Abstract
The present study examined whether the excitability of the corticospinal pathway and the GABA-mediated inhibitory circuits of the primary motor cortex that project onto the corticospinal neurons in the tonically contracting hand muscle are changed by tonic contraction of the adjacent hand muscle. The motor evoked potential (MEP) and cortical silent period (CSP) in the tonically contracting hand muscle were obtained while the adjacent hand muscle was either tonically contracting or at rest. The MEP and CSP of the first dorsal interosseous (FDI) muscle elicited across the scalp sites where the MEP is predominantly elicited in the FDI muscle were decreased by tonic contraction of the abductor digiti minimi (ADM) muscle. The centers of the area of the MEP and the duration of the CSP in the FDI muscle elicited across the sites where the MEP is predominantly elicited in the FDI muscle were lateral to those in the FDI muscle elicited across the sites where the MEP is elicited in both the FDI and ADM muscles. They were also lateral to those in the ADM muscle elicited either across the sites where the MEP is predominantly elicited in the ADM muscle, or across the sites where the MEP is elicited in both the FDI and ADM muscles. The decrease in the corticospinal excitability and the excitability of the GABA-mediated inhibitory circuits of the primary motor cortex that project onto the corticospinal neurons in the FDI muscle may be due either to (1) the interaction between the activity of the lateral area of the FDI representation and the descending drive to the ADM muscle, or (2) the decreased susceptibility of the primary motor area that predominantly projects onto the corticospinal neurons in the FDI muscle, which also plays a role in independent finger movement when both the FDI and ADM muscles act together as synergists.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Production of force by some fingers causes involuntary force production of the other fingers (Zatsiorsky et al. 2000). Moreover, force production by one finger decreases force production of the other fingers and cessation of force production by the same finger increases that (Li et al. 2003). Such interaction of motor outputs between the fingers must be partially mediated by horizontal interaction among motor cortical cells (Huntley and Jones 1991). Indeed, inactivation of the motor cortical cells decreases the independence of finger movements (Schieber and Poliakov 1998), indicating that motor cortical cells contribute to the balance between independent and coordinated finger movements.
Coordination of motor cortical or corticospinal activities between tonically contracting hand muscles has been investigated using transcranial magnetic stimulation (TMS). The amplitude of the motor evoked potential (MEP) in the tonically contracting abductor digiti minimi (ADM) muscle elicited at the hotspot was not changed by tonic contraction of the first dorsal interosseous (FDI) muscle (Poston et al. 2012). In contrast, the MEP amplitude averaged across the TMS sites around the hotspot of the representation of the tonically contracting muscle was changed by tonic contraction of another muscle. The average amplitude of the MEPs elicited across the agonist muscle representation in the arm was decreased by tonic contraction of the antagonist muscle (Aimonetti and Nielsen 2002). Similarly, the average amplitude of the MEPs elicited across the TMS sites around the hotspot of the representation of the tonically contracting FDI muscle was decreased by tonic contraction of the ADM muscle (Jono et al. 2015). In contrast, the average amplitude of the MEPs elicited across the TMS sites around the hotspot of the distal arm muscle representation was increased by tonic contraction of the proximal arm muscle (Tyč and Boyadjian 2011). Accordingly, the tonic contraction of one muscle does not affect the MEP of another tonically contracting muscle elicited at the hotspot, but it does affect the average amplitude of the MEPs elicited across the TMS sites around the hotspot. It is possible to elicit the MEP in one hand muscle not only at the hotspot, but also at some sites around the hotspot (Wassermann et al. 1992; Wilson et al. 1995). Therefore, a plausible explanation for the different findings between the study on the MEP elicited at the hotspot and the studies on the MEP across the TMS sites around the hotspot is that motor cortical cells, which play a role in hand muscle coordination, are unequally distributed across the TMS sites around the hotspot and relatively sparse at the hotspot. Accordingly, it seems reasonable to speculate that the TMS sites around the hotspot interact with the intracortical interneurons that mediate coordination between the hand muscles.
Different finger movements partially share functional somatotopy of the primary motor area (Sanes et al. 1995; Kleinschmidt et al. 1997; Beisteiner et al. 2001; Hlustik et al. 2001; Dechent and Frahm 2003). The representations of the muscles that overlap each other are thought to play a role in the coordination of finger movements, while the muscle representations that do not overlap each other play a role in independent movements (Cunningham et al. 2013). This hypothesis was studied in a previous investigation on the MEP map (Jono et al. 2015). In that study, the active TMS sites where TMS elicits MEP in the FDI and/or ADM muscles were categorized into two areas: the overlapped (OL) area, where TMS elicits MEP in both the FDI and ADM muscles, and the non-overlapped (NOL) area, where TMS elicits MEP predominantly in one of the two muscles. This previous study revealed that the MEP of the tonically contracting FDI muscle elicited across the NOL area was decreased by tonic contraction of the ADM muscle, although this effect was absent in the MEP elicited across the OL area. This finding indicates that the descending drive to the ADM muscle decreases the excitability of the corticospinal pathway that predominantly projects onto the FDI muscle, but does not change the excitability of the corticospinal pathway that projects onto both the FDI and ADM muscles.
Despite Jono et al.’s findings (2015), two issues remain to be elucidated. One is whether the effect of the tonic contraction of the hand muscle on the corticospinal excitability of the tonically contracting adjacent hand muscle is dependent on the muscle tested. Different interactions take place among the descending drives to the different fingers. While the index finger moves independently, the little finger moves together with the other fingers (Häger-Ross and Schieber 2000; Reilly and Hammond 2000; Slobounov et al. 2002b; Aoki et al. 2005). The motor-related cortical potential was found to be greater when force production of the ring finger was combined with force production of the other fingers, but smaller when force production of the index finger was combined with that of the other fingers (Slobounov et al. 2002b). In addition, the surround inhibition of the MEP is not equivalent between the hand muscles (Beck et al. 2008). Accordingly, the decrease in excitability of the corticospinal pathway that predominantly projects onto the tonically contracting FDI muscle induced by tonic contraction of the ADM muscle, found in the previous study (Jono et al. 2015), may not occur in the other hand muscles. Based on this view, we hypothesize that the descending drive to the ADM muscle interacts with the motor representation that predominantly projects onto the corticospinal neurons in the FDI muscle. This is because the ADM muscle moves the little finger, which is interactive with the other fingers, but the descending drive to the FDI muscle is not interactive with the motor representation that predominantly projects onto the corticospinal neurons in the ADM muscle; the FDI muscle moves the index finger, which frequently moves independently in daily motor tasks. If this hypothesis is correct, the MEP of the tonically contracting FDI muscle elicited in the NOL area is affected by tonic contraction of the ADM muscle, but the MEP of the tonically contracting ADM muscle in the NOL area is not affected by tonic contraction of the FDI muscle. The present study tested this hypothesis.
Another issue to be elucidated is whether tonic contraction of one hand muscle affects the inhibitory circuits of the primary motor cortex that project onto the corticospinal neurons in the adjacent hand muscle. The effect of the tonic contraction of the hand muscle on intracortical inhibition of the adjacent hand muscle representation has been investigated via observation of the MEP elicited at the hotspot. Studies have found that short-latency intracortical inhibition (SICI) of one-hand muscle representation is not changed either by tonic or phasic contraction of the adjacent hand muscle (Sohn and Hallett 2004a; Beck et al. 2008); long-latency intracortical inhibition, however, was decreased by phasic contraction of the adjacent hand muscle (Sohn and Hallett 2004a). Despite these findings, the effect of the tonic contraction of one hand muscle on the inhibitory circuits of the primary motor cortex that project onto the corticospinal neurons in the tonically contracting adjacent hand muscle has not been investigated. The long-lasting cortical silent period (CSP) represents the activity of the inhibitory circuits mediated by intracortical γ-aminobutyric acid (GABA) (Nakamura et al. 1997; Siebner et al. 1998; Chen et al. 2008). The duration of the CSP in the FDI muscle during a grip task was shown to be shorter than that during an index finger abduction task (Tinazzi et al. 2003). Furthermore, the CSP duration of the ADM muscle was shortened by phasic movement of the index finger, but was not changed by tonic movement of the index finger (Poston et al. 2012), indicating that excitability of the GABA-mediated inhibitory circuits of the primary motor cortex that project onto the corticospinal neurons in the hand muscle are changed in a task-dependent manner. Given these facts, the excitability of not only the corticospinal neurons but also the GABA-mediated inhibitory circuits of the primary motor cortex that project onto the corticospinal neurons in the tonically contracting hand muscle may be decreased by tonic contraction of the adjacent hand muscle. This hypothesis was also tested in the present study.
Materials and Methods
Participants
Ten healthy participants aged 28.9 ± 1.9 years (nine males and one female) participated in this study. All participants were right-handed according to the Edinburgh Handedness Inventory (Oldfield 1971). No participant had any history of orthopedic or neurological issues. The experimental protocol was explained to, and informed consent was obtained from, all individual participants included in the study. All procedures were approved by the Ethics Committee of Osaka Prefecture University and were conducted according to the Declaration of Helsinki.
Apparatus
Each participant was seated in a chair. The right forearm was in the prone position, and the right hand was placed on a table with the palm facing downward. The middle and ring fingers and the forearm were sandwiched by metal plates fixed to the table, so that the fingers and the forearm were maintained at the start position throughout each trial. Electromyographic (EMG) activities of the FDI and ADM muscles were recorded using Ag/AgCl surface electrodes configured in belly-tendon montages. The EMG signals were amplified and band-pass filtered (15 Hz–3 kHz) using an amplifier (MEG-1200; Nihon Kohden, Tokyo, Japan). A display with the targets of the EMG levels in the FDI and ADM muscles and a cursor indicating actual EMG levels was placed in front of the participant (Fig. 1d). A cursor along the vertical axis indicated the EMG level of the FDI muscle, while a cursor along the horizontal axis indicated the EMG level of the ADM muscle. The angles of the index and little fingers were recorded using electrogoniometers fixed over the fingers. The signals from the electrogoniometers were amplified via strain amplifiers (DPM-712B; Kyowa Dengyo, Tokyo, Japan). The EMG signals and the signals from the electrogoniometers were converted to digital signals at a sampling rate of 10 kHz using an A/D converter (PowerLab 800S; AD Instruments, Colorado Springs, CO, USA) and stored on a personal computer.
Motor tasks of the experiment. Black objects indicate the metal plates, while black and gray arrows indicate the direction of finger movement (a, b, c). The participant abducts the index finger when testing the FDI muscle (a) and abducts the little finger when testing the ADM muscle (b) in the TEST task. The participant abducts the index and little fingers simultaneously in the TEST + COND task (c). The participant abducts the finger or fingers with visual feedback of the EMG level on a display (d). A cursor (closed circle) indicating EMG levels in the FDI and ADM muscles and targets (opened circles) is indicated on the display. Target “A” indicates the point at which the FDI muscle contracts at 10 % of the MVC without contraction of the ADM muscle; target “B” indicates the point at which the ADM muscle contracts at 10 % of the MVC without contraction of the FDI muscle, while target “AB” indicates the point at which both the FDI and ADM muscles contract at 10 % of the MVC
TMS
TMS was delivered using a figure-of-eight coil (YM-133B; Nihon Kohden) connected to a magnetic stimulator (SMN-1200; Nihon Kohden). The maximum intensity of the coil was 0.96 T. The intersection of the coil was placed tangentially to the scalp with the two wings at a 45° angle to the sagittal plane, and a posterior-anterior electrical current in the brain was induced (Sakai et al. 1997). Hotspots where the MEP amplitude was maximum among the TMS sites were determined in each muscle. The resting motor threshold (RMT) of the FDI muscle—defined as the minimal intensity of the magnetic stimulator output producing MEP with an amplitude larger than 50 μV in at least five out of ten stimulations delivered over the hotspot—was determined (Groppa et al. 2012). The earlier component of the CSP is the spinal origin, but the late component of the CSP is the cortical origin (Fuhr et al. 1991; Inghilleri et al. 1993; Chen et al. 1999). Accordingly, the CSP long enough to involve the late component must be obtained to observe the inhibitory circuits of the primary motor cortex. Furthermore, since the CSP duration is dependent on TMS intensity (Inghilleri et al. 1993), the TMS intensity used for the present experiment was 120 % of the RMT in the FDI muscle (Daskalakis et al. 2003).
Motor Tasks
The participant abducted the index or little finger at maximum effort for 5 s to obtain an EMG trace while exerting maximum voluntary contraction (MVC) of the tested muscle. The MVC was considered to be the average amplitude of the rectified EMG trace in a time window of approximately 3 s during the period when the amplitude level was steady. The participant performed a motor task with visual feedback of the EMG levels in the FDI and ADM muscles (Fig. 1d). In the TEST task of the FDI muscle, the participant abducted the index finger so that the FDI muscle produced an EMG at 10 % of the MVC without contraction of the ADM muscle (0 % of MVC) by placing a cursor in the monitor over target “A” (Fig. 1a, d). In the TEST task of the ADM muscle, the participant abducted the little finger so that the ADM muscle produced an EMG at 10 % of the MVC without contraction of the FDI muscle (0 % of MVC) by placing the cursor over target “B” (Fig. 1b, d). In the TEST + COND task, the participant abducted the index and little fingers simultaneously so that the FDI and ADM muscles produced an EMG at 10 % of the MVC by placing the cursor over target “AB” (Fig. 1c, d).
Mapping Procedure
TMS was delivered over the map, which was a fixed 5 × 5 cm grid (25 points) where the hotspot was centered (Pascual-Leone et al. 1995; Malcolm et al. 2006; Reilly and Mercier 2008). Since a map containing 15 MEP points is reliable according to a previous study, we considered our map to be reliable (Littmann et al. 2013). A session was conducted on each TMS site for each task. The coil was placed over one of the 25-point mapping sites at each session. For each session, TMS was delivered six times consecutively with intervals from 3 to 5 s at each mapping site for each task. The average MEP area estimated from the six MEPs was considered reliable, based on a previous study in which the average size of the MEP estimated from five MEPs was not significantly different between sessions (Littmann et al. 2013). The tasks and the TMS sites were randomly altered from session to session. The interval between the sessions was approximately 30 s. The twenty-five points of the TMS sites centered at the hotspot of the FDI representation were stimulated during the TEST task for the FDI muscle; those centered at the hotspot of the ADM representation were stimulated during the TEST task for the ADM muscle, while the TMS sites in both the FDI and ADM muscles were stimulated during the TEST + COND task (Fig. 2). Several familiarizing sessions were conducted before the experiment. The mapping procedure was completed within 2 h with several intermissions.
Mapping sites. The circles indicate the TMS sites. The letter “F” indicates the hotspot of the FDI representation, while the letter “A” indicates the hotspot of the ADM representation. The TMS sites of the FDI muscle in the TEST task are enclosed by a solid thin line; those of the ADM muscle in the TEST task are enclosed by a dashed line, and those in the TEST + COND task are enclosed by a bold line
Data Analysis
The EMG traces were rectified, and six of the rectified EMG traces from each TMS site were averaged for each task. The MEP area and pre-stimulus background EMG (bEMG) amplitude were estimated from the rectified and averaged EMG trace. The average pre-stimulus bEMG amplitude in the time window between 110 and 10 ms before TMS was estimated. The onset of the MEP area was the period at which the post-stimulus EMG amplitude first exceeded the pre-stimulus bEMG amplitude, and the offset was the period at which the post-stimulus EMG amplitude was first below the pre-stimulus bEMG amplitude. The onset and offset were both visually determined. In order to estimate the MEP area, the area of the MEP in the time window between the onset and offset of the MEP was provisionally estimated. Then, the provisionally estimated MEP area was subtracted from the area of the bEMG in the time window between the onset and offset of the MEP, which was the mean pre-stimulus bEMG amplitude multiplied by the time between the onset and offset of the MEP. CSP duration was defined as the interval between the TMS artifact and the reappearance of bEMG activity after the TMS. The active TMS site, where an MEP larger than two-thirds of the maximal MEP area across the 25-point map of each muscle was elicited, was identified (Classen et al. 1998; Reilly and Mercier 2008). The overlapping area between the active TMS sites in the muscle and the sites in the adjacent muscle was designated as the OL area. Thus, the OL area represents the scalp sites where TMS elicits a large MEP in both the FDI and ADM muscles. The active TMS sites that were not categorized into the OL area were considered the NOL area. Thus, the NOL area represents the sites where TMS elicits a large MEP either in the FDI or ADM muscle. The center of gravity (COG) of the OL area and that of the NOL area in the MEP and CSP maps were estimated to determine the spatial distribution of the excitability of the MEP and CSP across each area (Classen et al. 1998; Hetu et al. 2011; Littmann et al. 2013; Wassermann et al. 1992).
One-way analysis of variance (ANOVA) was conducted to test the difference in means for a main effect: [4 (COG)]. Two-way ANOVA was conducted to test the difference in means for two main effects: [2 (task) * 25 (TMS site)], [2 (finger) * 2 (task)], [2 (muscle) * 2 (task)], or [2 (OL vs. NOL) * 2 (task)]. When ANOVA revealed a statistical difference, Bonferroni’s multiple comparison test was conducted. When two-way ANOVA revealed a significant interaction, a test of simple main effect was conducted. A paired t test was performed to examine the difference between two means. A one-sample t-test was conducted to test whether the velocity of finger movement was significantly different from the theoretical zero velocity. The alpha level was 0.05. Statistical analysis was conducted using the software package Ekuseru-Toukei 2012 (Social Survey Research Information Co., Tokyo, Japan). Data are expressed as the mean and standard error of the mean.
Results
Behavioral Data
The pre-stimulus bEMG amplitude in the tonically contracting tested muscle was similar between the tasks and among the TMS sites. In the FDI muscle, the pre-stimulus bEMG amplitude was not significantly different between the tasks [F(1, 9) = 3.18, p = 0.11] or among the TMS sites [F(24, 216) = 0.57, p = 0.95], and there was no significant interaction between the two main effects [F(24, 216) = 0.98, p = 0.49]. Similarly, in the ADM muscle, the pre-stimulus bEMG amplitude was not significantly different between the tasks [F(1, 9) = 0.28, p = 0.61] or among the TMS sites [F(24, 216) = 0.66, p = 0.88], and there was no significant interaction between the two main effects [F(24, 216) = 0.64, p = 0.90].
The angle of each finger in the pre-stimulus time window is shown in Fig. 3a. The angle of index finger abduction was 11.8 ± 2.0° during the TEST task and 10.1 ± 1.8° during the TEST + COND task. The angle of little finger abduction was 13.8 ± 1.8° during the TEST task and 14.0 ± 1.8° during the TEST + COND task. The angle of finger abduction was not significantly different between the fingers [F(1, 9) = 2.30, p = 0.16] or between the tasks [F(1, 9) = 3.52, p = 0.09], and there was a significant interaction between the two main effects [F(1, 9) = 7.59, p < 0.05]. A test of the simple main effect revealed that the angle of index finger abduction during the TEST + COND task was significantly smaller than that during the TEST task [F(1, 18) = 10.40, p < 0.05].
The angular velocity of each finger movement is shown in Fig. 3b. The angular velocity of each finger movement was similar between the tasks and between the fingers. The angular velocity of index finger abduction was 0.04 ± 0.04°/s during the TEST task and 0.02 ± 0.04°/s during the TEST + COND task. The angular velocity of little finger adduction was 0.00 ± 0.11°/s during the TEST task and 0.01 ± 0.03°/s during the TEST + COND task. The one sample t-test revealed that the angular velocity of index finger abduction was not significantly different from the theoretical zero velocity both during the TEST (p = 0.31) and TEST + COND tasks (p = 0.59), and that of little finger abduction was not significantly different from the theoretical zero velocity both during the TEST (p = 1.00) and TEST + COND tasks (p = 0.76). These statistical results using one-sample t-tests indicate that the fingers were almost stationary at a position in the pre-stimulus period.
Hotspot
The loci of the hotspots are shown in Fig. 4a. The hotspot of the FDI representation and that of the ADM muscle were close to each other. Moreover, in eight out of 10 participants, the locus of the hotspot of the FDI representation and that of the ADM muscle were identical. The hotspot was located 61.0 ± 2.3 mm lateral to and 15.0 ± 3.4 mm anterior to the vertex in the FDI muscle, and 62.0 ± 2.5 mm lateral to and 13.0 ± 3.3 mm anterior to the vertex in the ADM muscle. The TMS intensity at the RMT of the FDI muscle was 62.5 ± 1.6 %, and that of the ADM muscle was 63.1 ± 1.4 % of the maximum stimulator output. The MEP area and CSP duration were similar between the tasks and between the muscles, as shown in Fig. 4b, c. The MEP area at the hotspot was not significantly different between the muscles [F(1, 9) = 3.33, p = 0.10] or between the tasks [F(1, 9) = 0.47, p = 0.51], and there was no significant interaction between the two main effects [F(1, 9) = 1.11, p = 0.32]. The CSP duration at the hotspot was not significantly different between the muscles [F(1, 9) = 2.05, p = 0.19] or between the tasks [F(1, 9) = 2.01, p = 0.19], and there was no significant interaction between the two main effects [F(1, 9) = 1.92, p = 0.20].
COG of the OL and NOL Areas
The specimen record of the rectified EMG traces in each grid of the 25-point map is presented in Fig. 5. The COGs of the MEP are shown in Fig. 6a. The COG of the MEP in the FDI muscle elicited in the OL area was 3.8 ± 1.2 mm medial to and 3.7 ± 2.5 mm anterior to the hotspot, and that elicited in the NOL area was 4.2 ± 2.1 mm lateral to and 2.9 ± 2.6 mm anterior to the hotspot. The COG of the MEP in the ADM muscle elicited in the OL area was 5.0 ± 1.6 mm medial to and 5.5 ± 2.5 mm anterior to the hotspot, and that elicited in the NOL area was 6.9 ± 1.4 mm medial to and 3.9 ± 3.5 mm posterior to the hotspot. The COG of the MEP in the mediolateral axis was significantly different among the four areas [F(3, 35) = 8.93, p < 0.05]. A post hoc test revealed that the COG of the MEP in the FDI muscle elicited in the NOL area was significantly lateral to the COG of the MEP in the FDI muscle elicited in the OL area or the COG of the MEP in the ADM muscle elicited in the NOL or OL area (p < 0.05). In contrast, no significant difference in the COG of the MEP in the anterior-posterior axis was found among the four areas [F(3, 35) = 2.10, p = 0.12].
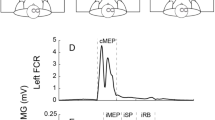
Specimen EMG traces. Each trace indicates the average of six rectified EMG traces in the time window between 110 ms before TMS and 240 ms after TMS. EMG traces in the upper panels are from the FDI muscle (a–c), while those in the lower panels are from the ADM muscle (d–f). An EMG response in each trace indicates an MEP
The COGs of the CSP are shown in Fig. 6b. Similar to the COG of the MEP, the COG of the CSP of the FDI muscle elicited at the NOL area was particularly lateral to the other COGs of the CSP. The COG of the CSP elicited in the OL area was 4.1 ± 1.3 mm medial to and 4.0 ± 2.5 mm anterior to the hotspot, while that elicited in the NOL area was 4.3 ± 2.2 mm lateral to and 3.0 ± 2.6 mm anterior to the hotspot in the FDI muscle. The COG of the CSP elicited in the OL area was 4.9 ± 1.6 mm medial to and 5.7 ± 2.4 mm anterior to the hotspot, and that elicited in the NOL area was 7.0 ± 1.4 mm medial to and 3.9 ± 3.5 mm posterior to the hotspot in the ADM muscle. The COG of the CSP in the mediolateral axis was significantly different among the four areas [F(3, 35) = 8.80, p < 0.05]. A post hoc test revealed that the COG of the CSP of the NOL area in the FDI muscle was significantly lateral to the other three areas (p < 0.05). In contrast, the COG of the CSP in the anterior-posterior axis was not significantly different among the four areas [F(3, 35) = 2.20, p = 0.10].
Task Effect on the OL and NOL Areas
The number of active TMS sites in the FDI muscle is shown in Fig. 7a. The number of active TMS sites was similar between the OL and NOL areas and between the tasks. The number of active TMS sites in the OL area was 4.1 ± 0.8 during the TEST task and 4.9 ± 0.8 during the TEST + COND task. The number of active TMS sites in the NOL area was 5.0 ± 1.3 during the TEST task and 5.5 ± 1.1 during the TEST + COND task. The number of active TMS sites was not significantly different between the tasks [F(1, 9) = 1.90, p = 0.20] or between the areas [F(1, 9) = 0.42, p = 0.53], and there was no significant interaction between the two main effects [F(1, 9) = 0.02, p = 0.89]. In contrast, in the ADM muscle, the number of active TMS sites in the OL area was higher than that in the NOL area, as shown in Fig. 7d. In the ADM muscle, the number of active TMS sites in the OL area was 4.4 ± 0.9 during the TEST task and 4.8 ± 0.9 during the TEST + COND task. The number of active TMS sites in the NOL area was 1.6 ± 0.3 during the TEST task and 0.9 ± 0.3 during the TEST + COND task. The number of active TMS sites was not significantly different between the tasks [F(1, 8) = 0.11, p = 0.75], but the number of active TMS sites in the NOL area was significantly smaller than that in the OL area [F(1, 8) = 18.02, p < 0.05], without significant interaction between the two main effects [F(1, 8) = 1.29, p = 0.29].
The number of the active TMS sites (a, d), the MEP area (b, e), and the CSP duration (c, f) in the OL and NOL areas. The upper panels represent data from the FDI muscle (a–c) and the lower panels represent data from the ADM muscle (d–f). Bars indicate the mean, and error bars indicate the standard error. An asterisk indicates a significant difference (p < 0.05)
Tonic contraction of the adjacent muscle decreased the area of the MEP elicited in the NOL area in the FDI muscle, but did not decrease this area in the ADM muscle, as shown in Fig. 7b, e. The MEP area was not significantly different between the tasks [F(1, 9) = 4.96, p = 0.05], but was significantly different between the OL and NOL areas [F(1, 9) = 21.16, p < 0.05], with significant interaction between the two main effects [F(1, 9) = 6.18, p < 0.05] in the FDI muscle. The test of the simple main effect revealed that the MEP area during the TEST + COND task was significantly smaller than that during the TEST task in the NOL area [F(1, 16) = 10.63, p < 0.05], and the MEP elicited in the NOL area was significantly smaller than that elicited in the OL areas during the TEST + COND task [F(1, 17) = 26.50, p < 0.05]. The MEP elicited in the NOL area was decreased by 18 % in the FDI muscle. In the ADM muscle, the MEP area was not significantly different between the tasks [F(1, 8) = 0.07, p = 0.80] or between the sites [F(1, 8) = 4.96, p = 0.06], and there was no significant interaction between the two main effects [F(1, 8) = 0.10, p = 0.76].
The tonic contraction of the adjacent muscle decreased the duration of the CSP elicited in the NOL area in the FDI muscle, but it did not do so in the ADM muscle, as shown in Fig. 7c, f. The duration of the CSP in the FDI muscle elicited in the OL area was 164.5 ± 9.6 ms during the TEST task and 160.0 ± 8.6 ms during the TEST + COND task. The duration of the CSP in the FDI muscle elicited in the NOL area was 161.2 ± 10.7 ms during the TEST task and 139.7 ± 9.9 ms during the TEST + COND task. The CSP duration was not significantly different between the tasks [F(1, 9) = 3.94, p = 0.08], but was significantly different between the sites [F(1, 9) = 7.63, p < 0.05]; there was a significant interaction between the two main effects [F(1, 9) = 5.67, p < 0.05]. The test of the simple main effect revealed that the duration of the CSP elicited in the NOL area during the TEST + COND task was significantly shorter than that elicited in the NOL area during the TEST task [F(1, 14) = 8.29, p < 0.05], and the duration of the CSP elicited in the NOL area was significantly shorter than that elicited in the OL area during the TEST + COND task [F(1, 17) = 13.29, p < 0.05]. The duration of the CSP in the FDI muscle elicited in the NOL area was decreased by 12 %. In the ADM muscle, the duration of the CSP elicited in the OL areas was 154.6 ± 6.6 ms during the TEST task and 140.4 ± 6.7 ms during the TEST + COND task. The duration of the CSP elicited in the NOL area was 157.1 ± 9.7 ms during the TEST task and 155.3 ± 7.9 ms during the TEST + COND task. The CSP duration in the ADM muscle was not significantly different between the tasks [F(1, 8) = 3.25, p = 0.11] or between the areas [F(1, 8) = 2.13, p = 0.18], and there was no significant interaction between the two main effects [F(1, 8) = 3.79, p = 0.09].
Discussion
The present study investigated whether the excitability of the corticospinal pathway and inhibitory circuits of the primary motor cortex that project onto the corticospinal neurons in the tonically contracting hand muscle is changed by tonic contraction of the adjacent hand muscle. The area of the MEP and duration of the CSP in the FDI muscle elicited by TMS over the NOL area were decreased by tonic contraction of the ADM muscle, but these decreases were absent in the MEP and CSP elicited across the OL areas of the FDI muscle, as well as in those elicited across both the OL and NOL areas of the ADM muscle.
Methodological Consideration
In a previous study, the effect of the tonic contraction of the ADM muscle on the MEP elicited across the active TMS sites of the MEP map in the tonically contracting FDI muscle was investigated (Jono et al. 2015). In this previous study, participants abducted their index and/or little fingers against rigid plates fixed over a table. When they abducted their fingers against the fixed rigid plates, cutaneous sensation was likely produced by counter force from the plates. In the present study, cutaneous sensation was minimal during the tasks, because the index and/or little fingers were freely abducted. Thus, cutaneous sensation must not be the cause of the change in the MEP area or CSP duration. When moving the fingers freely, proprioceptive afferents from the contracting muscle are produced. However, such proprioceptive afferents must not have been related to the changes in the MEP area or changes in the CSP duration either, because the velocity of finger motion was almost zero when TMS was delivered. While the MEP is dependent on the bEMG level (Devanne et al. 1997; Hasegawa et al. 2001), the CSP is not (Inghilleri et al. 1993; Kojima et al. 2013). Nevertheless, the pre-stimulus bEMG level must not have affected the MEP map in the tested muscle, because EMG activity was maintained at a certain level; indeed, the level was not significantly different among the TMS sites or among the tasks. The onset of fatigue-induced facilitation of the MEP occurs after 6 min of sustained contraction of the tested muscle with 20 % of the MVC, and that of the CSP occurs after 13 min of such contraction (Sacco et al. 1997). However, in the present study, each session terminated before the onset of fatigue: the participant sustained the target EMG level (10 % of MVC) for only about 30 s each session. Thus, the fatigue within each session must not have been the cause of the changes in the MEP area and CSP duration. In addition, fatigue across sessions must not have been the cause of the changes in the MEP area or CSP duration, because the effect of long-term fatigue was cancelled by random alternation of the TMS sites and tasks session by session. Abduction of the index finger in the TEST task of the FDI muscle was smaller than that in the TEST + COND task of the same muscle. This may have been related to the change in mechanical constraint induced by abduction of the little finger; the tension of the connective tissues in the hand increased during little finger abduction and increased the passive force pulling the index finger to the side of the little finger. The total sum of the number of active TMS sites in the OL and NOL areas was around seven in the present study. The number of active TMS sites in the hand muscles was from 20 to 40 when an amplitude of MEP larger than 10 μV was considered to be active (Wassermann et al. 1992), but it was around six when MEPs larger than two-thirds of the maximum MEP amplitude were considered to be active (Reilly and Mercier 2008). The small number of active TMS sites in the present study must have been due to the strict criteria for defining active TMS sites: only MEPs larger than two-thirds of the maximum MEP size were considered to be active, following the criteria of Reilly and Mercier (2008). Finally, the change in the number of active TMS sites must not have been the cause of the changes in the MEP area or CSP duration induced by tonic contraction of the adjacent muscle, because the number was not significantly different between the tasks.
Hotspot
The MEP and CSP elicited at the hotspot in the tonically contracting muscle were not affected by tonic contraction of the adjacent muscle. This finding was consistent with the previous finding that tonic contraction of the FDI muscle did not change the amplitude of the MEP or the duration of the CSP in the tonically contracting ADM muscle elicited at the hotspot (Poston et al. 2012). MEP size as a function of TMS intensity forms a sigmoidal curve (Devanne et al. 1997; Carrol et al. 2001). High-intensity TMS was delivered to elicit a sufficient length of CSP in the present study, as well as in the previous study by Poston et al. (2012). In our study, TMS with an intensity of 120 % of RMT was delivered during tonic contraction with 10 % MVC; in the previous study by Poston et al. (2012), TMS with an intensity of 130 % of RMT was delivered during tonic contraction with 5 % MVC. Accordingly, one may speculate that the excitability of the MEP in the tested muscle elicited by high-intensity TMS at the hotspot was not changed by tonic contraction of the conditioned muscle due to the ceiling effect resulting from the large MEP size. However, such a phenomenon was not likely, because even when TMS with intensity at the RMT was delivered over the hotspot, the MEP amplitude in the FDI muscle at rest was not changed by tonic contraction of the ADM muscle in a previous study (Jono et al. 2015).
Moreover, the amplitude of the MEP and the duration of the CSP in both the FDI and ADM muscles elicited at the hotspot were not changed by tonic contraction of the adjacent muscle, indicating that the changes in excitability of the corticospinal pathway and the GABA-mediated inhibitory circuits elicited at the hotspot induced by tonic contraction of the adjacent muscle were not dependent on the muscle tested. In previous studies, the MEP amplitude in the ADM or abductor pollicis brevis (APB) muscle elicited at the hotspot was decreased by phasic contraction of the FDI muscle (Sohn and Hallett 2004a, b; Beck et al. 2008; Poston et al. 2012), and the MEP amplitude in the ADM or APB muscle at rest elicited at the hotspot was facilitated by tonic contraction of the FDI muscle (Hess et al. 1986; Beck et al. 2008). These previous findings were derived from a task in which the conditioned muscle was phasically contracted or the tested muscle was at rest. In contrast, when the tested muscle was tonically contracted, the changes in the amplitude of the MEP and/or the duration of the CSP elicited at the hotspot induced by tonic contraction of the adjacent muscle were absent (Poston et al. 2012; Jono et al. 2015). Accordingly, it is likely that the excitability of the corticospinal pathway and the GABA-mediated inhibitory circuits of the primary motor cortex that project onto the corticospinal neurons in the hand muscle elicited at the hotspot is not modulated by the contraction of the adjacent muscle when the tested muscle is tonically contracted.
OL and NOL Areas
The MEP area and CSP duration of the FDI muscle elicited in the NOL area were decreased by tonic contraction of the ADM muscle, although such a decrease was not observed in either the MEP or CSP of the FDI muscle elicited in the OL area, or those of the ADM muscle elicited in either the OL or NOL area. These findings indicate that excitability of the corticospinal pathway and the GABA-mediated inhibitory circuits of the primary motor cortex that predominantly project onto the corticospinal neurons in the FDI muscle are specifically decreased by the descending drive to the ADM muscle. One question regarding this finding is why both the MEP area and CSP duration were similarly decreased by adjacent muscle contraction. Consistent with the present finding, both the amplitude of the MEP and the duration of the CSP in the tonically contracting muscle elicited at the hotspot were previously reported to be decreased by phasic contraction of the adjacent muscle (Poston et al. 2012). As speculated by Poston and colleagues in this previous study, the similar decreases in the MEP area and CSP duration induced by contraction of the adjacent muscle must be mediated by different neural mechanisms.
The CSP duration recorded in the present study was considered to represent the activity of the intracortical inhibitory circuits in the motor representation, as it was long enough to represent cortical activity (Fuhr et al. 1991; Inghilleri et al. 1993; Chen et al. 1999). More specifically, the long-lasting CSP represents the activity of the inhibitory circuits mediated by GABA (Nakamura et al. 1997; Siebner et al. 1998; Chen et al. 2008). Thus, the effect of the tonic contraction of the adjacent muscle on the CSP observed in the present study means that tonic contraction of the ADM muscle decreases the excitability of the GABA-mediated inhibitory circuits in the representation that predominantly project onto the corticospinal neurons in the FDI muscle.
On the other hand, various neural circuits that project onto the corticospinal neurons in the FDI muscle are possible candidates for the decrease in the MEP area. TMS transsynaptically activates the intracortical circuits, inducing descending volleys (the D-wave and I1, I2, and I3 waves). These volleys produce an MEP in the tested muscle. Each I-wave is mediated by different interneurons that project onto the corticospinal neurons (Di Lazzaro et al. 2004). Moreover, when the inhibitory interneurons are activated by the paired-TMS, the MEP is decreased no matter how long the inter-stimulus interval is (Chen et al. 1999), indicating that the activity of various inhibitory interneurons that project onto the corticospinal neurons decreases the MEP. The decrease in the long-lasting CSP duration observed in the present study reflects the decrease in excitability of the GABA-mediated inhibitory neurons acting for the CSP (Nakamura et al. 1997; Siebner et al. 1998; Chen et al. 2008). The SICI is inhibited during the CSP, indicating that the activity of the GABA-mediated inhibitory neurons acting for the CSP inhibits the SICI (Ni et al. 2007). In the present study, both the MEP area and CSP duration of the FDI muscle were decreased by tonic contraction of the ADM muscle. Given these findings, one possible explanation for the observed decrease in the MEP area of the FDI muscle induced by tonic contraction of the ADM muscle in the present study would be disinhibition of the SICI due to a decrease in excitability of the GABA-mediated intracortical inhibitory neurons acting for the CSP.
Muscle-specific Effect
In contrast to the findings in the FDI muscle, tonic contraction of the FDI muscle did not change the excitability of the corticospinal pathway or the GABA-mediated inhibitory circuits of the primary motor cortex that project onto the corticospinal neurons in the ADM muscle. The NOL area of the FDI muscle was particularly lateral to the OL area of the FDI muscle and lateral to the OL and NOL areas of the ADM muscle. Accordingly, the present finding might be explained by the viewpoint that the activity of the lateral area of the FDI representation is particularly interactive with the descending drive to the ADM muscle. An alternative explanation for the muscle-specific effect of adjacent muscle contraction may be a difference in supraspinal control between the index and little fingers. The index finger moves independently in daily activities. Such independence of the index finger is supported by previous findings that involuntary force production of the fingers induced by force production of the index finger is smaller than that induced by force production of the little finger (Häger-Ross and Schieber 2000; Reilly and Hammond 2000; Slobounov et al. 2002a, b), and that the tapping speed of the index finger is faster than that of the little finger when single-finger or double-finger tapping is performed (Aoki et al. 2005). The FDI and ADM muscles become the synergists when the index and little fingers are abducted together, because simultaneous abduction of the index and little fingers is common in daily activities, such as when spreading out the fingers. When those muscles act as the synergists, an isolated descending drive to the FDI muscle may be detrimental for the coordinated activity of these muscles. Therefore, the decrease in the MEP and CSP elicited in the NOL area of the FDI muscle induced by tonic contraction of the ADM muscle can be reasonably explained by the decrease in susceptibility of the circuits of the primary motor cortex that project onto the corticospinal neurons in the relatively independent FDI muscle to prevent isolated activity of the muscle causing independent index finger movement, when the FDI muscle acts together with the ADM muscle as the synergist.
Conclusion
The area of the MEP and the duration of the CSP in the FDI muscle elicited across the sites where the MEP is predominantly elicited in the FDI muscle were decreased by tonic contraction of the ADM muscle. In contrast, the MEP area and CSP duration of the FDI muscle elicited across the sites where the MEP is elicited in both the FDI and ADM muscles, and those of the ADM muscle elicited both across the sites where the MEP is predominantly elicited in the ADM muscle and the sites where the MEP is elicited in both the FDI and ADM muscles, were not changed by tonic contraction of the adjacent hand muscle. These area-dependent and muscle-specific decreases in the MEP area and CSP duration may reflect the fact that the activity of the lateral area of the FDI representation interacts with the descending drive to the ADM muscle, or the fact that the susceptibility of the motor representation that projects onto the FDI muscle, which plays a role in independent finger movement, is decreased when both the FDI and ADM muscles act together as synergists.
References
Aimonetti JM, Nielsen JB (2002) Cortical excitability and motor task in man: an investigation of the wrist extensor motor area. Exp Brain Res 143(4):431–439. doi:10.1007/s00221-002-1010-3
Aoki T, Furuya S, Kinoshita H (2005) Finger-tapping ability in male and female pianists and nonmusician controls. Mot Control 9(1):23–39
Beck S, Richardson SP, Shamim EA, Dang N, Schubert M, Hallett M (2008) Short intracortical and surround inhibition are selectively reduced during movement initiation in focal hand dystonia. J Neurosci 28(41):10363–10369. doi:10.1523/JNEUROSCI.3564-08.2008
Beisteiner R, Windischberger C, Lanzenberger R, Edward V, Cunnington R, Erdler M, Gartus A, Streibl B, Moser E, Deecke L (2001) Finger somatotopy in human motor cortex. Neuroimage 13(6 Pt 1):1016–1026. doi:10.1006/nimg.2000.0737
Carroll TJ, Riek S, Carson RG (2001) Reliability of the input-output properties of the cortico-spinal pathway obtained from transcranial magnetic and electrical stimulation. J Neurosci Methods 112(2):193–202. doi:10.1016/S0165-02700100468-X
Chen R, Lozano AM, Ashby P (1999) Mechanism of the silent period following transcranial magnetic stimulation evidence from epidural recordings. Exp Brain Res 128(4):539–542. doi:10.1007/s002210050878
Chen R, Cros D, Curra A, Di Lazzaro V, Lefaucheur JP, Magistris MR, Mills K, Rösler KM, Triggs WJ, Ugawa Y, Ziemann U (2008) The clinical diagnostic utility of transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol 119(3):504–532. doi:10.1016/j.clinph.2007.10.014
Classen J, Knorr U, Werhahn KJ, Schlaug G, Kunesch E, Cohen LG, Seitz RJ, Benecke R (1998) Multimodal output mapping of human central motor representation on different spatial scales. J Physiol 512(Pt 1):163–179. doi:10.1111/j.1469-7793.1998.163bf.x
Cunningham DA, Machado A, Yue GH, Carey JR, Plow EB (2013) Functional somatotopy revealed across multiple cortical regions using a model of complex motor task. Brain Res 1531:25–36. doi:10.1016/j.brainres.2013.07.050
Daskalakis ZJ, Molnar GF, Christensen BK, Sailer A, Fitzgerald PB, Chen R (2003) An automated method to determine the transcranial magnetic stimulation-induced contralateral silent period. Clin Neurophysiol 114(5):938–944. doi:10.1016/S1388-2457(03)00038-5
Dechent P, Frahm J (2003) Functional somatotopy of finger representations in human primary motor cortex. Hum Brain Mapp 18(4):272–283. doi:10.1002/hbm.10084
Devanne H, Lavoie BA, Capaday C (1997) Input–output properties and gain changes in the human corticospinal pathway. Exp Brain Res 114(2):329–338. doi:10.1007/PL00005641
Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, Insola A, Tonali PA, Rothwell JC (2004) The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol 115(2):255–266. doi:10.1016/j.clinph.2003.10.009
Fuhr P, Agostino R, Hallett M (1991) Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalogr Clin Neurophysiol 81(4):257–262. doi:10.1016/0168-5597(91)90011-L
Groppa S, Oliviero A, Eisen A, Quartarone A, Cohen LG, Mall V, Kaelin-Lang A, Mima T, Rossi S, Thickbroom GW, Rossini PM, Ziemann U, Valls-Solé J, Siebner HR (2012) A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol 123(5):858–882. doi:10.1016/j.clinph.2012.01.010
Häger-Ross C, Schieber MH (2000) Quantifying the independence of human finger movements: comparisons of digits, hands, and movement frequencies. J Neurosci 20(22):8542–8550
Hasegawa Y, Kasai T, Tsuji T, Yahagi S (2001) Further insight into the task-dependent excitability of motor evoked potentials in first dorsal interosseous muscle in humans. Exp Brain Res 140(4):387–396. doi:10.1007/s002210100842
Hess CW, Mills KR, Murray NMF (1986) Magnetic stimulation of the human brain: facilitation of motor responses by voluntary contraction of ipsilateral and contralateral muscles with additional observations on an amputee. Neurosci Lett 71(2):235–240. doi:10.1016/0304-3940(86)90565-3
Hetu S, Gagne M, Reilly KT, Mercier C (2011) Short-term reliability of transcranial magnetic stimulation motor maps in upper limb amputees. J Clin Neurosci 18(5):728–730. doi:10.1016/j.jocn.2010.09.011
Hlustik P, Solodkin A, Gullapalli RP, Noll DC, Small SL (2001) Somatotopy in human primary motor and somatosensory hand representations revisited. Cereb Cortex 11(4):312–321. doi:10.1093/cercor/11.4.312
Huntley GW, Jones EG (1991) Relationship of intrinsic connections to forelimb movement representations in monkey motor cortex: a correlative anatomic and physiological study. J Neurophysiol 66(2):390–413
Inghilleri M, Berardelli A, Cruccu G, Manfredi M (1993) Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol 466:521–534
Jono Y, Chujo Y, Nomura Y, Tani K, Nikaido Y, Hatanaka R, Hiraoka K (2015) The effect of tonic contraction of the hand muscle on the motor cortical representation of the contracting adjacent muscle. Somatosens Mot Res 32(2):114–121. doi:10.3109/08990220.2014.994738
Kleinschmidt A, Nitschke MF, Frahm J (1997) Somatotopy in the human motor cortex hand area. A high-resolution functional MRI study. Eur J Neurosci 9(10):2178–2186. doi:10.1111/j.1460-9568.1997.tb01384.x
Kojima S, Onishi H, Sugawara K, Kirimoto H, Suzuki M, Tamaki H (2013) Modulation of the cortical silent period elicited by single- and paired-pulse transcranial magnetic stimulation. BMC Neurosci 14:43. doi:10.1186/1471-2202-14-43
Li S, Latash ML, Zatsiorsky VM (2003) Finger interaction during multi-finger tasks involving finger addition and removal. Exp Brain Res 150(2):230–236. doi:10.1007/s00221-003-1449-x
Littmann AE, McHenry CL, Shields RK (2013) Variability of motor cortical excitability using a novel mapping procedure. J Neurosci Methods 214(2):137–143. doi:10.1016/j.jneumeth.2013.01.013
Malcolm MP, Triggs WJ, Light KE, Shechtman O, Khandekar G, Gonzalez Rothi LJ (2006) Reliability of motor cortex transcranial magnetic stimulation in four muscle representations. Clin Neurophysiol 117(5):1037–1046. doi:10.1016/j.clinph.2006.02.005
Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H (1997) Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J Physiol 498(3):817–823. doi:10.1113/jphysiol.1997.sp021905
Ni Z, Gunraj C, Chen R (2007) Short interval intracortical inhibition and facilitation during the silent period in human. J Physiol 583(Pt 3):971–982. doi:10.1113/jphysiol.2007.135749
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9(1):97–113. doi:10.1016/0028-3932(71)90067-4
Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M (1995) Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol 74(3):1037–1045
Poston B, Kukke SN, Paine RW, Francis S, Hallett M (2012) Cortical silent period duration and its implications for surround inhibition of a hand muscle. Eur J Neurosci 36(7):2964–2971. doi:10.1111/j.1460-9568.2012.08212.x
Reilly KT, Hammond GR (2000) Independence of force production by digits of the human hand. Neurosci Lett 290(1):53–56. doi:10.1016/S0304-3940(00)01328-8
Reilly KT, Mercier C (2008) Cortical topography of human first dorsal interroseus during individuated and nonindividuated grip tasks. Hum Brain Mapp 29(5):594–602. doi:10.1002/hbm.20421
Sacco P, Thickbroom GW, Thompson ML, Mastaglia FL (1997) Changes in corticomotor excitation and inhibition during prolonged submaximal muscle contractions. Muscle Nerve 20(9):1158–1166. doi:10.1002/(SICI)1097-4598(199709)20:9<1158:AID-MUS11>3.0.CO;2-P
Sakai K, Ugawa Y, Terao Y, Hanajima R, Furubayashi T, Kanazawa I (1997) Preferential activation of different I waves by transcranial magnetic stimulation with a figure-of-eight-shaped coil. Exp Brain Res 113(1):24–32. doi:10.1007/BF02454139
Sanes JN, Donoghue JP, Thangaraj V, Edelman RR, Warach S (1995) Shared neural substrates controlling hand movements in human motor cortex. Science 268(5218):1775–1777. doi:10.1126/science.7792606
Schieber MH, Poliakov AV (1998) Partial inactivation of the primary motor cortex hand area: effects on individuated finger movements. J Neurosci 18(21):9038–9054
Siebner HR, Dressnandt J, Auer C, Conrad B (1998) Continuous intrathecal baclofen infusions induced a marked increase of the transcranially evoked silent period in a patient with generalized dystonia. Muscle Nerve 21(9):1209–1212. doi:10.1002/(SICI)1097-4598(199809)21:9<1209:AID-MUS15>3.0.CO;2-M
Slobounov S, Johnston J, Chiang H, Ray W (2002a) The role of sub-maximal force production in the enslaving phenomenon. Brain Res 954(2):212–219. doi:10.1016/S0006-8993(02)03288-2
Slobounov S, Johnston J, Chiang H, Ray WJ (2002b) Motor-related cortical potentials accompanying enslaving effect in single versus combination of fingers force production tasks. Clin Neurophysiol 113(9):1444–1453. doi:10.1016/S1388-2457(02)00195-5
Sohn YH, Hallett M (2004a) Surround inhibition in human motor system. Exp Brain Res 158(4):397–404. doi:10.1007/s00221-004-1909-y
Sohn YH, Hallett M (2004b) Disturbed surround inhibition in focal hand dystonia. Ann Neurol 56(4):595–599. doi:10.1002/ana.20270
Tinazzi M, Farina S, Tamburin S, Facchini S, Fiaschi A, Restivo D, Berardelli A (2003) Task-dependent modulation of excitatory and inhibitory functions within the human primary motor cortex. Exp Brain Res 150(2):222–229. doi:10.1007/s00221-003-1448-y
Tyč F, Boyadjian A (2011) Plasticity of motor cortex induced by coordination and training. Clin Neurophysiol 122(1):153–162. doi:10.1016/j.clinph.2010.05.022
Wassermann EM, McShane LM, Hallett M, Cohen LG (1992) Noninvasive mapping of muscle representations in human motor cortex. Electroencephalogr Clin Neurophysiol 85(1):1–8. doi:10.1016/0168-5597(92)90094-R
Wilson SA, Thickbroom GW, Mastaglia FL (1995) Comparison of the magnetically mapped corticomotor representation of a muscle at rest and during low-level voluntary contraction. Electroencephalogr Clin Neurophysiol 97(5):246–250. doi:10.1016/0924-980X(95)80001-8
Zatsiorsky VM, Li ZM, Latash ML (2000) Enslaving effects in multi-finger force production. Exp Brain Res 131(2):187–195. doi:10.1007/s002219900261
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jono, Y., Iwata, Y., Mizusawa, H. et al. Change in Excitability of Corticospinal Pathway and GABA-Mediated Inhibitory Circuits of Primary Motor Cortex Induced by Contraction of Adjacent Hand Muscle. Brain Topogr 29, 834–846 (2016). https://doi.org/10.1007/s10548-016-0499-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-016-0499-x