Abstract
Current models of the basal ganglia assume a firing-rate code for information processing. We have applied five complementary computing methods to assess firing patterns in 188 cells of the substantia nigra in the anaesthetized rat. Fractal firing activity was found in 100% of nigral cells projecting to the superior colliculus, in 51% of cells projecting to the thalamus and in 33% of cells projecting to the pedunculopontine nucleus, but was practically absent in dopaminergic nigrostriatal neurons (3%). The finding of fractal firing patterns may lead to a better understanding of the normal operational mode and pathological manifestations of the basal ganglia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The substantia nigra (SN) is a key structure in the control of input and output neuronal activity of the basal ganglia (BG) (Gerfen et al. 1990; Parent and Hazrati 1995; Obeso et al. 2000). It is composed of two cell groups: a dopaminergic group that projects to the striatum (ST) and a GABAergic group that projects to the thalamus, superior colliculus (SC) and pedunculopontine nucleus (PPTg) (González-Hernández and Rodríguez 2000). The current model of BG assumes a firing-rate code (the time between consecutive spikes is the only significant variable) for information processing in the SN. However, a firing-pattern code involving a complex relationship between several successive spikes may also be operative (Abeles 1991, Wilson et al. 1977; Terman et al. 2002). We evaluated the latter possibility by studying the stochastic properties of SN cell activity over a large range of time scales, paying special attention to those measures that reveal the existence of fractal activity. A fractal is an object composed of sub-units that resemble the larger scale structures, a property known as scale-invariance or self-similarity (Mandelbrot 1997). Fractals with scale-invariance have been found in both the space (e.g. morphology of the tracheobronchial tree) and time (signals with a similar fluctuation in multiple time scales) domains (Goldberger 1996). Fractals in the time domain may be identified in the electrophysiological activity of neurons when spike firing shows clustering, with clusters of spikes within larger clusters and so forth, over a range of cluster sizes (Teich et al. 1997). In this study, we used the multi-spike interval histogram (MIH) and the recurrence plot (Faure and Korn 1997) to unravel the existence of the long-term correlation characteristic of fractal series. Additionally, the fractal grouping of spikes was confirmed by the Fano (FF) and Allan (AF) factors (Fano 1947; Allan 1966) and the periodogram (Lowen and Teich 1996).
Methods
The activity of SN neurons was recorded in male Sprague-Dawley rats (300–350 g) using previously reported procedures (Rodríguez and González-Hernández 1999). Since fractal behaviour has been described in visual and auditory neurons (Lowen and Teich 1996), and these sensory systems may influence SN cells (Schultz and Romo 1990), the experimental set up was prepared so as to minimize the effect of the environment on brain activity. Thus, the recording sessions were performed under chloral hydrate anaesthesia (400 mg/kg i.p.) in a sound-proofed, dark room and body temperature maintained at 36.5–37.5 °C. The extracellular activity of SN neurons was recorded with glass electrodes filled with 2 M NaCl containing 2% Pontamine Sky Blue (6–9 MΩ at 1,000 Hz). All recordings were obtained in the ventral mesencephalon (2.8–3.4 mm anterior to lambda, 2.0–2.5 mm lateral to the midline and 6.5–7.5 under the cortical surface; Paxinos and Watson 1988). The brain signal was amplified and filtered (200–5,000 Hz) in a CAN96T model (Telcan, Tenerife, Spain). The signal was digitized and stored in a Pentium-based computer using a 16-bit analogue-to-digital converter (LTI-C30, Tecnología de la Información, Madrid, Spain). Only recordings showing single-unit activity were used. Action potentials were considered as belonging to the same neuron when their spike shape, which was analysed by using both hardware (SD1 spike discriminator, Tucker-Davis Technologies, Gainesville, Fla., USA) and software (hybrid multi-layer artificial neural network; Garcia-Baez et al. 1998) procedures, remained stable during the recording session. The collision test was used to classify SN cells according to their antidromic activation from the caudate nucleus (0.0 mm anterior to bregma, 3.2 mm lateral to midline and 5 mm ventral to the brain surface), ventral thalamus (−2.5 mm posterior to bregma, 1.5 mm lateral to midline and 6.5–7 mm ventral to the brain surface), SC (1.5 mm anterior to lambda, 1.5–2.5 mm lateral to midline and 4.0–4.5 mm ventral to the brain surface) and PPTg (0.7 mm anterior to lambda, 1.5–2.5 mm lateral to midline and 7.5 mm ventral to the brain surface). The neuronal recording was considered valid for further analysis when it contained spike activity corresponding to only one cell (Garcia-Baez et al. 1998), showed an antidromic response to stimulation of one nucleus only and the duration of recording was of a minimum of 4 min.
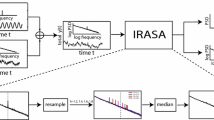
The recurrence plot of inter-spike intervals (ISI) is a useful graphic tool for the detection of complex structures in a signal (Takens 1981; Faure and Korn 1997; Abarbanel 1996). It was computed with delayed vectors formed by embedding the ISI series in 3-dimensional space (Takens 1981). The components of these 3-dimensional vectors were the values of the ISIs taken every τ=2 intervals. The value of τ was chosen by mutual information criteria to avoid spurious correlations between consecutive components of the vectors (Abarbanel 1996). Traditional recurrence plots are constructed on an all-or-nothing basis by placing a point in the position (i, j) whenever the vector i is closer to vector j than a fixed distance r. Instead, in this study, we used an extended version of this graphic tool, which provides a more comprehensive description of the dynamic (Manetti et al. 1999). A 2-dimensional plot was created by filling the position (i, j) with a colour that represented the distance between the vectors i and j. Finally, a bicubic spline smoothing procedure was used to fit the mesh surfaces of this 2-dimensional figure, a procedure that, despite the fact that it produces a slight asymmetry in the image (and causes the 45° line, where the embedding vector is compared with itself, to nearly disappear from view), often increases the visual clarity. Thus, clusters of points with similar colours in the recurrence plot reflect epochs of the signal with similar dynamics. Therefore, the existence of a long term correlation—such as a fractal dynamic—should cause a definite set of clusters to appear, whereas a random signal should exhibit a homogeneous distribution of colours.
Sequence of rates (SR) represents the modification of firing rate over time. The SR was calculated by counting the number of spikes found in contiguous non-overlapping time windows of duration T. The shuffled sequence of rates (SSR) is the SR of shuffled inter-spike intervals (SISI). The MIH is a probability-density function of the time elapsed between two spikes that has a number n of spikes between them. The MIH can be calculated for n=1, 2, 3…, the ISI histogram being the MIH when n=0. The expected distribution of accumulated intervals of a random point process is the negative binomial distribution (distribution of the number of Bernoulli trials before n potentials are found). The Chi-square test was used to identify differences between the MIH of original and surrogate ISIs. When accumulation of ISIs produced MIH with a dispersion higher than expected by random (negative binomial distribution) and higher in original than in the shuffled ISIs-sequences, data were interpreted as indicating that the natural order of ISIs increases the firing rate variability, which is a characteristic expected for fractal point processes. This possibility cannot be observed with the ISI histogram because it has no information about the relationship between successive ISIs.
A main requirement for identifying fractal activity is the observation of the power law that characterizes self-similarity. The power law was explored by analysing the variance of firing rate for an increasing observation window T. Three complementary approaches were used, the FF (Fano 1947), AF (Allan 1966) and the periodogram (Lowen and Teich 1996). The FF time curve is the ratio of the count variance to the count mean for increasing T. The AF time curve is the ratio of the event-number Allan variance (the mean of the square values of differences between successive observation windows) to twice the mean for increasing T. The periodogram curve estimates the power spectral density of a point process. It was calculated with the discrete Fourier transform for a 500-ms observation window. The finding of a power law function in the FF, AF and periodogram that vanished after the random shuffling of ISIs (constructed according to and as described by Teich, 1992) was used to identify fractality in firing activity. Differences between original and shuffled data were statistical when the FF and the AF time curves were not included within the range mean±1 SD of data shuffled 100 times (P<0.05, t-test, df=100).
Results
In response to antidromic stimulation, 188 SN-neurons, out of 475 tested, were identified according to their projection (Table 1). The recurrence plots showed distinctive clusters (Fig. 1C), that clearly decreased (Fig. 1D) after the random shuffling of ISI sequences (surrogate ISI). This indicates a complex non-random dynamic with long-term correlations suggesting "fractal behaviour" (similar clusters for different pairs of vectors that are far apart from each other). This possibility was subsequently demonstrated by different procedures (Fig. 2).
An example of an extended recurrence plot for a fractally firing nigrocollicular neuron (C) and its shuffled version (D). The sequences of the original (A) and shuffled (B) inter-spike intervals (ISI) are shown above (axis: number of ISI; ordinate: duration of the ISI). The colour of each point represents the distance (in ms) in a 3-dimensional space between two vectors corresponding to the ISIs displayed in the position x for vector one and y for vector two. The colour distribution shows long-term correlation clusters (C) that suggest the typical scale-invariance of fractals and that decreases after the random shuffling of ISI (D). Often a slight clustering is still apparent after ISI shuffling, which is probably induced by the finite size of the data sample
An example of a nigrocollicular neuron showing the basic characteristics of the fractal firing activity. As expected, the variance of the sequence of rates (SR) was lower for long-duration time windows (T), decreasing from T=100 to T=5,200 ms. However, this decrease (shuffled sequence of rates, SSR) was more marked after shuffling the ISIs (shuffled inter-spike intervals; SISIs) Thus, for any T value the variance of both the sequence of rates and the multi-spike interval histogram (MIH) was higher in the original than in the shuffled ISIs. The Allan factor (A), Fano factor (B) and periodogram (C) are shown at left. Original intervals are shown by solid lines, shuffled intervals by dotted lines (data were shuffled 100 times: the mean is indicated by the central dotted line and the standard deviation by the upper and lower dotted lines) and expected values for a random point process by discontinuous lines. For values of T>100 ms, the variance of the MIH was higher in the original than in the surrogate data. The AF, FF and periodogram show the typical power law dependence that characterizes the fractal activity
As Teich (1992) has pointed out, simple inspection of the firing rate plot for an increasing time window T (between 100–5,200 ms in the example in Fig. 2) shows a higher variance in the original (SR in Fig. 2) than in the shuffled (SSR in Fig. 2) ISIs. As expected for a fractal pattern, this difference increases with the amplitude of T. This high variability was verified with the MIH that was more dispersed in the original (Fig. 2 shows the MIH for accumulations of 2–100 ISIs) than in the shuffled (Fig. 2) data (curves distribution was compared with a Chi-square test). Thus, the natural order of the ISI sequence increased the variance of the mean firing rate, indicating fractality. The FF, AF and periodogram showed for an increasing observation window T a power law characteristic of a fractal pattern. This is shown in the example of Fig. 2 for observation windows that exceeded 100 ms for the AF (Fig. 2A) and FF (Fig. 2B) respectively. The periodogram showed a power law reduction of the power spectral density that corresponds with a fractal pattern (Fig. 2C). The power law vanished in all tests after the random shuffling of ISIs.
Some 40 out of 188 cells (21.3%) fulfilled the requisites for identifying a fractal pattern in the ISI sequences. (1) The variance of the MIH was higher in original, than in shuffled ISIs; (2) the recurrence plot suggested scale-invariance; (3) a power law was found in both the FF and AF and this vanished after the ISIs shuffling; (4) the power spectral density decreased as a power law function of the frequency in the original but not in shuffled ISIs. None of the remaining 148 neurons exhibited fractality with any of the four methods employed here. The presence of a fractal pattern was not evenly distributed in the different SN cell groups. It was present in 100% of nigrocollicular cells, 51% of nigrothalamic cells, 33% of nigropeduncular and 3.3% of nigrostriatal cells (Fig. 3)
Discussion
The prevailing model of the BG is mainly based on the assumption that neuronal processing is carried out by inhibitory/excitatory interaction between cells, the firing rate being the substrate for information transfer. A putative role for oscillatory neuronal activity and pattern of discharges in the BG is gaining increasing attention (Wilson et al. 1977; Wichmann and DeLong 1996; Terman et al. 2002; Farmer 2002). However, information may be coded in firing patterns in many different manners, giving rise to a large variety of possible codes whose identification is difficult with the usual methods. Using different mathematical algorithms we have recognized a fractal pattern in the firing activity of BG cells in the rat.
The common recognition of fractal-like patterns in physical and biological systems may cast doubt upon the importance and specificity of such activity when referred to neuronal functioning. The possibility of a noise-induced fractal pattern was considered in preliminary analyses (data not shown) conducted in our laboratory. A computerized simulation of different types of random point process and the inter-event intervals generated with a natural source of noise (nucleus disintegration of Ra226) were not associated with fractal patterns when analysed with the four mathematical methods applied in the experiment reported here. On the other hand, these methods showed homogenous and coherent results in neurons, with no cell showing "fractal patterns" with one method but not with the other three. More over, the fractal pattern was not ubiquitously present in SN cells. A net difference within cell populations was observed, ranging from 100% of GABAergic nigro-collicular neurons to 3% of DAergic nigro-striatal neurons showing fractal activity. Overall, it appears unlikely that an unspecific source of noise would be associated with such cellular distribution and specificity.
The origin of fractal patterns in the basal ganglia is not clear. Fractal activity has been identified in sensory areas of the cortex, which, projecting to the BG (Teich et al. 1997; Lowen and Teich 1996), could induce fractal firing in SN cells. The present findings do not support this possibility because our recordings were performed in anaesthetized rats under conditions of sensory deprivation. SN reticulata cells are integrated within several cortico-subcortical circuits (i.e. oculomotor, prefrontal, etc.) (Parent and Hazrati 1995; Middleton and Strick 1997), which could be the origin of fractal activity in SN cells. On the other hand, fractal patterns have been found in the opening and closing of ion channels (Liebovitch and Töth 1990) and in quantal neurotransmitter secretion (Lowen et al. 1997). Accordingly, fractal activity could be the result of a sub-cellular component of SN cells. Whether the origin of a fractal pattern of neuronal firing in the SN cells is "extrinsic" (i.e. related to circuits) or "intrinsic" (i.e. related to membrane properties and intracellular events) remains to be established. Regardless of its intimate mechanisms of origin, we believe the fractal pattern here described for SN firing reflects an operational mode that could be relevant to various functions of the BG.
One of the main characteristics of fractal images is the spatial-invariance which make it possible for an object to be reproduced by magnifying a portion of it (self-similarity). Self-similarity in the case of fractal signals make it possible for the information that they are carrying to be recovered at any moment with any sampling time (e.g. music recordings with fractal characteristics should sound similar even when reproduced at different velocities). This time-invariance could be useful for systems that, like the BG, receive and use a large load of sensory stimuli (Marsden 1982; Middleton and Strick 1997) to organize different motor patterns in parallel (Alexander et al. 1986; Middleton and Strick 2000), each of them with a specific time-cadence (for example walking, saluting and speaking simultaneously). In this regard, fractal firing of SN cells could be on the basis of the fractal dynamic observed in different motor patterns. This is the case for animal and human locomotion patterns (Goldberger et al. 1990; Hausdorff et al. 1995) that could be influenced by the fractal activity found in nigro-PPTg neurons (PPTg is considered as a locomotion region of the mesencephalon; Ross and Sinnamon 1984; Masdeu et al. 1994). The fractal dynamic also prevents persistence and perseveration, thus facilitating the exploration of all possibilities available to a system. Perseveration must be prevented in some BG functions such as the control of oculo-motor behaviour (Hikosaka and Wurtz 1983) and the switching of attention (Schultz 1998; Redgrave et al. 1999). The firing rate of nigro-collicular cells have a key role in orienting ocular movements toward salient visual stimulus (Hikosaka and Wurtz 1983) but probably it is not efficient in generating the random exploration of the visual field observed under basal conditions. The fractal firing found in 100% of nigro-collicular cells could allow a random exploration of the visual field, preventing, in the absence of salient stimulus, the repetitive exploration of the same visual region. The striking lack of fractal activity in DA cells may also be relevant for BG function. A fractal pattern of firing requires both a high variability of ISIs (variation coefficient of DA cells in Table 1 is half that expected by random) and the grouping of short or long ISIs (see MIH in Fig. 2). Dopaminergic neurons exhibit a relatively constant firing to keep extracellular DA levels in the striatum stable (Gonon 1998; Grace 1991), thus avoiding marked variations in the motor state (Obeso et al. 2000). It appears that the essential modulatory function of the DA nigro-striatal system would not fit with the characteristics of fractal activity. Some motor disturbances associated with BG disorders may also be associated with fractality. Loss of fractal activity promotes underlying periodic rhythms in different biological systems (Sugihara et al. 1987). Resting tremor in Parkinson´s disease is associated with rhythmical neuronal firing in the BG and thalamus not observed under normal condition (Zirh et al. 1997). We may hypothesize that a putative loss of fractal activity in nigrothalamic cells could be related with facilitation of rhythmical discharges and tremor onset.
In conclusion, the present study reports fractal dynamic in the unit activity of basal ganglia cells. The characteristics of fractal signals suggest that fractal firing could be particularly useful in the BG where very diverse information coming from all the cortex converge without apparent time-coherence.
References
Abarbanel HDI (1996) Analysis of observed chaotic data. Springer, Berlin Heidelberg New York
Abeles M (1991) Corticonics: neural circuits of the cerebral cortex. Cambridge University Press, New York
Alexander GE, Strick PJ, DeLong MR (1986) Parallel organization of functionally segregated circuits linking the basal ganglia and cortex. Annu Rev Neurosci 9:357–381
Allan DW (1966) Statistics of atomic frequency standards. Proc. IEEE 54:221–230
Fano U (1947) Ionization yield of radiations. II. The fluctuations of the number of ions. Phys Rev 72:26–29
Farmer S (2002) Neural rhythms in Parkinson's disease. Brain 125:1175–1176
Faure P, Korn H (1997) A random dynamic component in the synaptic noise of central neuron. Proc Natl Acad Sci USA 94:6506–6511
Garcia-Baez P, Suarez-Araujo CP, Rodríguez J, Rodríguez M (1998) Unsupervised classification of neural spikes with a multilayer artificial neural network. J Neurosci Methods 82:59–73
Gerfen CR, Engber TR, Mahan LC, Suzel Z, Chase TN, Monsma FR, Sibbley DR (1990) D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250:1429–1432
Goldberger AL (1996) Non-linear dynamics for clinicians: chaos theory, fractals, and complexity at the bedside. Lancet 347:1312–1314
Goldberger AL, Rigney DR, West BJ (1990) Chaos and fractals in human physiology. Sci Am 262:42–49
Gonon FG (1988) Nonlinear relationship between impulse flow and dopamine released by rat midbrain dopaminergic neurons as studied by in vivo electrochemistry. Neuroscience 24:19–28
González-Hernández T, Rodríguez M (2000) Compartimental organization and chemical profile of dopaminergic and GABAergic neurons in the substantia nigra of the rat. J Comp Neurol 421:107–135
Grace AA (1991) Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: an hypothesis for the etiology of schizophrenia. Neuroscience 41:1–24
Hausdorff JM, Peng CK, Ladin Z, Wei JY, Goldberger AL (1995) Is walking a random walk? Evidence for long-range correlations in the stride interval of human gait. J Appl Physiol 78:349–358
Hikosaka O, Wurtz RH (1983) Visual and oculomotor functions of monkey substantia nigra pars reticulata. I. Relation of visual and auditory responses to saccades. J Neurophysiol 49:1230–1301
Liebovitch LS, Töth TI (1990) Using fractals to understand the opening and closing of ion channels. Ann Biomed Eng 18:177–194
Lowen SB, Teich MC (1996) The periodogram and Allan variance reveal fractal exponents greater than unity in auditory-nerve spike trains. J Acoustic Soc Am 99:3585–3591
Lowen SB, Cash SS, Mu-ming Poo, Teich MC (1997) Quantal neurotransmitter secretion rate exhibits fractal behavior. J Neurosci 17:5666–5677
Mandelbrot B (1997) The fractal geometry of nature. Clotet-Tusquets Publishers, Barcelona
Manetti C, Ceruso MA, Giuliani A, Webber CL, Zbilut JP (1999). Recurrence quantification analysis in molecular dynamics. Ann NY Acad Sci 879:258–266
Marsden CD (1982) The mysterious motor function of the basal ganglia. Neurology 32:514–529
Masdeu JC, Alampur U, Cavaliere R, Tavoulareas G (1994) Astasia and gait failure with damage of the pontomesencephalic locomotor region. Ann Neurol 35:619–621
Middleton FA, Strick PL (1997) New concepts about the organization of basal ganglia output. Adv Neurol 74:57–68
Middleton FA, Strick PJ (2000) Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Rev 31:236–250
Obeso JA, Rodríguez-Oroz MC, Rodríguez M, DeLong MR, Olanow W (2000) Pathophysiology of levodopa-induced dyskinesias in Parkinson's disease: problems with the current model. Ann Neurol 47 (suppl. 1):22–34
Parent A, Hazrati L-N (1995) Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Rev 20:91–127
Paxinos G, Watson C (1988) The rat brain. Academic Press, Sydney
Redgrave P, Prescott T, Gurney K. (1999) Is the short-latency dopamine response too short to signal reward error?. Trends Neurosci 22:146–151
Rodríguez M, González-Hernandez T (1999) Electrophysiological and morphological evidence for a GABAergic nigrostriatal pathway. J Neurosci 19:4682–4694
Ross GS, Sinnamon HM (1984) Forelimb and hindlimb stepping by anesthetized rat elicited by electrical stimulation of the pons and medulla. Physiol Behav 33:201–208
Schultz W (1998) Predictive reward signal of dopamine neurons. J Neurophysiol 80:1–27
Schultz W, Romo R (1990) Dopamine neurons of the monkey midbrain: contingencies of responses to stimuli eliciting immediate behavioral reactions. J Neurophysiol 63:607–624
Sugihara G, Allan W, Sobel D, Allan KD (1996) Nonlinear control of heart rate variability in human infants. Proc Natl Acad Sci USA 93:2608–2613
Takens F (1981) Detecting strange attractors in turbulence. In: Warwick DA, Young LS (eds.) Dynamical systems and turbulence (Lecture notes in mathematics series, vol. 898). Springer, Berlin Heidelberg New York, pp 366–381
Teich MC (1992) Fractal neuronal firing patterns. In: McKenna T, Davis J, Zornetzer S (eds) Single neuron computation. Academic Press, Boston, pp 589–625
Teich MC, Heneghan C, Lowen SB, Ozaki T, Kaplan E (1997) Fractal character of the neural spike train in the visual system of the cat. J. Opt Soc Am [A] 14:529–546
Terman D, Rubin JE, Yew AC, Wilson CJJ (2002) Activity patterns in a model for the subthalamopallidal network of the basal ganglia. J Neurosci 22:2963–2976
Wichmann T, DeLong MR (1996) Functional and pathophysiological models of the basal ganglia. Curr Opin Neurobiol 6:751–758
Wilson CJ, Young SJ, Groves PM (1977) Statistical properties of neuronal spike trains in the substantia nigra: cell types and their interactions. Brain Res 136:243–260
Zirh TA, Lenz FA, Reich SG, Dougherty PM (1997) Patterns of bursting occurring in thalamic cells during parkinsonian tremor. Neuroscience 83:107–121
Acknowledgements
This work was supported by the Consejería de Educación del Gobierno de Canarias (PI2001/049), and the Ministerio de Ciencia y Tecnología del Gobierno Español (SAF2002-03382 and FISP.I.020194), Spain.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodríguez, M., Pereda, E., González, J. et al. Neuronal activity in the substantia nigra in the anaesthetized rat has fractal characteristics. Evidence for firing-code patterns in the basal ganglia. Exp Brain Res 151, 167–172 (2003). https://doi.org/10.1007/s00221-003-1442-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-003-1442-4