Abstract
We investigated the behaviour of vision-deprived human subjects who try to maintain their horizontal alignment in space on a rotating platform by stepping about their own axis in counter-direction (‘podomotor counter-rotation’), and we ask which of two alternative hypotheses best explains this behaviour. (1) The feedback hypothesis assumes that the podomotor counter-rotation is controlled by negative feedback of vestibular signals from the horizontal canals. (2) The reconstruction hypothesis holds that the vestibular cue first is combined with leg proprioceptive afferents signalling the individual’s rotation on the platform (‘podokinesthetic cue’) in a way that reconstructs the platform’s motion in space for internal representation; a negative (direction-inverted) copy of this representation then would drive the counter-rotation. Subjects were exposed to three different velocity profiles of platform rotation: VC, constant velocity rotation with sudden onset and offset; VS, sinusoidal rotation; VN, pseudorandom noise sequences. The subjects’ response (i.e., their active self-rotation on the platform) to the onset and offset of VC rotations was reminiscent of a first-order lead system. Specifically, after rotation onset subjects immediately began to step on the platform in opposite direction; initially, the velocity of this response matched that of platform rotation, leading to a fairly good stabilisation of subjects’ alignment in space. However, this response declined exponentially; consequently, subjects began to increasingly rotate in space along with the platform, ultimately stepping in place on the platform. After rotation offset, subjects immediately began to step around on the now stationary platform so as to continue their previous rotation in space; this response again declined exponentially until subjects became gradually stable again with respect to space. Within subjects, the time constant (τ) of these responses was similar for onset and offset. Across subjects it exhibited a conspicuous variability, ranging from 7 s to virtually infinity. The responses to VS and VN rotations were closely correlated to what could be predicted for each individual from his τ during VC on the assumption of a first-order lead system. We conclude that the mechanism stabilising body orientation basically is linear (no prediction with sinusoidal rotation, no extrapolation of constant velocity rotation). A comparison of the experimental results with simulations of the feedback hypothesis and of the reconstruction hypothesis suggests that the reconstruction hypothesis is a more likely description of the underlying processing of the vestibular and podokinesthetic cues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The vestibular apparatus is a key source of information for controlling the stability of posture and gaze, and likewise for self-orientation in space. The attempt to understand vestibular-aided posture control generally rests on an interpretation in terms of reflexes based on negative feedback loops. For example, the vestibulo-collic reflex (which stabilises head position in space during body rotations) has been described as a system that acts toward nullifying the vestibular signal and, thereby, attenuating the very input that drives it (Outerbridge and Melvill Jones 1971). Superposition of vestibular and proprioceptive reflexes has been invoked to explain differential postural reactions, for example to whole body movements on the one hand, and isolated head movements on the other; a review of such presumed reflex interactions is given by Roberts (1995).
Whereas the reflex concept of vestibular stabilisation bears the connotation of a fairly direct wiring between receptor and effectors, spatial orientation is generally viewed as requiring considerable processing of the vestibular input and being ‘far away’ from postural mechanisms. However, in recent years, a systems theory inspired analysis of how the vestibular signals interact with other afferents for the perception of self-motion and object motion has led to the idea that vestibular-based perception and at least some vestibular reflexes share common principles (Mergner et al. 1997). Outstanding among these principles is the notion of an internal (neural) representation of the individual’s kinematic and kinetic situation serving as a primordial reference for both perception and motor action.
As a prerequisite for such a reconstruction, the intervention of a vestibular ‘eigenmodel’ has been postulated. To briefly summarise the rationale of this postulate (which has been extensively discussed in previous work: Mergner and Becker 2003), consider a blindfolded individual standing on a rotatable, though stationary, platform whose head is turning about the vertical axis. This situation gives rise to a vestibular canal signal that records head rotation with respect to inertial space without discriminating whether the head (and/or the trunk) is rotating relative to the stationary platform or en bloc with the platform. To obtain information about the kinematic state of the platform, the proprioceptive signals of head-on-trunk and trunk-versus-platform rotation must be subtracted from this vestibular signal. Ideally, these signals would cancel each other, thereby indicating platform stationarity. In reality, however, these signals match each other only at ‘high’ frequencies (≥0.1 Hz), whereas at low frequencies (rotations at quasi-constant velocity) there are only proprioceptive but no vestibular signals (the latter behaving like a first-order high-pass system). Therefore, subtracting the unprocessed proprioceptive signals from the vestibular message would lead to an erroneous perception of support (i.e., platform) motion during low frequency rotations. To prevent such errors, axial proprioceptive signals are thought to be first processed by a filter endowing them with the dynamic characteristics of the vestibular channel (vestibular eigenmodel). Modified in this way, they would, upon subtraction, exactly cancel all those vestibular signals that were caused by axial motions of the body relative to the platform. Put in general terms, the difference between the vestibular afferents and the ‘vestibularly transformed’ proprioceptive afferents is the best available estimate of the support’s kinematic state. Under the ecologically most relevant scenario of a motionless support this estimate is always veridical, yielding a value of zero; it is thought to constitute an internal reference for both the conscious perception of how the various body segments move in space and for the unconscious evaluation of these movements in view of body stabilisation. With regard to both purposes it is assumed that, paralleling the physical stack of superimposed mechanical systems (support, trunk, head, eyes), a second set of proprioceptive signals, now with full low frequency capabilities, is added to this reference; for example, adding to the reference signal proprioceptive information on trunk-versus-feet rotation would provide our sample individual with an internal estimate of trunk rotation relative to space (for details see Mergner et al. 1997).
While the above notion of a vestibular eigenmodel leading to an internal estimate of support motion (or stability) has been extensively studied in experiments probing the perception of self- and object-motion (e.g., Mergner et al. 1992), less attention has been directed at examining its postulated role for reflex-like mechanisms of body stabilisation. The scenario evoked above, of a vision-deprived individual at the centre of a rotatable platform, lends itself readily to such an examination: we can ask this individual to try to stabilise himself with respect to space whenever the platform moves, that is, to step about his own axis in the opposite direction so as to exactly compensate for the platform rotation and to remain aligned with his initial straight-ahead direction. This task allows us to test two alternative hypotheses:
-
1.
The feedback hypothesis explains stabilisation by a negative feedback scheme set to minimise the vestibular signal; that is, the vestibular afferents assume the role of an error signal that is fed back, with appropriate amplification and inverted sign, to the podomotor system, which, in turn, would act to reduce vestibular stimulation and, hence, the individual’s rotation in space.
-
2.
The reconstruction hypothesis, on the other hand, proceeds from the aforementioned reference signal, i.e., from an internal reconstruction of the platform’s rotation in space obtained by combining vestibular and leg proprioceptive afferents in the way outlined above. The podomotor system then would mirror image (with inverted sign) this internal representation of platform rotation and, thus, stabilise the individual in space.
The two hypotheses predict different time-courses of the individual’s reaction. These differences should be detectable in his step responses, which are elicited if the platform suddenly starts a constant velocity rotation (‘step’ referring to the angular velocity profile and not to the individual’s stepping on the platform). In the present study, we have therefore subjected vision-deprived human subjects to angular velocity steps of different magnitudes and recorded their counter-rotation on the platform. However, the use of constant velocity stimuli might invite an interference of cognitive mechanisms; for example, an individual, from knowing or guessing the velocity profile, could decide to maintain a constant pace of counter-rotation throughout the period of stimulation and thus override the response of his ‘hard-wired’ mechanisms. To control for this possibility, we presented our subjects also with sinusoidal motions of the platform, on the assumption that it would be fairly difficult to produce a sinusoidal counter-rotation of appropriate amplitude from volition. Yet, it is well known that sinusoidal stimuli invite temporal prediction in many behavioural tasks, including some vestibular-based behaviour (stabilisation of posture: McIlroy and Maki 1994). Therefore, to exclude that an individual would manipulate (consciously or unconsciously) the phase of his response, we used random motions of the platform as a third type of stimulation. As we shall show, the responses to all three patterns of platform motion hint at an essentially similar way of interaction between vestibular and proprioceptive afferents, which can be best understood in terms of the reconstruction hypothesis.
Material and methods
Subjects
Fourteen subjects aged 19–61 years (nine male, five female) participated in the experiments; among them were 12 paid volunteers recruited from a class of undergraduate students, and two of the authors. All were free of any known neurological pathology. Subjects gave their informed consent after having learned the goals and procedures of the experiment, which had been approved by the local ethics committee.
Equipment and procedures
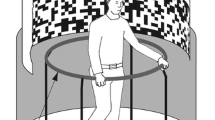
Subjects stood at the centre of a computer-controlled turning platform. Platform position was recorded by means of a 360°-potentiometer and dedicated software that would indicate its angular displacement across an arbitrary number of turns. The platform’s centre was surrounded by an elevated rim of 45 cm in diameter, which subjects could sense with their feet and which helped them to remain centred when actively stepping on the platform. Subjects were equipped with an orthopaedic neck collar to minimise head versus trunk rotations, and with a helmet that was coupled via a flexible, yet torsionally rigid, hose to an overhead potentiometer recording the rotation of their heads with respect to space. Integrated into the helmet was a wireless headphone for communication and for presenting a masking noise during trials.
Prior to each trial, subjects were asked to align their current orientation with a standard direction in space (‘space’ being represented by the laboratory environment). Lights then were switched off and a warning tone announced the upcoming trial. Upon hearing this tone, subjects started to step in place while the platform was still stationary. After 3 s, platform rotation started and subjects were to step as smoothly as possible about their vertical axis into the opposite direction in such way as to feel stationary in space; specifically, subjects were instructed to imagine the previously seen standard direction and to try and maintain their alignment relative to this direction. Subjects were to continue their stepping, even if they thought the platform had become motionless, until a second tone signalled the end of a trial.
Three different velocity profiles of platform rotation were used:
- VC:
-
Constant velocity rotation (20°/s or 40°/s, left and right directions alternating) lasting 120 s (ten subjects) or 80 s (four subjects), followed by an after-period of same length during which the platform was stationary (Fig. 1); thereafter the end-of-trial tone was sounded. The start and stop of the rotation was smoothed by multiplying the onset and the offset of the velocity signal with the first and second half, respectively, of a ‘raised cosine’ of 1 Hz (Mergner et al. 2001), resulting in acceleration and deceleration periods each of 0.5 s.
- VS:
-
Four periods of sinusoidal rotation at 12 mHz and 40°/s peak velocity. The start and stop of this rotation was smoothed by multiplying the first and last half period with a raised cosine of corresponding duration (42 s; Fig. 2).
- VN:
-
Three different epochs of pseudorandom noise sequences with ‘1/f characteristics’, each lasting 520 s (Fig. 4). In all three epochs the power density of the platform velocity decreased as the inverse of the frequency up to the cut-off frequency fmax, and the distribution of the amplitudes was approximately Gaussian with peak values of up to ±60°/s; the three periods differed with respect to fmax: VN1 60 mHz, VN2 90 mHz, and VN3 120 mHz. The same three epochs were presented to each subject; they had been selected for their smooth start and stop from a manifold of noise sequences with similar statistical properties.
In a variant of the experiment, performed only with sinusoidal rotation, subjects were instructed to compensate the platform rotation in a discontinuous way. Specifically, subjects would periodically let the platform rotate them in space for a short distance while they tried to estimate the resulting angular displacement as accurately as possible, and then turn back in a single leap to offset the sensed displacement. Their angular position in space, therefore, would assume the appearance of a nystagmic pattern.
The experiments were grouped into four sessions, each of about 30 min duration, which were carried out on 2 separate days. Between sessions of the same day, subjects were given a break during which they could leave the platform. Each session started with four VC trials (two velocities by two directions), followed either by one of the VN trials or by the two VS trials (continuous compensation always first). The order of presentation of VC trials within sessions, and likewise the order in which sessions were carried out, was randomised across subjects. The direction of the first VC trial of a session was always announced, and subjects knew that the directions of the following trials would alternate. At the conclusion of each session an interview was conducted to learn whether subjects were confident of having successfully maintained their spatial orientation and whether they had experienced any difficulty keeping their balance during rotation.
Data acquisition and analysis
Both platform (PS) and head angular positions (HS) with respect to space were digitised at a rate of 50 Hz and stored in computer files. The signal of head versus platform position (HP) was obtained offline by letting HP=HS−PS (note that because of the neck collar, HS and HP approximately equal body-in-space and body-on-platform position, respectively). Quantitative analyses were mostly performed on the velocity signals ps, hs, and hp obtained by digital differentiation of PS, HS, and HP, respectively. To attenuate the high-frequency contributions from the subjects’ stepping, these signals were limited to 0.5 Hz by digital filtering (FIR low-pass, 0.1 dB ripple in pass-band, 60 dB attenuation in stop-band).
Subjects’ podomotor responses (hp) during VC rotations were fitted by exponentials (details to be given later), while VS and VN responses were characterised by (1) the phase (Φ) of hp with respect to ‘ideal’ counter-rotation (the one that would achieve perfect stabilisation), and by (2) the gain (GR) of subjects’ residual rotation in space (hs; ‘error’). Phase values, Φ, were read from the 12-mHz component of the transfer functions (T) between response velocity hp and stimulus velocity ps (focussing on 12-mHz allowed a comparison between VN and VS as this was the frequency of VS rotations). T was calculated as T=C/A, where C and A represent the cross-spectrum between hp and ps, and the auto-spectrum of ps, respectively, obtained by means of fast Fourier transforms (FFT). Residual gain values GR were obtained in a similar way, from the transfer functions between hs and ps. Finally, to test the assumption that the podomotor response behaves like of a first-order lead system, we used the time constants (τ) obtained from the exponential VC fits to calculate, for each subject, the phase ΦC of hp at f=12 mHz expected under this assumption [ΦC=90°−arctan(2π·f·τ)], and likewise the expected gain of the residual rotation in space (GRC). As they refer to the same frequency, these parameters can be compared to the corresponding values from VS (ΦS, GRS) and VN (ΦN; GRN), respectively. The average relationship across subjects between any two of these phase (or gain) parameters, say between ΦC and ΦS, was characterised by (1) the coefficient of correlation, and (2) the line that minimises (in the least-squares sense) the orthogonal distance (δ) between it and each of the data points in an x-y scatter plot of the two parameters; specifically, if δxi and δyi represent the horizontal and vertical deviations of the i-th subject from that line, the fit minimises Σδi2 where δi2=(δxi2·δyi2)/(δxi2+δyi2). This approach was chosen instead of calculating the regression of, for example, ΦS on ΦC (or vice versa) because both phase values are supposed to reflect the same process so that there is no basis for considering one as an independent and the other as a dependent variable.
Results
Constant velocity (VC) trials
Qualitative observations
After the start of constant velocity (VC) trials all subjects clearly felt the platform rotation under their feet and began to compensate for it quite well so that they remained approximately stable in space. However, as the rotation continued, all subjects except one gradually slowed down their counter-rotation and eventually stepped in place relative to the platform while rotating with the platform in space, as illustrated by trace hs in Fig. 1. Simultaneously, subjects ceased to perceive the platform rotation. To see how closely the perception and the compensation of platform rotation are correlated, the authors, acting as subjects in a supplementary experiment, explicitly signalled when they felt no more platform rotation; interestingly, their perception of platform rotation subsided earlier than their compensatory circling relative to the platform (grey vertical lines in Fig. 1).
Constant velocity (VC) rotation. Sample trial from one subject showing angular velocities of platform (ps) and head (hs) with respect to space, and of head relative to platform (hp).The subject’s active counter-rotation on the platform (podomotor reaction) is reflected by hp, while hs represents his residual rotation in space (error); note that during platform standstill hs and hp are identical. Trace hs was smoothed, for the purpose of this figure, by a moving average of width 0.4 s (3dB pass band ≈ 1.1 Hz) to reduce ripple caused by subject’s stepping. Trace hp was obtained by letting hp=hs−ps; exponential fit to hp (bold continuous curve) has a time-constant of 23.2 s. Directional bias after decline of compensatory reactions to onset and offset of platform rotation are represented by hp0 and hp1, respectively. Grey vertical lines represent instant of button press delivered when subject ceased to perceive a platform rotation. Note that the illustrated subject (#002) participated only in the supplementary experiments aimed at studying this perception and does not figure in Table 1
While stepping in place relative to the rotating platform, many subjects, including the authors, experienced a strange sensation as if they were walking in a slightly drunk condition. Conceivably, this can be attributed to the fact that each body sway engendered unusual forces in this condition (centrifugal forces that rapidly rise as the centre of mass becomes eccentric, combined with Coriolis forces). Yet, in spite of these sensations, naive subjects were sure to be stationary in space.
When the platform stopped after 80 or 120 s, subjects experienced a sudden rotation of the platform into the opposite direction and started to compensate for the apparent rotation; as a result, they initially continued to rotate in space by actively circling on the now physically stationary platform. This active turning then gradually slowed down again until subjects eventually were stepping in place on the stationary platform.
Retrospectively, 9 of the 14 subjects thought they always had maintained their orientation quite well throughout the onset and the offset of the rotation up to the end of a trial. They were astonished, thereafter, to see a deviation of 180° for example, not realising that the actual error typically amounted to more than ten turns. The other five subjects mostly reported that they had briefly lost their spatial alignment, often because their stepping was not swift enough during the sudden onset or offset of the 40°/s stimuli or because they had stumbled. Noticeably also, a number of subjects mentioned that a main cue directing their behaviour was the awareness of platform rotation, which they accordingly attempted to counteract, and some noted that this awareness was “in the feet”.
To emphasise the podomotor response proper of the subjects, Fig. 1 also plots hp, the velocity of subjects’ counter-rotation relative to the platform (hp=hs−ps). In most subjects, this velocity consisted of two ‘well behaved’ transients linked to the onset and the offset of platform rotation, respectively, with time-courses that closely resembled exponentials (as in Fig. 1), except for an often quite large modulation at the pace of subjects’ stepping. The exponential character of the podomotor response can also be appreciated from Fig. 6C, which shows individual averages of hs across all 16 VC trials (2 velocities × 2 directions × 4 repetitions; hs normalised to the direction and the magnitude of platform velocity). However, occasionally more erratic behaviour occurred in some subjects, such as sudden accelerations or decelerations of their counter-rotation in mid-trial; retrospective questioning supports the suspicion that one reason for these abrupt changes may be conscious ‘corrections’ (in reality often changes for the worse) by the subject, secondary to a perception of performing wrongly. Also, some subjects exhibited a marked directional bias that caused them to slowly circle on the platform in always the same direction after the decay of their transient response (hp0, hp1 in Fig. 1).
In one subject (#110) the counter-rotation exhibited no signs of transientness; this subject almost perfectly maintained his spatial orientation by turning relative to the platform at the appropriate velocity throughout the whole epoch of platform rotation, and by stepping in place immediately after the platform stopped (Fig. 6C, trace #110). Below we will argue that there were no unsolicited cues that could have caused this behaviour (see Discussion). Finally, another subject (#108) exhibited a behaviour that may be relevant for the distinction between the reconstruction and the feedback hypotheses: he frequently overcompensated for the platform rotation during the very first few seconds, thus rotating in space against the direction of the platform (Fig. 6C, trace #108).
Quantitative description
In view of its predominantly exponential appearance, the time-course of subjects’ velocities relative to the platform (hp) was fitted (in the least-squares sense) by functions of the type -(Ghp·vC+hp0)·exp(−t/τ)+hp0 (onset response) and (Ghp·vC−{hp1−hp0})·exp(−t/τ)+hp1 (offset), where vC represents stimulus velocity (±20°/s, ±40°/s) and where hp0 and hp1 account for the directional bias exhibited by a number subjects during or after platform rotation, respectively (see Fig. 1; in most cases hp0≈hp1).
Responses with clearly non-exponential character were excluded from this analysis (12 cases out of 224 onset responses, 8 out of 224 offset responses); when visual inspection suggested only a temporary deviation from the exponential course, the deviating epoch was excluded from the fit (six onset responses, 17 offset responses). Finally when no decay of the compensatory circling could be detected after rotation onset or when, correspondingly, the subject began to step in place immediately after the cessation of the rotation, we arbitrarily set the time-constant to a value of 250 s to avoid a value of infinity (this situation applied to nearly all trials of subject #110 as well as to five trials of a second subject).
The time-constants obtained in this way were averaged across the four repetitions of each of the four conditions (2 velocities × 2 directions). Because the interindividual distribution of these averages was clearly not normal, Friedman’s non-parametric analysis of variance (ANOVA) was invoked to test for possible effects of the velocity and the direction of platform rotation. As these tests failed to detect any significant effects, grand averages across all conditions were formed for the onset and offset responses, respectively, and were compared using the Wilcoxon test for matched pairs. According to this test, the time-constants of the onset and offset responses did not significantly differ from each other. In fact, their across-subjects averages were virtually identical (mean onset 56.4 s, offset 58.2 s; median onset 38.9 s, offset 36.3 s). For comparisons with the results of the VS and VN experiments we therefore averaged the onset and offset time-constants for each subject; these global averages, which are listed in Table 1, ranged from 6.6 s to 250 s (recall that the latter value is a place-holder for values close to infinity). Also listed in Table 1 are the gain values Ghp of the podomotor reaction to the onset of the platform rotation; these values are scattered about a mean of almost unity (mean 0.97, median 0.96). As evident from Table 1, not only subject #108 mentioned above, but also other subjects initially overcompensated platform rotation (Ghp>1), albeit to a much lesser degree and for shorter time. Finally, in spite of similar time-constants (see above), the gain values of the offset responses were, on average, smaller (mean 0.83, median 0.84) than, but clearly correlated (r=0.67) to, those of the onset responses (data not shown in the table).
Sinusoidal trials
During sinusoidal turning subjects periodically lost and regained the perception of platform rotation under their feet. The velocity of their compensatory counter-rotation (hp) exhibited a clearly periodical, albeit not always strictly sinusoidal, character, which, by the same token, also held for hs, the signal of head velocity in space (see example in Fig. 2A). As depicted in Fig. 3A, within subjects there was a close correspondence between the phase of this counter-rotation (ΦS, ordinate) and the phase calculated, on the assumption of a first-order lead system, from the time-constant (τ) of their VC responses (ΦC, abscissa), with the coefficient of correlation reaching r=0.93 and the slope of the ‘orthogonal-fit’ line characterising the magnitude of ΦS in relation to ΦC (see Data acquisition and analysis section of Methods) being close to unity (0.96).
Sinusoidal (VS) rotation. Sample trials from one subject (#105) showing angular velocity of platform (ps) and head (hs, residual rotation) with respect to space, and of the head relative to the platform (hp, podomotor reaction). Traces hs were smoothed by moving average of width 0.4 s (pass band ≈ 1.1 Hz); traces hp were obtained by letting hp=hs−ps. A Continuous counter-rotation. B Discontinuous (‘nystagmic’) counter-rotation. For the sake of clarity, hp has been omitted in the first half of the plot and hs from the second half
Scatter plots comparing individual phase and gain values during sinusoidal (VS) and random(VN1) rotation to those calculated from responses during constant velocity (VC) rotation. A Sinusoidal rotation (12 mHz): phase lead ΦS of counter-rotation (ordinate) as function of phase lead ΦC calculated from VC (abscissa); each symbol represents one subject; oblique line shows ‘orthogonal’ fit (see Data acquisition and analysis section of Methods); equation of fit in lower right corner. B Sinusoidal rotation: gain of residual head rotation in space GRS (error) as a function of gain GRC calculated from VC, with presentation as in A. C,D phase ΦN and residual gain GRN at 12 mHz during random rotation, analogous to A and B, respectively
An analogous comparison of the gain of the counter-rotation during VS to that calculated from VC-responses is not very enlightening; at our stimulation frequency of 12 mHz, the variations in gain that can be expected from the observed variations of τ are fairly small in the presumed first-order lead system and, hence, do not allow for a meaningful analysis of correlation. Therefore, we focussed our analysis on the gain GR of the residual rotation in space (hs; ‘error’ occurring in spite of the counter-rotation), for which a considerably larger variation can be expected. Figure 3 B indicates a tight correspondence (r=0.88) between the values of the residual gain calculated from τ (GRC, abscissa) and those obtained by analysis of the error during VS rotation (GRS, ordinate), and identical average magnitudes (slope of orthogonal distance fit 1.00).
Noticeably, the one subject who maintained his counter-rotation in the VC paradigm throughout the period of rotation (τ close to infinity) also stabilised himself in a nearly perfect manner upon sinusoidal rotation. His turning on the platform was almost exactly in phase with the ideal signal and admitted a residual head-in space rotation of only 7%.
A sample record of the discontinuous, ‘nystagmic’ mode of stabilisation during sinusoidal rotation is shown in Fig. 2B. This mode had been introduced to test for the possibility that subjects might sense their rotation in space more easily, and hence stabilise themselves more accurately, if they were not confused by their concurrent compensatory stepping on the platform. However, upon retrospective questioning only a minority of the subjects thought that the discontinuous task was easier (4 out of 14). The phase leads during this task correlated well with those during continuous stepping (r=0.74) but were by about 20% smaller (difference not significant), whereas the error (residual rotation hs) was almost identical in the two conditions (r=0.90; slope of orthogonal distance fit 1.06).
Random velocity (VN) trials
A sample record of a subject’s performance during random rotations of the platform is shown in Fig. 4. Typically, and not unexpectedly, this subject and most others compensated almost perfectly for the high-frequency components of platform rotation. Indeed, according to the transfer functions (T; see Data acquisition and analysis section of Methods) between response velocity hp and stimulus velocity ps, at frequencies ≥30 mHz, the spectral components of the counter-rotation had an average phase close to zero (which is the ‘ideal’ value) and a gain of 0.9 (median values) and, correspondingly, a residual hs rotation of only 10%. Below this frequency, the phase exhibited an increasing lead while the gain changed only marginally down to the lowest frequencies for which reliable results could be expected on the basis of the subjects’ coherency spectra (VN1 9 mHz, VN2 and VN3 15 mHz).
Random noise (VN) rotation; sample trial from one subject (#105) showing angular velocity of platform (ps), of head with respect to space (hs, residual rotation), and of head relative to platform (hp, podomotor reaction). Trace hs was smoothed by moving average of width 2 s (pass band ≈ 0.22 Hz); trace was hp obtained by letting hp=hs−ps. To avoid squeezing of the curves only the first two-thirds of the trial are shown
For a comparison with the results of VC, we considered the 12 mHz component of VN1 trials. The scatter plots in Fig. 3C,D display the relationship between this component and the response calculated from the time-constant τ measured in the VC paradigm. The phase lead of the subjects’ counter-rotation (ΦN, Fig. 3C) was well correlated (r=0.92) with the lead derived from τ (ΦC), but clearly smaller on average (slope of orthogonal distance fit 0.65). Friedman’s ANOVA, followed by a Wilcoxon test for matched pairs indicates that ΦN was, in fact, significantly smaller than both ΦC and ΦS (p<0.025). Likewise, the gain of the residual rotation (GRN) was well correlated (r=0.91) with, but smaller (by 16%) than, GRC calculated from τ (Fig. 3D); this 16% mean difference in magnitude was not significant (Friedman’s ANOVA), though.
Discussion
In the present experiments we wished to learn whether the task of stabilising one’s spatial alignment on a rotating platform without the help of visual cues is solved by a direct retroaction of horizontal canal signals upon the leg motor system (feedback hypothesis) or by drawing on an internal reference signal that would reconstruct the platform’s motion in space from canal and leg proprioceptive afferents (reconstruction hypothesis). The interest in distinguishing these two possibilities is motivated by the recent suggestion that perceptual processes and reflex-like mechanisms of posture stabilisation share common or similar internal representations of relevant physical facts (Mergner and Becker 2003). Platform, or more generally support, motion is such a relevant fact, and its internal representation appears to assume the role of a reference signal for the perception of both self-motion and object motion.
Both hypotheses assume an ‘automatic’ processing of afferent information without intervention of cognitive (or ‘top-down’) mechanisms and can, therefore, be represented by simple ‘wiring schemes’ (see details below and Fig. 5). However, at the outset of the present experiments it was not clear whether this assumption would apply since psychophysical studies into vestibular navigation suggest that many individuals can, for example, ignore the decay of vestibular afferents during constant velocity rotation and behave as if they were extrapolating its initial magnitude (Becker et al. 2002; Jürgens et al. 2003). An analogue behaviour in the present experiments could not be excluded a priori: subjects, from knowing that the platform will turn at constant speed, might deliberately maintain a constant pace of stepping during VC trials. Two pieces of evidence speak against the possibility that such a behaviour occurred.
First, when the authors acted as subjects and explicitly tried to apply this strategy, they failed to achieve an enduring counter-rotation. Their efforts were overruled by the behaviour required to actually feel stationary in space, which inevitably resulted in a slowly declining velocity of counter-rotation.
Second, to check for a conceivable top-down influence, we have used, besides constant velocity rotations (condition VC), also sinusoidal and random velocity motions (conditions VS and VN). Within subjects, these different conditions exhibited a close correspondence when the phase of subjects’ responses and the gains of their residual velocity in space were calculated from the time-constant of VC responses on the assumption of a first-order lead system (ΦC and GRC) and compared with the corresponding values obtained with VS (ΦS, GRS) and VN (ΦN, GRN), respectively (cf. Fig. 3). These observations strongly suggest that, by and large, the stabilisation response indeed conforms to the behaviour of a first-order lead system irrespective of the particular type of velocity profile applied to the platform. In particular, subjects did not profit from the predictable character of VC and/or VS stimuli. This would have resulted in smaller values of ΦC and/or ΦS compared with ΦN, whereas in reality the smallest Φ-values were observed during the random VN stimulation. Therefore, it is unlikely that there was a systematic interference of cognitive mechanisms during constant velocity or sinusoidal rotations. This is not to say that there were no conscious interventions at all. For example, abrupt changes, sometimes back and forth, were occasionally observed in the velocity of subjects’ counter-rotations which, upon retrospective questioning, appeared to be linked to an individual’s sudden feeling of being ‘wrong’ and his attempt to correct.
It is true, however, that with regard to the mean magnitudes of Φ and GR there were differences between condition VN (random rotation trials) and the other two conditions (note the less-than-unity slopes of the regression lines in Fig. 3C,D). Possible reasons will be discussed below. Notwithstanding these differences, the subjects’ responses to VC rotations provide fair descriptions of their stabilisation performance in general, and we will concentrate mainly on these in the following.
Interpretation in terms of a model
The two hypotheses that we want to contrast are best discussed by referring to the signal flow diagrams shown in Fig. 5. Before we describe these hypotheses and possible variants in turn, some general observations are appropriate.
Diagramatic representations of feedback and reconstruction hypotheses. A Feedback hypothesis. Stabilisation results from negative feedback of head velocity in space (hs) as sensed by the vestibular system. hsdes desired head-in-space velocity (=set point), bp body rotation on platform, hp head rotation relative to platform (=bp, as long as there is no head versus trunk torsion), ps platform rotation relative to space (=disturbance to be compensated by feedback), ves vestibular pathway, hs′ central vestibular representation of hs, G loop gain of feedback circuit, Mot podomotor system (arbitrarily assumed to behave like a first-order delay system with a time constant of 0.5 s), vest Syst vestibular system with time-constant τ*=8 s. B Reconstruction hypothesis. A central representation (ps′′) of platform rotation in space is reconstructed from vestibular (ves) and podokinesthetic (pod) information. hp′ proprioceptive (mostly podokinesthetic) signal of body and head rotation relative to platform after transformation by vestibular eigenmodel, ps′ central representation of ps resulting from summation of hs′ and −hp′, ps′′ improved representation of ps after central prolongation of time constant (box τ*→τ), G gain of counter-rotation (for optimal stabilisation G must equal unity). Remaining symbols as in A
The diagrams in Fig. 5 describe the relationship between the platform rotation and the subjects’ counter-rotations in the velocity domain, giving the theoretical results a similar format as used for rendering the experimental results in Figs. 1, 2, 4 and 6C. Accordingly, they consider the subjects’ velocity in space as the signal to be controlled. This approach does not contradict the instruction given to the subjects, which implies a control of angular position (or displacement). Indeed, both schemes can be transposed into the position domain, without affecting their transfer characteristics, by replacing velocities ps, hs, and bp with positions PS, HS, and BP, respectively, and by considering the internal signals (symbols with single or double dash in Fig. 5) as representations of position rather than velocity. We note that such representations must exist as testified by the ability of humans to navigate, but do not consider how exactly the required neural integration of the vestibular velocity signals takes place.
In the following, we repeatedly will have to refer to the magnitude of the peripheral vestibular time-constant (τ*) in humans. From the data of Fernandez and Goldberg (1971) who observed a value of 5.7 s in squirrel monkey and from the comparisons between the dimensions of human and simian canals reported by Igarashi (1967), we surmise that τ* is in the range between 5 and 10 s and will arbitrarily assume a value of 8 s.
Finally, unless otherwise stated, we will consider head-versus-platform velocity (hp) as being identical to body-versus-platform velocity (bp) and use these two parameters interchangeably, depending on the context.
Feedback hypothesis
The feedback hypothesis is depicted in Fig. 5A. It proceeds from a negative feedback circuit that tries to clamp head velocity in space (hs) at the desired set value hsdes=0 in spite of the disturbance by platform velocity ps. Head-in-space velocity results from the imposed platform rotation and the individual’s active rotation relative to the platform (hp=ps+bp). It is measured by the vestibular canal system, which delivers a high-pass version (hs′) of hs, shaped by the system’s peripheral time-constant τ*. This measurement then is compared to the set value (hsdes) of the feedback loop; the resulting error signal, after appropriate amplification (box G in Fig. 5A), drives the compensatory action of the podomotor system (box Mot).
The response of the feedback system in Fig. 5 A does not match the behaviour of our subjects (compare panels A and C of Fig. 6). With moderate values of G (1≤G≤8) there always occurs a clear error (i.e., a residual head-in-space rotation hs) right from the beginning of platform motion because counter-rotation bp never reaches the full amplitude of disturbance ps (Fig. 6A). In contrast, in many of our subjects the gain of the initial counter-rotation (Ghp) closely scattered about unity, so that there was virtually no initial error (cf. Table 1); one subject even clearly overcompensated platform rotation during the first few seconds after its onset (Ghp>1). Only a minority of our subjects exhibited overtly undersized responses with errors of more than 10%, comparable with those depicted in Fig. 6A for G≤8.
Constant velocity (VC) rotations: comparison between experimental responses and model simulations. A responses according to feedback hypothesis (Fig. 5A), assuming a peripheral time-constant of τ*=8 s. Each curve corresponds to a different gain value G (G=1, 2, 4, ..., 32); initial error and time-constant of response vary inversely with respect to each other as G varies. B responses according to reconstruction hypothesis (Fig. 5B), assuming a gain (G) of unity. Each curve corresponds to a different value of time-constant τ (τ=8, 16, 24, 32, 64 and 512); grey curve depicts overcompensation when gain exceeds unity (G=1.2, τ=32 s). C Normalised averages of podomotor reaction as measured by head velocity relative to platform (hp). Each curve corresponds to one subject and is average of up to 16 trials (two velocities, two directions, four repetitions). Here, hp is plotted with inverted sign (unlike in Fig. 1) to better visualise its relationship to platform velocity (dashed curve). Two subjects are highlighted (grey curves): #108 initially overcompensates platform rotation, #110 exhibits a time-constant near infinity
It is true, though, that the simulations in Fig. 6A represent an oversimplification in that they do not account for the inertial momentum of the body; this momentum causes a passive body counter-rotation (torsion about ankle, knee and hip joints) during the initial platform acceleration. However, more detailed simulations (not shown) indicate that the inclusion of this transient passive stabilisation does not much improve the similarity between the responses of the negative feedback scheme in Fig. 6A and the experimentally obtained averages in Fig. 6C: although there is a brief period after rotation onset during which the (passive) counter-rotation indeed matches platform rotation, the compensatory velocity hp rapidly returns to the level of the active response thereafter. Given gain values of about 8 or less, such a short-lived full compensation cannot explain the many cases with an initial gain of hp close to unity (Ghp in Table 1) that were derived from fits to the whole period of platform rotation.
On the other hand, with large values of G, the response of the model becomes more similar to the subjects’ behaviour in that there will be only a small residual rotation at the onset of the platform rotation. However, this improved similarity is traded for an unrealistically large time-constant τ. In fact, with the feedback scheme in Fig. 5A, τ is inversely proportional to hsi, the initial value of the residual head-in-space rotation:
This inverse relationship between τ and the initial error is clearly evident from the family of curves in Fig. 6A. According to Eq. 1, a reduction of hsi to, say, 10% of ps would result in τ=80 s (τ*≈8 s); however, most of our subjects exhibited smaller time constants and, at the same time, had residual velocities hsi of less than 10% (cf. Table 1). Moreover, a plot of τ versus hsi (not shown) failed to reveal any systematic relationship between these two parameters. Therefore, the proportional feedback scheme in Fig. 5A is an unlikely representation of our subjects’ behaviour. We note, however, that the prolongation effect of feedback upon the overall time constant of a system expressed by Eq. 1 has been invoked to explain why head stabilisation during body rotation (vestibulo-collic reflex) exhibits a clearly better low-frequency performance than the vestibulo-ocular reflex (VOR), which is a system without feedback (Outerbridge and Melvill Jones 1971).
Another difficulty with the scheme in Fig. 5A becomes apparent by examining the magnitude of the position error (deviation from initial position) that an internal monitoring of angular displacement (HS’=∫hs’·dt, assuming an ideal neural integrator) would detect. With any reasonable magnitude of G, HS’ will reach fairly large values; for example, with G=16, HS’ would reach a value of about 180° by the end of a 120-s period of rotation at 40°/s. Given that HS’ in some way also determines the subjects’ perception of body in space position, we would have to explain the paradox that subjects feel stationary although the error would seem to be far above the threshold for detection of angular displacement.
Rather than by increasing G, a reduction of the initial error hsi can also be achieved by adding a differentiating element with gain G·τ* in parallel to G (creating a so-called PD-controller). While such an arrangement does not decouple τ from hsi (in contrast to our experimental evidence, cf. above), it results in a smaller increase of τ for the same decrease of the error and can, therefore, produce relatively realistic responses. Nonetheless, we doubt that it is an appropriate representation of information processing in real subjects since both theory and simulations warn that the concurrence of the required high differentiating gain (G·τ*) and the transport delays inherent to any biological system will lead to oscillations.
Finally, we also have explored the possibility that vestibular feedback acts via an integrating controller instead of a proportional one (gain element G replaced by an integrator). However, the responses of such a scheme do not match the experimental results either: after a slow start they continue at constant velocity throughout the period of platform rotation, contrary to the response in the vast majority of our subjects (only subject #110 exhibited a similar behaviour except that he had a more sudden start).
In summary, it is difficult to explain our subjects’ responses in terms of circuits based on the negative feedback principle. While these circuits can produce a corrective output (a counter-rotation in the present case) only if there is an error to drive them, the initial response of most subjects did, on average, exhibit no error that could have entertained such an output. Moreover, feedback control entails a reverse proportionality between the amount of residual error and the time-constant of the counter-rotation that does not seem to hold for the observed responses.
Reconstruction hypothesis
The reconstruction hypothesis is outlined in Fig. 5B. The basic idea here is that by subtracting measurements of head-versus-platform rotation (hp) from those of head-in-space rotation (hs), an internal ‘reconstruction’ of the platform’s rotation in space (ps) can be obtained (ps=hs−hp). Once ps is known, the podomotor system would produce an output in the opposite direction having exactly the magnitude of ps, i.e., a perfect counter-rotation.
Measurements of hp mainly are provided by the ‘podokinesthetic’ system which, conceivably, delivers a compound of leg proprioceptive afferents, somatosensory information from the soles (resulting from shearing forces as subjects turn on their feet), and podomotor efference copies; however, to the extent that torsions occur between the head and the feet (as during angular accelerations), other axial proprioceptive signals (neck, hips, etc.) will also contribute. Although we largely ignore the details of the transfer functions of these various sensory systems, it can be taken for granted that they profoundly differ from the high-pass characteristics of the vestibular system in that they also can also transmit very low frequencies and, therefore, signal constant velocity rotations over extended periods of time. As a consequence, vestibular and podokinesthetic afferents (vest and pod, respectively, in Fig. 5B) cannot be combined directly, but must first be ‘homogenised’. Figure 5B suggests that this is achieved by a vestibular eigenmodel. This eigenmodel, which in the present context is part of the podokinesthetic pathway, emulates the transfer characteristics of the vestibular pathway. It transforms the primordial podokinesthetic afferent signal into a ‘vestibularly coloured’ representation of head-versus-platform rotation (hp′) by conferring on it high-pass properties similar to those of the vestibular message hs′. By subtracting hp′ from hs′, an internal reconstruction of platform rotation in space then is obtained (ps=hs′−hp′), which is vestibularly coloured, too. When the platform is stationary, this reconstruction is always veridical (i.e., ps′=0, independent of how the body moves relative to the platform). When the platform rotates, it correctly renders the high-frequency components of the rotation but fails to signal the low-frequency components because of its vestibular character.
In summary, the podokinesthetic pathway through the vestibular eigenmodel serves to eliminate exactly those components from the vestibular message that are caused by the movements of the head relative to the platform, i.e., by subjects’ counter-rotations and axial torsions; therefore, what is left (ps′), represents the contribution stemming from the platform rotation itself. Using control-systems terminology, an equivalent description states that the podokinesthetic pathway neutralises the feedback around the vestibular pathway and therefore renders the system open-loop with regard to the platform input (ps). Such a view is reminiscent of certain models of the smooth pursuit eye movement system, which suggest that an efference copy of the oculomotor drive eliminates the effect of visual feedback to create an internal percept of how the target moves in space, which, in turn, is thought to drive the motor output (‘perceptual feedback hypothesis’ (Yasui and Young 1975).
In the present context we propose that it is the internal percept of platform rotation in space (ps′) that is driving, with inverted sign, the podomotor system (ignoring for the moment box τ*→τ and its output ps′′ in Fig. 5B). Because there is effectively no feedback, a tracking gain (G) of exactly unity is required for accurate compensation of platform rotation (accurate in the sense that bp exactly matches the reconstructed platform rotation ps′). Slight deviations from this value in either direction would nicely account for the occurrence of both undershoots and overshoots of subjects’ initial responses (i.e., for positive and negative errors, respectively).
On the other hand, large deviations of G from unity would entail correspondingly large residual head-in-space rotations and, hence, lead to suprathreshold vestibular stimulation. Conceivably, in such a case, the resulting vestibular message would exert a corrective influence upon the podomotor command (not shown in the figure); thus, the reconstruction hypothesis does not preclude vestibular feedback as an auxiliary mechanism.
Because of the open-loop character implied by the reconstruction hypothesis the podomotor response to the sudden onset of a constant velocity platform rotation is similar to the step response of the vestibular system. Hence, from what has been described up to now, one would expect the counter-rotation to decline along a time-constant of about 8 s (peripheral time-constant τ*). This is clearly wrong; as evident from Table 1, the experimentally observed time-constant had a median value of 36 s (mean 59 s), and some subjects exhibited much larger values. However, it is well known that already at the level of the brain stem, neural mechanisms act to increase the short peripheral value of the time-constant (‘velocity storage’; Raphan et al. 1979). Although textbooks suggest that these mechanisms only double the time-constant, at best (τ=10–15 s; Leigh and Zee 1999), others have observed population means of almost 25 s (Peterka et al. 1990), with individual values ranging from 15 to 50 s. Also, many psychophysical observations suggest that central mechanisms increase the time-constant to values ranging between 15 and 20 s (Guedry 1974;Young 1981) or even up to 45 s (Mittelstaedt and Mittelstaedt 1996). Therefore, to explain the present results on the basis of our reconstruction hypothesis, we postulate a central stage that upgrades the effective time constant of the internal platform signal ps′ from its low peripheral value (τ*) to the much larger values (τ) that characterise subjects’ perception and counter-rotation (box τ*→τ in Fig. 5B). An analogous postulate occurs in recent models of self- and object-motion perception by Mergner and collaborators (“partial reconstruction of low frequency content” (Mergner et al. 1991). The introduction of this stage should not be seen as a mere trick to force the ‘correct’ response upon the reconstruction hypothesis model. We hold that it reflects a specific processing in the human orientation system that is characterised by a large intraindividual variability that cannot be explained as an emergent property of the over-all structure of the model itself—note that the upgrading of τ* ranges from nonexistent (subject #109, cf. Table 1) to virtually infinity (#110). In view of the likewise extended range of the individual time-constants of the VOR and of their fairly elevated mean reported by Peterka et al. (1990, see above), it does not seem unlikely that this processing shares much of the circuitry upgrading the vestibular signal for its use with the VOR.
As shown in Fig. 6B, upon variation of the efficiency of the upgrading mechanism and, hence, of τ, the reconstruction model produces a family of response curves that closely parallel the individual averages of our subjects in Fig. 6 C. As explained above, with small variations of G, the model also would replicate the slight overshoot or undershoot of the initial response that characterises individual subjects (see curve G=1.2 in Fig. 6B).
Finally, by focussing on the proprioceptive cues of mainly podokinesthetic origin, the reconstruction hypothesis is in good agreement with the subjects’ introspective impressions that the rotations about their ankle, knee and hip joints tells them the platform rotation and, thence, by how much they should step to compensate for it. Kinesthetic information from these joints could be provided by receptors in the joint capsules, by fusiform activity from the muscles acting on these joints, and by cutaneous receptors adjacent to the joints and on the soles (part of the counter-rotation occurs by a pivoting movements of the feet while in contact with the platform surface). Studies into the perceptions of body sway (Fitzpatrick and McCloskey 1994), of support pitch or roll (Teasdale et al. 1999), and of self-rotation in yaw (Mergner et al. 1993) concur in concluding that the perceptual acuity of this proprioceptive and somatosensory information is considerably better than that of vestibular canal and graviceptive afferents.
Sinusoidal and random velocity rotations
During sinusoidal rotations of 12 mHz, both the phase leads of subjects’ responses and their residual head-in-space rotations were, on average, similar to those calculated from their constant-velocity responses (Fig. 3A,B). Being linear, the scheme in Fig. 6B, which instantiates our reconstruction hypothesis, therefore is also applicable to the case of sinusoidal platform rotation without any further change.
On the other hand, at the same frequency of 12 mHz the phase leads during random rotations were significantly smaller (by about 35% on average) and there was somewhat less residual hs rotation than was predicted from VC responses. We considered the possibility that these improvements were caused by a threshold mechanism related to the central prolongation of the time-constant (stage τ*→τ). It can be argued that the recovery of the low-frequency content by this operation entails the risk of generating inappropriate signals from vestibular noise and, hence, a delusion of support motion during standstill; because in reality such delusions rarely occur, it has been inferred that spurious noise is fended off by the action of a threshold (see Appendix in Mergner et al. 2001). This threshold could have attenuated the low-frequency (12 mHz) signal that occurred during sinusoidal stimulation, whereas the additional high-frequency components arising during random rotations could have carried a larger proportion of the 12-mHz component across it. However, detailed simulations based on the same VN profiles as used in the experiments failed to support this conjecture. We are left to speculate that the repeated occurrence of high accelerations and turnarounds in the random condition has a priming effect upon the central prolongation of the time-constant.
As already pointed out, our observation of similar phase leads during sinusoidal and constant velocity rotations and of smaller phase leads during random rotations is incompatible with the occurrence of a prediction based on regular temporal features. This conclusion is reminiscent of results obtained in studies of the vestibulo-ocular reflex where comparisons between responses to random and sinusoidal rotations also failed to reveal signs of a predictive mechanism (Furman et al. 1979; Peterka et al. 1990; Bouveresse et al. 1998). The lack of a predictive intervention is of particular relevance with regard to subject #110: we were surprised to see that he apparently was able to increase τ to a value close to infinity during constant velocity (VC) rotations. Because VC trials were presented on two different days and were interleaved with those of other subjects, it is unlikely that a methodological imperfection had provided a directional cue, and retrospective questioning also did not support this possibility. Therefore, a first guess was that the subject used a cognitive strategy of consciously perpetuating his initial pace of stepping during VC rotations, a guess seemingly supported by the subject’s commenting “I tried to continue at the same pace; I thought the platform was always turning”. However, such a strategy, or any other strategy drawing on regular features, is not feasible with random rotations of the platform where subject #110 also achieved a nearly perfect compensation down to the lowest frequencies. Note also that the subject’s comment does not preclude the possibility that his endeavour to continue at the same pace was only secondary to the perception of a continuing rotation, a perception that could have been entertained by the reconstruction mechanism. We conclude that all responses of subject #110 fit into the scheme outlined in Fig. 5B, while we admit to be unaware how exactly his (τ*→τ) upgrading mechanism achieved a value of ≈∞.
Action equal to perception?
Our reconstruction scheme does not distinguish between perception and action. Signal ps′′ in Fig. 6 B can likewise be viewed as representing the perception of platform rotation in space or as being a motor control signal. This is probably an oversimplification. In supplementary experiments in which the authors served as subjects, it was observed that the perception (of platform rotation) ceased earlier than the action (counter-rotation on platform, Fig. 1). This observation does not invalidate the reconstruction hypothesis, though. It merely suggests that (1) the internal reconstruction of platform rotation in space is used in slightly different ways by the perceptual and motor systems, respectively, and (2) the motor response is not driven by conscious perception in the manner of a continuous one-to-one copy. The latter contention obviously does not exclude the possibility that other perceptions interfere with the motor response, mostly in an intermittent, discontinuous way. For example, when subjects suddenly gain the impression of performing wrongly (for whichever reason), they abruptly accelerate or decelerate their counter-rotation. All-in-all, however, such conscious ‘corrections’ (which did not always improve stability) were rare and limited to a few subjects only.
Conclusions
The performance of human subjects during the attempt to maintain their spatial alignment on a rotating platform in the absence of vision is, in our view, best explained by the reconstruction hypothesis outlined in Fig. 5B. According to this hypothesis, the motor response compensating for platform rotation is driven by an internal reconstruction of the platform’s rotation in space, based on a subtractive combination of vestibular and podokinesthetic information. An alternative but equivalent description holds that this subtractive combination offsets all vestibular feedback caused by the subject’s circling on the platform, thus creating an open-loop system whose input is platform rotation and whose output is the subject’s compensatory action.
Why should evolution have preferred control by an effectively open-loop system to a direct feedback control by vestibular afferents? To be effective (in the sense of achieving small errors within a short time), vestibular feedback requires that the error (the vestibularly sensed head-in-space rotation) be considerably amplified. In conjunction with the delays that inevitably arise in the nervous system this entails a danger of unstable operation, which may be one reason to avoid pure feedback control. Another, and probably more important, aspect is that, for a flexible control of behaviour, the central nervous system should not only be informed that ‘something’ is happening (in our case an undesired rotation of head and body in space) but also why it is happening (because the platform is rotating). Understanding the ‘why’ calls for an internal representation of the physics (mainly kinematics and kinetics) of the scenario in which the individual is engaged and allows a distinction between extraneous events (e.g., platform rotation) and self-generated ones (rotation relative to the platform). In a generalised scenario, not only podokinesthetic but also axial proprioceptive information will contribute to this representation. The flexibility of such an approach becomes apparent when the individual attempts to reduce (or to increase) his axial torsion (head-to-body, body-to-feet) while engaged in the counter-rotation: although stimulating the vestibular system, these rotations will have no effect on the ongoing compensatory response which, therefore, can proceed independent of these adjustments.
On the other hand, the postulated open-loop control has an obvious drawback: its accuracy depends critically on both the correct combination of the vestibular and podokinesthetic cues (including their appropriate homogenisation by the vestibular eigenmodel), and on the correct translation of the resulting representation of platform motion into the podomotor command (gain G in Fig. 5B). These concerns can be alleviated by noting that, ecologically, the most important function of the hypothesised reconstruction of support motion is to ensure that we perceive our support as stationary when we are moving on firm ground (unlike in the present experiments). Errors of processing would cause a delusion of ground movement, in conflict with visual and contextual information; if occurring systematically, such a conflict is likely to lead to an adaptive correction of the underlying processing error. Moreover, simulations suggest that slight errors in the emulation of the vestibular time constant by the vestibular eigenmodel do not much affect the characteristics of counter-rotation. Yet, small errors do indeed occur as witnessed by the small overshoots and undershoots of the initial counter-rotation in responses to the onset of VC stimuli.
The present discussion is not meant to deny the existence of directly acting vestibular reflexes for postural control. Their advantage is to be fast (no time wasted for computations and for transfer to and from computing stages) and veridical (within the limits set by the dynamic characteristics of the vestibular system). In case the open-loop control advocated here derails because of computational errors, the vestibular cue would detect the resulting instability and act as a ‘safety brake’ by eliciting a rapid corrective intervention of its own. It therefore can be viewed as a feedback that short-circuits the open-loop structure in Fig. 5B in case of large errors.
References
Becker W, Nasios G, Raab S, Jürgens R (2002) Fusion of vestibular and podokinesthetic information during self-turning towards instructed targets. Exp Brain Res 144:458–474
Bouveresse A, Kalfane K, Gentine A, Eichhorn JL, Kopp C (1998) Pseudorandom rotational stimuli of the vestibulo-ocular reflex in humans: normal values of the transfer function. Acto Otorhinolaryngol Belg 52:207–214
Fernandez C, Goldberg JM (1971) Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. II. Response to sinusoidal stimulation and dynamics of peripheral system. J Neurophysiol 34:661–675
Fitzpatrick RC, McCloskey DI (1994) Proprioceptive, visual and vestibular thresholds for the perception of sway during standing in humans. J Physiol 478:173–186
Furman JM, O’Leary DP, Wolfe JW (1979) Application of linear system analysis to the horizontal vestibulo-ocular reflex of the alert rhesus monkey using pseudorandom binary sequence and single frequency sinusoidal stimulation. Biol Cybern 33:159–165
Guedry FE (1974) Psychophysics of vestibular sensation. In: Kornhuber HH (ed) Handbook of Sensory Physiology, vol VI/2, Vestibular system. Springer, Berlin Heidelberg New York, pp 1–154
Igarashi M (1967) Dimensional study of the vestibular apparatus. Laryngoscope 77:1806–1817
Jürgens R, Nasios G, Becker W (2003) Vestibular, optokinetic, and cognitive contribution to the guidance of passive self-rotation toward instructed targets. Exp Brain Res 151:90–107
Leigh RJ, Zee DS (1999) The neurology of eye movements. Oxford University Press, New York Oxford, p 45
McIlroy WE, Maki BE (1994) The ‘deceleration response’ to transient perturbation of upright stance. Neurosci Lett 175:13–16
Mergner T, Becker W (2003) A modeling approach to the human spatial orientation system. Ann N Y Acad Sci 1004:303–315
Mergner T, Siebold C, Schweigart G, Becker W (1991) Human perception of horizontal trunk and head rotation in space during vestibular and neck stimulation. Exp Brain Res 85:389–404
Mergner T, Rottler G, Kimmig H, Becker W (1992) Role of vestibular and neck inputs for the perception of object motion in space. Exp Brain Res 89:655–668
Mergner T, Hlavacka F, Schweigart G (1993) Interaction of vestibular and proprioceptive inputs. J Vestib Res 3:41–57
Mergner T, Huber W, Becker W (1997) Vestibular-neck interaction and transformation of sensory coordinates. J Vestib Res 7:347–367
Mergner T, Nasios G, Maurer C, Becker W (2001) Visual object localisation in space. Interaction of retinal, eye position, vestibular and neck proprioceptive information. Exp Brain Res 141:33–51
Mittelstaedt M-L, Mittelstaedt H (1996) The influence of otoliths and somatic graviceptors on angular velocity estimation. J Vestib Res 6:355–366
Outerbridge JS, Melvill Jones G (1971) Reflex vestibular control of head movement in man. Aerosp Med 42:935–940
Peterka RJ, Black FO, Schoenhoff MB (1990) Age-related changes in human vestibulo-ocular and optokinetic reflexes: Pseudorandom rotation tests. J Vestib Res 1:61–71
Raphan T, Matsuo V, Cohen B (1979) Velocity storage in the vestibulo-ocular reflex arc (VOR). Exp Brain Res 35:229–248
Roberts TDM (1995) Understanding balance. Chapman and Hall, London, p 121ff
Teasdale N, Nougier V, Barraud P-A, Bourdin C, Debû B, Poquin D, Raphel C (1999) Contribution of ankle, knee, and hip joints to the perception threshold for support surface rotation. Percept Psychophys 61:615–624
Yasui S, Young LR (1975) Perceived visual motion as effective stimulus to pursuit eye movement system. Science 190:906–908
Young LR (1981) Perception of the body in space: mechanisms. In: Geiger SR (ed) Handbook of physiology. Section 1: The nervous system, vol III. American Physiological Society, Bethesda, pp 1023–1066
Acknowledgements
We are pleased to acknowledge the help of Dr. Grigorios Nasios who conducted some of the experiments. Thanks are due also to Ralph Kühne for developing and maintaining every sort of electronic hard- and software, and to Bruno Glinkemann for his shop work. Supported by Deutsche Forschungsgemeinschaft grant Be 783/3.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Diekmann, V., Jürgens, R. & Becker, W. Maintaining spatial body alignment on a rotating platform by means of active counter-circling: role of vestibular and podokinesthetic afferents. Exp Brain Res 158, 504–518 (2004). https://doi.org/10.1007/s00221-004-1921-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-004-1921-2