayen et al. 2014). However, this change in response control might also reflect the instrumental encoding of the relationship between the action (specific body turn in the maze)-outcome (A-O) association (Balleine and Dickinson 1998; Killcross and Coutureau 2003).
The failure of animals with lesions of the MD to show any sensitivity to devaluation is consistent with previous data, which used either pure instrumental (Corbit et al. 2003; Ostlund and Balleine 2008; Pickens 2008) or Pavlovian (Pickens 2008) setups, which all indicate that the MD plays a general role in the representation of outcome value, resulting from Pavlovian or instrumental contingencies. It must be noted that this deficit is unlikely to result from a deficit in discriminating the devalued vs. non-devalued rewards since MD rats behaved as the control Sham animals during the consumption test which followed devaluation. This deficit is also unlikely to be the result of general changes in the motivational control of action since MD rats displayed a similar breaking point as assessed in a progressive ratio procedure (see “Experiment 3”). These results may be integrated in the broader framework of neural circuits underlying response control. Indeed, previous research has demonstrated a major dissociation among prefrontal areas innervated by the MD (Groenewegen 1988; Hoover and Vertes 2007). This research has shown the medial prefrontal and the orbitofrontal cortex to be key elements of the circuit mediating the use of instrumental value (Corbit and Balleine 2003; Coutureau et al. 2009; Killcross and Coutureau 2003; Tran-Tu-Yen et al. 2009), and Pavlovian value (McDannald et al. 2014), respectively.
In our spatial goal-directed task ATN lesions appear to produce similar deficits as MD lesions. Given the available data, it is, however, unlikely that such an effect results from similar cognitive deficits. Indeed, previous findings have demonstrated that ATN lesions did not impact the sensitivity to instrumental devaluation (Corbit et al. 2003). In addition, available data suggest that ATN rats, like Sham rats, are able to attribute a predictive value to both discrete and complex Pavlovian stimuli (Aggleton et al. 1996, 2011; Dupire et al. 2013; Ward-Robinson et al. 2002). The impact of ATN lesions on changing outcome value in a pure Pavlovian setup is, however, unknown and further studies are warranted. Nevertheless, since ATN rats are sensitive to devaluation in an instrumental setup (Corbit et al. 2003), the most parsimonious interpretation is that ATN deficits do not originate from an inability to use outcome value to guide behavior but rather from an initial failure of the place–reward encoding resulting from a major deficit in the encoding of spatial information.
The role of thalamic nuclei in spatial memory
Spatial working memory was assessed by a non-matching to place procedure known to be sensitive to hippocampal system damage (Aggleton et al. 2009; Warburton et al. 2000, 2001). In particular, the detrimental effect of ATN lesions on such spatial reinforced alternation is well documented (Aggleton et al. 2011; Warburton et al. 1997; Wilton et al. 2001). Furthermore, the use of a cross-maze enables to vary the start point form the sample to the test runs, which limits the efficacy of egocentric strategies and encourages the animals to use distal cues to guide behavior. Under these conditions, expected to particularly challenge hippocampal functions, the spatial impairment in ATN rats is known to be even more pronounced (Aggleton et al. 1996; Loukavenko et al. 2007; Ulrich et al. 2014). The present data fully confirmed this major deficit as ATN rats performed very poorly throughout all phases of testing without showing any sign of improvement. They actually performed so poorly that, contrary to Sham and MD rats, their performance did not degrade further when a delay was introduced between the sample and the test runs. By comparison, the performance of MD rats could not be distinguished from that of Sham rats. They achieved high level of performance even when confronted to spatial or temporal challenges. MD lesions were previously reported to spare performance in such non-matching to place procedure (Hunt and Aggleton 1998a; Hunt et al. 1994) but to our knowledge, this study is the first to provide a full and explicit comparison between the effects of both thalamic lesions. Our data, therefore, unequivocally indicate a role for the ATN but not the MD in spatial working memory. Interestingly, one study reported that MD lesions produced a deficit when rats were required to perform a matching to place procedure, which is contrary to the animals’ innate preference to alternate (Hunt and Aggleton 1998b). However, this impairment was not interpreted as a working memory deficit but as an increase in perseverative behavior, possibly retarding task acquisition.
Conclusion
Altogether, the present data, therefore, formally demonstrate the existence of a major functional dissociation within the limbic thalamus in the control of spatially guided goal-directed behavior. These results emphasize the crucial role of the MD in the representation of outcome value to guide behavior. Given that these thalamic nuclei share abundant anatomical connections with both frontal and temporal regions, which have also been involved in goal-directed behavior, it is of great importance to clarify the specific contribution of the MD. Indeed, given that this brain region has been largely involved in many psychiatric diseases, such as schizophrenia, animal studies can be expected to greatly enhance our understanding the neural circuit of goal-directed behavior, which rely upon homologous regions in rodents and higher primates (Balleine and O’Doherty 2010).
References
Aggleton JP, Hunt PR, Nagle S, Neave N (1996) The effects of selective lesions within the anterior thalamic nuclei on spatial memory in the rat. Behav Brain Res 81:189–198
Aggleton JP, Poirier GL, Aggleton HS, Vann SD, Pearce JM (2009) Lesions of the fornix and anterior thalamic nuclei dissociate different aspects of hippocampal-dependent spatial learning: implications for the neural basis of scene learning. Behav Neurosci 123:504–519. doi:10.1037/a0015404
Aggleton JP, Amin E, Jenkins TA, Pearce JM, Robinson J (2011) Lesions in the anterior thalamic nuclei of rats do not disrupt acquisition of stimulus sequence learning. Q J Exp Psychol (Hove) 64:65–73. doi:10.1080/17470218.2010.495407
Babb SJ, Crystal JD (2006) Episodic-like memory in the rat. Curr Biol 16:1317–1321. doi:10.1016/j.cub.2006.05.025
Balleine BW, Dickinson A (1998) Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology 37:407–419
Balleine BW, O’Doherty JP (2010) Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology 35:48–69. doi:10.1038/npp.2009.131
Balleine B, Ball J, Dickinson A (1994) Benzodiazepine-induced outcome revaluation and the motivational control of instrumental action in rats. Behav Neurosci 108:573–589
Balleine BW, Killcross AS, Dickinson A (2003) The effect of lesions of the basolateral amygdala on instrumental conditioning. J Neurosci 23:666–675
Corbit LH, Balleine BW (2003) The role of prelimbic cortex in instrumental conditioning. Behav Brain Res 146:145–157
Corbit LH, Janak PH (2010) Posterior dorsomedial striatum is critical for both selective instrumental and Pavlovian reward learning. Eur J Neurosci 31:1312–1321. doi:10.1111/j.1460-9568.2010.07153.x
Corbit LH, Muir JL, Balleine BW (2003) Lesions of mediodorsal thalamus and anterior thalamic nuclei produce dissociable effects on instrumental conditioning in rats. Eur J Neurosci 18:1286–1294
Coutureau E, Marchand AR, Di Scala G (2009) Goal-directed responding is sensitive to lesions to the prelimbic cortex or basolateral nucleus of the amygdala but not to their disconnection. Behav Neurosci 123:443–448. doi:10.1037/a0014818
Dickinson A (1985) Actions and habits: the development of behavioural autonomy. Phil Trans R Soc Lond B 308:67–78
Dupire A, Kant P, Mons N, Marchand AR, Coutureau E, Dalrymple-Alford J, Wolff M (2013) A role for anterior thalamic nuclei in affective cognition: interaction with environmental conditions Hippocampus 23:392–404. doi:10.1002/hipo.22098
Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ (2005) Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol 492:145–177. doi:10.1002/cne.20738
Groenewegen HJ (1988) Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience 24:379–431
Hayen A, Meese-Tamuri S, Gates A, Ito R (2014) Opposing roles of prelimbic and infralimbic dopamine in conditioned cue and place preference. Psychopharmacology. doi:10.1007/s00213-013-3414-0
Holmes NM, Marchand AR, Coutureau E (2010) Pavlovian to instrumental transfer: a neurobehavioural perspective. Neurosci Biobehav Rev 34:1277–1295. doi:10.1016/j.neubiorev.2010.03.007
Hoover WB, Vertes RP (2007) Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct 212:149–179. doi:10.1007/s00429-007-0150-4
Hunt PR, Aggleton JP (1998a) An examination of the spatial working memory deficit following neurotoxic medial dorsal thalamic lesions in rats. Behav Brain Res 97:129–141
Hunt PR, Aggleton JP (1998b) Neurotoxic lesions of the dorsomedial thalamus impair the acquisition but not the performance of delayed matching to place by rats: a deficit in shifting response rules. J Neurosci 18:10045–10052
Hunt PR, Neave N, Shaw C, Aggleton JP (1994) The effects of lesions to the fornix and dorsomedial thalamus on concurrent discrimination learning by rats. Behav Brain Res 62:195–205
Jankowski MM et al (2013) The anterior thalamus provides a subcortical circuit supporting memory and spatial navigation. Front Syst Neurosci 7:45. doi:10.3389/fnsys.2013.00045
Killcross S, Coutureau E (2003) Coordination of actions and habits in the medial prefrontal cortex of rats. Cereb Cortex 13:400–408
Loukavenko EA, Ottley MC, Moran JP, Wolff M, Dalrymple-Alford JC (2007) Towards therapy to relieve memory impairment after anterior thalamic lesions: improved spatial working memory after immediate and delayed postoperative enrichment. Eur J Neurosci 26:3267–3276. doi:10.1111/j.1460-9568.2007.05879.x
Marchand A, Faugere A, Coutureau E, Wolff M (2013) A role for anterior thalamic nuclei in contextual fear memory. Brain Struct Funct 219 (5):1575–1586. doi:10.1007/s00429-013-0586-7
McDannald MA, Jones JL, Takahashi YK, Schoenbaum G (2014) Learning theory: a driving force in understanding orbitofrontal function. Neurobiol Learn Mem 108:22–27. doi:10.1016/j.nlm.2013.06.003
Mitchell AS, Browning PG, Baxter MG (2007) Neurotoxic lesions of the medial mediodorsal nucleus of the thalamus disrupt reinforcer devaluation effects in rhesus monkeys. J Neurosci 27:11289–11295. doi:10.1523/JNEUROSCI.1914-07.2007
Ostlund SB, Balleine BW (2005) Lesions of medial prefrontal cortex disrupt the acquisition but not the expression of goal-directed learning. J Neurosci 25:7763–7770. doi:10.1523/JNEUROSCI.1921-05.2005
Ostlund SB, Balleine BW (2008) Differential involvement of the basolateral amygdala and mediodorsal thalamus in instrumental action selection. J Neurosci 28:4398–4405. doi:10.1523/JNEUROSCI.5472-07.2008
Parkes SL, Balleine BW (2013) Incentive memory: evidence the basolateral amygdala encodes and the insular cortex retrieves outcome values to guide choice between goal-directed actions. J Neurosci 33:8753–8763. doi:10.1523/JNEUROSCI.5071-12.2013
Pickens CL (2008) A limited role for mediodorsal thalamus in devaluation tasks. Behav Neurosci 122:659–676. doi:10.1037/0735-7044.122.3.659
Smith KS, Virkud A, Deisseroth K, Graybiel AM (2012) Reversible online control of habitual behavior by optogenetic perturbation of medial prefrontal cortex. Proc Natl Acad Sci USA 109:18932–18937. doi:10.1073/pnas.1216264109
Tanaka SC, Balleine BW, O’Doherty JP (2008) Calculating consequences: brain systems that encode the causal effects of actions. J Neurosci 28:6750–6755. doi:10.1523/JNEUROSCI.1808-08.2008
Tran-Tu-Yen DA, Marchand AR, Pape JR, Di Scala G, Coutureau E (2009) Transient role of the rat prelimbic cortex in goal-directed behaviour. Eur J Neurosci 30:464–471. doi:10.1111/j.1460-9568.2009.06834.x
Ulrich K, Aitken PN, Abraham WC, Dalrymple-Alford JC, McNaughton N (2014) Effects of thalamic lesions on repeated relearning of a spatial working memory task. Behav Brain Res 261:56–59. doi:10.1016/j.bbr.2013.12.002
Valentin VV, Dickinson A, O’Doherty JP (2007) Determining the neural substrates of goal-directed learning in the human brain. J Neurosci 27:4019–4026. doi:10.1523/JNEUROSCI.0564-07.2007
van Groen T, Kadish I, Michael Wyss J (2002) Role of the anterodorsal and anteroventral nuclei of the thalamus in spatial memory in the rat. Behav Brain Res 132:19–28
Warburton EC, Baird AL, Aggleton JP (1997) Assessing the magnitude of the allocentric spatial deficit associated with complete loss of the anterior thalamic nuclei in rats. Behav Brain Res 87:223–232
Warburton EC, Morgan A, Baird AL, Muir JL, Aggleton JP (1999) Does pretraining spare the spatial deficit associated with anterior thalamic damage in rats? Behav Neurosci 113:956–967
Warburton EC, Baird AL, Morgan A, Muir JL, Aggleton JP (2000) Disconnecting hippocampal projections to the anterior thalamus produces deficits on tests of spatial memory in rats. Eur J Neurosci 12:1714–1726
Warburton EC, Baird A, Morgan A, Muir JL, Aggleton JP (2001) The conjoint importance of the hippocampus and anterior thalamic nuclei for allocentric spatial learning: evidence from a disconnection study in the rat. J Neurosci 21:7323–7330
Ward-Robinson J, Wilton LA, Muir JL, Honey RC, Vann SD, Aggleton JP (2002) Sensory preconditioning in rats with lesions of the anterior thalamic nuclei: evidence for intact nonspatial ‘relational’ processing. Behav Brain Res 133:125–133
Wilton LA, Baird AL, Muir JL, Honey RC, Aggleton JP (2001) Loss of the thalamic nuclei for “head direction” impairs performance on spatial memory tasks in rats. Behav Neurosci 115:861–869
Wolff M, Gibb SJ, Dalrymple-Alford JC (2006) Beyond spatial memory: the anterior thalamus and memory for the temporal order of a sequence of odor cues. J Neurosci 26:2907–2913
Wolff M, Gibb SJ, Cassel JC, Dalrymple-Alford JC (2008a) Anterior but not intralaminar thalamic nuclei support allocentric spatial memory. Neurobiol Learn Mem 90:71–80. doi:10.1016/j.nlm.2008.01.007
Wolff M, Loukavenko EA, Will BE, Dalrymple-Alford JC (2008b) The extended hippocampal-diencephalic memory system: enriched housing promotes recovery of the flexible use of spatial representations after anterior thalamic lesions. Hippocampus 18:996–1007. doi:10.1002/hipo.20457
Yin HH, Ostlund SB, Knowlton BJ, Balleine BW (2005) The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci 22:513–523. doi:10.1111/j.1460-9568.2005.04218.x
Acknowledgments
This work was supported by the CNRS and the Conseil Régional d’Aquitaine. F. A. is supported by the Ministère de l’Enseignement Supérieur. We thank D. Panzeri, N. Argenta, and J. Huard for their help in animal care.
Author information
Authors and Affiliations
Corresponding authors
Additional information
F. Alcaraz and F. Naneix contributed equally.
M. Wolff and E. Coutureau contributed equally as senior authors.
Rights and permissions
About this article
Cite this article
Alcaraz, F., Naneix, F., Desfosses, E. et al. Dissociable effects of anterior and mediodorsal thalamic lesions on spatial goal-directed behavior. Brain Struct Funct 221, 79–89 (2016). https://doi.org/10.1007/s00429-014-0893-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-014-0893-7