Abstract
Sichuan dark brick tea (Camellia sinensis) and Sichuan Fuzhuan brick tea have significantly different aroma characteristics although both of them have almost the same processing methods. Thus, these two types of tea were used as the research materials to determine the differences in their aroma compounds. The volatile compounds in the two types of tea were identified and quantified by headspace solid-phase microextraction coupled with gas chromatography–mass spectrometry (HS-SPME–GC–MS), results showed that they both had 37 common volatile compounds. Then the aroma-active components were identified by odour activity value (OAV). It was found that SFBT had 20 aroma-active components, of which β-ionone had the largest OAV (199547.72). SDBT has 21 aroma-active ingredients (including all 20 aroma-active components of SFBT), of which β-ionone again has the largest OAV (114800.66). Finally, the aroma profile differences between the two tea samples were studied by aroma profile tests, and the results showed that the main aroma differences of SDBT and SFBT were caused by β-ionone, epoxydihydrolinalool II, methyl salicylate, geranylacetone, nerolidol, benzaldehyde, benzyl acetate, nonanal, trans,trans-2,4-heptadienal and 1-octen-3-ol, in addition, defined SFBT’s ‘fungi flower aroma’ and SDBT’s ‘aged fragrance’ from the level of aroma monomer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Based on the sensory flavour and processing procedure, teas are classified as green, black, oolong, white, dark or yellow tea [10]. Sichuan Dark brick tea (SDBT) (Camellia sinensis) and Sichuan Fuzhuan brick tea (SFBT) are two important types of Chinese dark tea [31] which have 300 years of drinking history in China [68]. These two types of dark tea are generally distinguished by ‘Jin Hua’ (Eurotium cristatum) [22], which is generated by a unique “Fa-Hua” process used in the process of making the Fuzhuan brick tea, which promotes the reproduction and metabolism of the Eurotium cristatum [12], and a type of yellow mycelium (‘Jin Hua’) will be formed on the surface and inside of the tea. This also promotes the formation of the unique flavour quality of Fuzhuan brick tea [60].
SDBT and SFBT show features of “aged fragrance” and “fungi flower aroma”, respectively, even though both of them are considered as dark tea and show the basic aroma characteristics of dark tea. To be more specific, the volatile aroma components of SDBT and SFBT are significantly different, even though their raw fresh leaves are the same and the processing used for these two types of tea is similar (with only two different processing steps).
It was reported that the “aged fragrance” is slowly formed by oxidation during processing [73, 76], while the “fungi flower aroma” is formed by the special process called “Fa-Hua”—fermentation by Eurotium cristatum [42, 69, 71]. However, no study on the aroma components and odour characteristics of the aged fragrance and the fungi flower aroma has been reported, even though more than 600 aroma components have been found in tea [72].
It is very important to study the differences in the aroma components of SDBT and SFBT, not only because these differences have not yet been studied but also because the differences in these aroma components can reveal the secondary metabolism of Eurotium cristatum in the process of dark tea fermentation and the effects of these metabolites on the aroma quality of dark teas.
Over the past years, only the conventional sensory evaluation method has been used to characterize the aroma of tea [32, 33]. This method can distinguish the different types of tea but cannot determine the effect of a substance on the aroma. However, there are some new and efficient research methods which have emerged in recent studies on food flavour, such as odour activity value method [3, 11] and aroma descriptive profile test [26, 32, 33], which could evaluate the contributions of individual aroma compounds to the overall aroma profile in food flavour. These methods have enabled progress in the analysis of the main aroma components of foods [11, 58, 59]. But in terms of researches of tea aroma, recent studies have focused on green tea and black tea; there are only a minority of reports aiming to investigate the aroma compounds of dark tea, which remain to be fully characterized.
Therefore, to study the differences in the aroma components of SDBT and SFBT, and the secondary metabolism of Eurotium cristatum in the process of dark tea fermentation as well as the effects of these metabolites on the aroma quality of above dark teas, first, the headspace solid-phase microextraction coupled with gas chromatography–mass spectrometry (HS-SPME–GC–MS) method was used to study the aroma components of SDBT and SFBT, then the odour activity value (OAV) calculation and aroma profile test methods were used to study the aroma characteristics of “aged fragrance” and “fungi flower aroma”.
Materials and methods
Tea samples
Fresh leaves were picked from a tea garden on Huaqiu mountain in Qionglai City, Sichuan Province (N30°23′, E103°17′), in June 2017. The tea trees were 5 years old, the picking standard was one bud and three leaves, and the tea cultivar was Hua Qiu No. 1. All the fresh leaves of the same batch were picked on 1 day (50–60 kg), and then the tea samples were immediately produced. The processing site was Huaqiu Tea Industry Co. Ltd. in Sichuan. The production process is shown in Fig. 1, and the specific process parameters are as follows:
Process parameters of SDBT
Fixing, which step that enzyme inactivation was achieved at high temperatures (200 °C for setting and 85 ± 2 °C for actual leaves temperature) and short time(110 ± 5 s), using a rotary continuous fixation machine.
Kneading, which step leaf cells were broken by a knead machine (55 ± 1r/min for rotation rate setting and the duration was 40 min).
Post-fermentation, which step that post-fermentation was achieved in a proving room (the moisture content of leaves was 55%, 45 °C for temperature setting and the duration was 22 h), then the tea material was pressed into brick shape with a mould (24 cm × 18 cm × 10 cm).
Ageing, which step that tea bricks were dried to 16% moisture content and then aged in a dry ventilated room (20 °C for ambient temperature and relative humidity was 55%) in 90 days.
Drying, which step that put aged tea bricks in a drying room and dried until the moisture content was less than 12% (temperature rose from 40 to 75 °C at a constant speed for 8 days).
Process parameters of SFBT
Fixing, kneading and post-fermentation were the same as SDBT.
Fa-Hua (fungal fermentation), which step that tea bricks were placed in a fermentation room (28 °C for ambient temperature and relative humidity was 80%) for natural fermentation for 10 days.
Drying, which step that put aged tea bricks in a drying room and dried until the moisture content was less than 12% (temperature rose from 40 to 75 °C at a constant speed in 12 days).
The tea samples were stored under vacuum at − 40 °C after manufacturing. The SDBT and SFBT samples which were used in the experiment are shown in Fig. 2.
Chemicals
The benzyl alcohol, benzaldehyde, benzyl acetate, 6-methyl-5-hepten-2-one, methyl salicylate, trans, trans-2,4-heptadienal, linalool, geraniol, β-ionone, 1-nonanal, nerolidol, hexyl hexanoate, 1-octen-3-ol, linalool oxide II, and indole standards were analytical reagents with at least 98% purity (Rhawn reagent, Shanghai, China), and ethyl decanoate (99.99% purity, used for quantitative analysis), C5–C16 normal paraffin standards (99.99% purity, used for RI calculation) was purchased from Sigma-Aldrich (Shanghai, China).
Equipment
HS-SPME was used to extract the aroma compounds. The SPME fibre was 50/30 μm DVB/CAR/PDMS whose length was 1 cm (Sigma-Aldrich, Shanghai, China), and the fibre penetration depth into the headspace was 2 cm. The size of the extraction bottle was 15 mL. The GC–MS was an Agilent 7890A/5975C-GC/MSD.
HS-SPME and GC–MS analysis
Extraction of aroma-active components
The HS-SPME method was chosen to isolate the aroma-active components; a tea sample (3.0 g) with 30 µg ethyl decanoate as an internal standard (10 mg/kg tea sample) was placed in an extraction bottle (15 mL volume). The sample was equilibrated in a thermostatic water bath for 10 min at 50 °C, and then sampled for 30 min in the head space. After that, SPME fibre was withdrawn and directly introduced to the GC–MS, and the process was repeated three times.
GC–MS instrument setup and analytical conditions
GC–MS analyses were performed on an Agilent 7890A/5975C-GC/MSD inert detector operating in EI mode in a 69.9 eV [75] chromatographic column: capillary-column chromatography DB-5 ms (30 m × 250 μm × 0.25 μm). The sampling was manual, no shunt was used, the sample was at a constant temperature, and the temperature of the injection port and GC–MS direct interface were 250 °C and 280 °C, respectively. Temperature programming: the column temperature was 50 ~ 250 °C; the starting column temperature was 50 °C which was held for 3 min, and then increased to 150 °C at a rate of 2 °C/min, held for 2 min, then increased to 250 °C at a rate of 2.5 °C/min and held for 4 min. Helium was used as a carrier gas at a flow rate of 1.0 mL/min. The ionic source temperature was 230 °C, the quadrupole temperature was 150 °C and the scanning quality range was set at 20 ~ 700 amu.
Qualitative and quantitative analysis of aroma compounds
Qualitative analysis: The NIST standard spectral library (https://www.nist.gov/) and retention indices (RIs) from other literatures were used to match ion mass spectra, and then completed the qualitative analysis of aroma components.
The RI calculation formula was
where RI are the retention indices of analyte, n the number of carbon atoms of n-alkanes before the analyte, RTx the retention time of the analyte, RTn the retention time of n-alkanes before the analyte, RTn+1 is the retention time of n-alkanes after the analyte.
Quantitative analysis: the internal standard method was used to quantify the volatile flavour compounds in the tea aroma [17]. Ethyl decanoate was selected as the internal standard (10 mg/kg tea sample). The calculation formula was
where Wi is the analyte concentration in mg/kg, Ws the concentration of the internal standard in mg/kg, Ai the analyte area and As is the peak area of the internal standard.
Calculation method of odour activity value
Aroma description data were picked from the Leffingwell and Associates Threshold Value Database (http://www.leffingwell.com/) and the FEMA GRAS Database (https://www.femaflavour.org/) [1],and then data from other literatures and monographs were used to determine the odour threshold (OT) and odour quality values. The contents of the aroma-active compounds were analysed on a gas chromatography–mass spectrometry (GC–MS) system. In addition, OAV is defined as the ratio of concentration to odour threshold, and the calculation formula of OAV was [19]
where C was the concentration of a single volatile component, OT is the odour threshold of the same volatile component.
If the OAV was equal to or greater than one, then the single volatile component had some influence on the overall aroma, and the higher the OAV is, the higher the contribution to the overall aroma [3, 18]
Training the sniffers
This method was based on Jiancai Zhu and Ye Liu’s method with some improvements [26, 32, 33, 80].
Sixteen assessors aged 23–28 years (half men, half women) were systematically trained for 4 months to recognize the aromas of substances naturally found in tea to define their aroma characteristics, detection thresholds and intensities. The training consists of two stages.
Stage 1: recognition training and intensity testing of single aroma components
Seventeen aroma compounds that are naturally found in dark tea with high frequency were identified (see Table 4 for details) and formulated as tenfold olfactory threshold water solutions. The test personnel were required to accurately identify the samples, and training was repeated to an accuracy of 95%.
Then the 17 aroma components were prepared into 0-fold (control check), 1-fold, 5-fold, 10-fold, 100-fold and 500-fold olfactory threshold aqueous solutions, and the test personnel were asked to sort the disordered solutions of a single aroma according to the order of intensity and scored them using a 7-point scoring method (above 7-points were 0, 0.5, 1, 1.5, 2, 2.5 and 3, respectively, in which 0-point is odourless and 3 points is the strongest solution). The training should be repeated to an accuracy of 99%.
Stage 2: mixed aroma recognition training and intensity testing
The above 17 aroma components were divided into 6 groups, floral, fruity, sweet, woody, fatty and mushroom earthy, and 2 aroma components were selected from each group. Then every two aromas are mixed together, and every pair was blended with each of the 12 aroma components (from a different group). It was required that the test personnel accurately distinguish the two types of aroma components (accuracy rate > 85%). At the same time, the tester needed to score the intensity of the aroma, the strength of the stronger components of the aroma is scored on a scale of 1 and the strength of the weaker aroma on a scale of 0–1.
Preparation of the dark tea for sniffing
According to the sensory analyses and quantitative determinations of the sample aromas, six characteristic aromas were chosen to describe the aroma profile: floral (representation of linalool, geraniol and β-ionone), fruity (representation of benzaldehyde and hexyl hexanoate), sweet (representation of methyl salicylate), woody (representation of nerolidol), mushroom earthy (representation of 1-octen-3-ol) and fatty (representation of 1-nonanal and trans,trans-2,4-heptadienal).
The method is based on the method of And, C. S., Liu, Y, and Isleten, M. H with slight adjustment (4; Liu et al. 30, 31; 22). Two tea samples of 10.0 g were weighed into a triangulated flask (250 mL volume), heated for 10 min in a water bath at 50 ± 1 °C to ensure that the aroma diffuses into the bottle to saturate the space, and then immediately sniffed in a sensory panel room at 21 ± 1 °C. To overcome the memory effects of a single aroma compound, every compound was sniffed only once, and the sniffing trials were performed 10 min apart.
Sensory evaluations were carried out by a sensory panel. They were asked to score the aroma qualities of floral, fruity, sweet, woody, mushroom earthy and fatty on a scale from 0 to 3 (where 0 means no smell and 3 points was the strongest). The samples were analysed in triplicate by each panellist, and the average score was used to draw the aroma profile.
Statistical analysis
Excel 2016 and the SPSS 25 software package were used to analyse the data, and the Excel visual plug-in XLSTAT 2018 was used to draw tables.
Results and analyses
Identification with GC–MS and PCA analysis results of aroma compounds in SDBT and SFBT
The overall aroma characteristics of SDBT are pure added aged fragrance and long lasting, and those of SFBT are pure added fungi flower aroma, strong and long lasting (results from early research, not cited in this paper). Although both of them show dark tea’s aromas, in accordance with traditional SDBT and SFBT, there are significant differences in their aroma characteristics under the premise that the materials and the production processes were both consistent, the differences between them may be caused by the unique technology “Fa Hua”. For further study of the aroma differences between SDBT and SFBT, their aroma components’ contents were detected by GC–MS.
According to Table 1, 37 volatile aroma components were detected in SDBT and SFBT. Among them, the components with the highest contents in SDBT were olivetol (15.55 mg/kg), linalool (14.01 mg/kg) and β-ionone (11.94 mg/kg), and those with the lowest contents were (E)-3,7-dimethylocta-1,3,6-triene (0.76 mg/kg), l-calamenene (0.99 mg/kg) and cis-3-hexenyl acetate (1.04 mg/kg). The components with the highest contents in SFBT were methyl salicylate (24.49 mg/kg), geranylacetone (21.73 mg/kg) and β-ionone (20.75 mg/kg), and those with the lowest contents were (E)-3,7-dimethylocta-1,3,6-triene (0.58 mg/kg), l-calamenene (0.74 mg/kg) and 2-acetyl pyrrole (0.84 mg/kg). Although the aroma components of SDBT and SFBT were highly consistent, their contents varied greatly. It is worth noting that the aroma components with the lowest contents in both SDBT and SFBT were (E)-3,7-dimethylocta-1,3,6-triene and l-calamenene.
The volatile aroma components in Table 1 were also analysed by PCA [24] (Fig. 4), and their detailed data are shown in Table 2. The results showed that the main sources of the difference between SDBT and SFBT were linalool, benzyl acetate, β-ionone, methyl salicylate, olivetol, geraniol, phenethyl alcohol, nerolidol, β-cyclocitral, (2,6,6-trimethyl-2-hydroxycyclohexylidene)acetic acid lactone, benzaldehyde and geranylacetone.
PCA score plot depicting the distribution of aroma compounds detected by GC–MS analysis and sensory attributes in tea samples for the two principal components. Compounds represented from Table 1
However, the PCA analysis did not take into account the threshold values of the aroma components. In other words, a low content of certain volatile aroma components may have a great impact on the overall aroma due to their low threshold values; on the contrary, a high content of other compounds may have little effect. Therefore, it is necessary to study further more about the contribution of each aroma component through OAV calculation.
Odour activity value (OAV) calculation and aroma description of the olfactory test results
Table 3 showed that there were 21 compounds whose OAVs are greater than 1 in SDBT. This result indicated that among the 37 detected components, only 21 components contributed to the overall aroma and were the aroma-active components of SDBT. According to the values of the aroma activity, from large to small, these components were β-ionone; linalool; benzyl acetate; 1-octen-3-ol; β-cyclocitral; trans,trans-2,4-heptadienal; geraniol; linalool oxide II; 1-nonanal; nerolidol; linalool oxide I; methyl salicylate; cis-3-hexenyl acetate; geranylacetone; hexyl hexanoate; benzaldehyde; 2,6,6-trimethyl-2-hydroxycyclohexylidene; indole; 6-methyl-5-hepten-2-one; (–)-alpha-terpineol and phenethyl alcohol. In terms of SFBT, there were 20 compounds whose OAVs were greater than 1 in it; these components contributed to the overall aroma and were the aroma-active components of SFBT. According to the values of the aroma activity from large to small, they were β-ionone; linalool; benzyl acetate; β-cyclocitral; trans,trans-2,4-heptadienal; 1-octen-3-ol; linalool oxide II; linalool oxide I; methyl salicylate; 1-nonanal; nerolidol; geranylacetone; geraniol; cis-3-hexenyl acetate; 6-methyl-5-hepten-2-one; hexanoate; 2,6,6-trimethyl-2-hydroxycyclohexylidene; indole; benzaldehyde; (–)-alpha-terpineol and phenethyl alcohol.
There were 11 volatiles among the aroma-active components of SDBT and SFBT for which the difference of the OAV ratio is greater than 0.5. The OAVs of β-ionone (199547.72), geranylacetone (362.15), linalool oxide II (1262.68), linalool oxide I (855.99), methyl salicylate (408.24) and 6-methyl-5-hepten-2-one (32.07) in SFBT were 74, 363, 127, 103, 151 and 185% higher than those in SDBT, respectively. In SDBT, the OAVs of geraniol (1055.13), phenethyl alcohol (1.02), benzyl acetate (4574.33), benzaldehyde (28.60) and 1-octen-3-ol (2438.46) were higher by 274, 158, 92, 109 and 89% than those in SFBT, respectively.
Using OAV analysis, we can further explore whether and how the active components contribute to the overall aroma. However, the specific influences on the aroma profiles of SDBT and SFBT need to be further explored through the aroma profile test.
The results of the panel training
Table 4 shows that after the first stage of identification training, the testers had the lowest recognizability (96%) for the four aroma components geraniol; linalool oxide I; trans,trans-2,4-heptadienal and 1-nonanal, and there were ten aroma components which reached 100% recognition rate. This result indicated that the identification of the single aroma components met the expected training target. After the first stage of intensity testing, the testers could correctly distinguish different concentration gradients of aroma components and provide aroma intensity scores. The training to judge the intensity of a single aroma component, thus, achieved the desired training objectives.
Figure 5 shows that after the second stage of the identification training, the testers’ recognition of any 2 aroma components (60 groups) of different flavour types after mixing was at least 85.4%, with an average recognition of 94.8%, showing that it achieved the desired training purpose.
It is found that a mixture containing nerolidol (90.5%), 1-octen-3-ol (92.7%), geraniol (93.6%) and benzaldehyde (94.6%) had the lowest recognition rate. Among them, nerolidol and geraniol had the lowest aroma intensities, only 0.34 (1st) and 0.46 (2nd), respectively, and 1-octen-3-ol and benzaldehyde had the highest aroma intensities, reached 0.97 (1st) and 0.94 (2nd), respectively.
The aroma profile of SDBT and SFBT
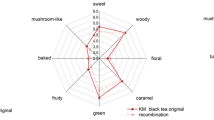
The aroma profiles were obtained through a 1.2.5 aroma profile evaluation experiment, as shown in Fig. 6.
The results (Fig. 6) of the aroma profile evaluation test showed that SDBT’s scores of floral, fruity, sweet, fatty, mushroom earthy and woody were 0.6, 1.2, 1.3, 1.8, 2.6 and 1.6, and SFBT’s scores were 1.1, 1.3, 1.9, 1.4, 1.7 and 1.2, respectively. The SDBT was more prominent than the SFBT in the aspects of fatty, woody and mushroom earthy, while in the aspects of floral and sweet, SFBT was more obvious.
The connection between the aroma profiles’ differences and aroma substance contents of the two tea samples, analysed by combining Fig. 3 with Table 3, is shown by the following:
First, as shown in Fig. 6, the two tea samples had similar basic aroma contours. Combined with that, as shown in Table 4, the SDBT and SFBT had 20 common active aroma components (the OAV of phenylethyl alcohol in SFBT is 0.4), and the highest OAV values in these two tea samples were both β-ionone (114800.66 and 199547.72, respectively), β-linalool and benzyl acetate followed. This result indicated that β-ionone, β-linalool and benzyl acetate had the greatest contributions to the aromas of SDBT and SFBT, and β-linalool, β-cyclocitral, cis-3-hexenyl acetate, hexyl hexanoate, (2,6,6-trimethyl-2-hydroxycyclohexylidene)acetic acid lactone, E-2-E-4-heptadienal, nonaldehyde, nerolidol, indole and terpene alcohol had similarly high OAVs in the two tea samples. Therefore, these results implied that both SDBT and SFBT showed analogical basic aroma characteristics of ‘dark tea’ because of above similar 13 aroma components.
In addition, the results of the aroma profile test showed that the SFBT had more development in sweetness than SDBT (Fig. 6), while compared with Table 3, it was found that all sweet aroma components in SFBT with an OAV > 1 were more prominent than those in SDBT, including linalool oxide II, linalool oxide I and methyl salicylate (Table 2), which had OAVs in SFBT of 1262.68, 855.99, 408.24, respectively; these 2 results, thus, were consistent. At the same time, the woody aroma of SDBT was more prominent than those of SFBT (Fig. 6), which was also consistent with the results in Table 3 that the OAV of nerolidol (423.28) and indole (18.63) in SDBT was greater than that in SFBT.
In terms of fruitiness, the scores of the two tea samples were close to each other (Fig. 6).The higher OAV values of phenylmethyl acetate (OAV = 4574.33), β-cyclocitral (OAV = 1633.31), hexyl hexanoate (OAV = 35.08), benzaldehyde (OAV = 28.60) and (2,6,6-trimethyl-2-hydroxycyclohexylidene)acetic acid lactone (OAV = 23.14) provided the SDBT with some fruit aromas such as apple peel and cherry. While SFBT also had prominent fruit aromas due to the higher OAV of 6,10-dimethyl-5,9-undecadien-2-one (OAV = 362.15).
In terms of mushroom earthy, the score of the SDBT was much higher than that of the SFBT (Fig. 6), which was closely related to the high OAV of 1-octen-3-ol (OAV = 2438.46) (Table 3).
Additionally, the fatty aroma of the SDBT was more prominent than that of SFBT (Fig. 6), and this result was in accordance with the higher OAV of nonaldehyde in SDBT (OAV = 525.81) (Table 3), but it was contradictory to the OAV of (E,E)-2,4-hepadienal, which will be further elaborated in the discussion.
Different OAVs in β-ionone, geraniol and phenethyl alcohol caused the different floral scores between the SDBT and the SFBT (Fig. 6). The high OAV of β-ionone (OAV = 199547.72) in the SFBT was close to the sweet floral, and the high OAVs of Geraniol (OAV = 1055.13) and phenethyl alcohol (OAV = 1.02) were close to the green floral in the SDBT, which was consistent with the study of Ashu Gulati on Kangra orthodox black tea [26]. The floral score of the SDBT was 50% lower than that of the SFBT; this result was related to the masking of the green floral aroma-active components of linalool and geraniol by 1-octen-3-ol and benzaldehyde, which will be further elaborated in the discussion.
In summary, SDBT performed better than SFBT in the woody, mushroom earthy and fatty fragrances represented by nerolidol, 1-octen-3-ol and nonanal, respectively, which were the main components of the aroma characteristics of “aged fragrance”. Compared with SDBT, SFBT showed better aspects of “floral”, represented by β-ionone, and ‘sweet’, represented by linalool oxide II and methyl salicylate, which led to the formation of the aroma characteristics of “green floral fragrance”.
Discussion
Causes of the main odour-active compound differences between the two types of dark tea
The results showed that the OAVs of β-ionone, geranylacetone, linalool oxide II, linalool oxide I, methyl salicylate and 6-methyl-5-hepten-2-one in SFBT are higher than those in SDBT. Therefore, it was considered that those substances mentioned above were the main active compounds that caused SFBT to show a stronger fungi flower aroma fragrance than SDBT. This difference was probably caused by the “Fa-Hua” (fungal fermentation) process.
β-ionone and geranylacetone were produced from the oxidization of β-carotene [14, 66] (Fig. 7). The evidence showed that there were high volumes of β-ionone and geranylacetone and lower volumes of β-cyclocitral and (2,6,6-trimethyl-2-hydroxy-cyclohexylidene)acetic acid lactone, from which can be deduced that the breaking of the double bonds of carbon between C9 and C10 and between C7 and C8 and C13 and C14 of β-carotene produced β-ionone and geranylacetone, respectively. This happened during the “Fa-Hua” (fungal fermentation) process and was caused by microorganism metabolism. In contrast, the SDBT had no “Fa-Hua” processing but rather had a drying process with high temperature. These processing characteristics may caused the β-carotene to break the C–C bond between C8 and C9 and produce (2, 6,6-trimethyl-2-hydroxy-cyclohexylidene)acetic acid lactone, and it may also caused the β-carotene to break the C = C bond between C7 and C8 and produce β-cyclocitral.
Geranylacetone, proved by experiments, originates from the degradation of carotenoid (Fig. 8) [23]. M Ibdah proved that geranylacetone was produced from the expressed product of CmCCD1, which caused oxidative chemical degradation at the C9–C10 bond of the carbon chain of phytoene. Based on the above experimental results and the experimental data of this research, the following hypothesis was proposed: during the “Fa-Hua” (fungal fermentation) of SFBT, Eurotium cristatum caused the upregulated gene expression of the CmCCD1 gene family and produced secondary metabolites which helped to produce geranylacetone; however, this hypothesis needs further experimental proof.
Schematic diagram of degradation of carotenoids to produce geraniol acetone in melon [23]
There was an experimental evidence proving that epoxydihydrolinalool I, epoxydihydrolinalool II [67] and methyl salicylate originated from the enzymatic hydrolysis of their primeveroside precursor substances. Benzyl alcohol originated from the hydrolysis of β-d-glucoside and is oxidized to produce benzaldehyde, which shared the same pathway with phenethyl alcohol oxidation to produce phenylacetaldehyde. In many experiments on the addition of exogenous Eurotium cristatum into tea or a water extract of tea, the hydrolysis of primeverosides was commonly observed. Therefore, we considered that the vital activities of Eurotium cristatum increased the hydrolysis efficiency of β-primeverosides.
Considering all the presented information, we had some assumptions as follows: [1] The vital activities of Eurotium cristatum increase the hydrolysis efficiency of β-primeverosides, which offers the possibility of producing more β-primeverosidase or changing the environmental condition of the enzyme catalysis. This change causes volume increases in epoxydihydrolinalool I, epoxydihydrolinalool II and methyl salicylate which leads SFBT to have more such substances than SDBT (Fig. 9) [5]. When the SDBT was aged, the β-d-glucosidase hydrolysed the precursors of benzyl alcohol with glucoside and produced benzyl alcohol, which is further converted to benzaldehyde or benzyl acetate. This results in higher contents of benzaldehyde and benzyl acetate in SDBT than SFBT. (Fig. 10).
Geraniol and phenethyl alcohol can originate from the enzymatic hydrolysis of primeveroside precursor substances, but the analysis showed a contrary result. Geraniol and phenethyl alcohol had higher volumes in SDBT than SFBT. This difference may be rooted in the enzyme specificity variance of the β-primeverosides and β-d-glucosidase [20]. For instance, β-primeverosides had a lower hydrolytic activity to geraniol-6-O-α-l-arabinofuranose-β-bioside-d-glucopyranoside [46]. In addition, geraniol may undergo enzymatic isomerization and transform into linalool, and phenethyl alcohol may also oxidize to phenylacetaldehyde and phenylacetic acid. The above reactions may cause unpredictable changes in the geraniol and phenethyl alcohol contents.
Benzyl acetate and 1-octen-3-ol are present in high volumes in aged dark teas, such as aged Pu’er tea and aged Qing brick tea. This high volume is due to the same reason as discussed in the comparison between SDBT and SFBT. Benzyl acetate originates from the esterification of benzyl alcohol. 1-Octen-3-ol may be a secondary metabolite of fungi (Fig. 11) [70]. Xue inoculated microbes into wheat koji. 1-Octen-3-ol was detected in the fungi group. 1-Octen-3-ol follows the LOX pathway and is produced from the oxy-cracking of linoleic acid with the catalysis of lipoxygenase and hydroperoxide lyase [43, 44]. We speculated from the evidence above that a secondary metabolite of the fungi increases the catalytic efficiency and produces more 1-octen-3-ol, which leads to the conclusion that SDBT has more 1-octen-3-ol than SFBT.
Relationship between OAVs of main odour-active compounds and aroma states
Though linalool had a higher OAV than most odour substances, the tea did not show the characteristic aroma of linalool. The results of the mixed aroma recognition training and intensity tests also supported this finding (Fig. 5), i.e. the aroma intensity of the floral fragrance was lower than that of the other fragrances. Miettinen’s research provided a clue to explain this finding—the volatilization of linalool and the sensitivity of the nose towards linalool will decrease when there are lipoids in the solution [41]. Lipoids exist in the fresh leaves, constituting 8% of the dry weight of the tea, including fats and glycerides. However, there is no clear clue about the volume of lipoids and their changes during the process. It is necessary to do more research about the influences of lipoids towards linalool.
Substances with a soil fragrance have a masking action towards those with a floral fragrance. 1-Octen-3-ol shows the same relationship towards linalool and geraniol. The mixture of these three substances with a volume of ten times the threshold shows the main odour of 1-octen-3-ol rather than the other two. In Fig. 5, 1-octen-3-ol also has the highest aroma intensity (0.97), and when 1-octen-3-ol is mixed with β-ionone and geraniol, the correct recognition rate was only 0.86 and 0.85, respectively, and the result was consistent with the above hypothesis. This result means that 1-octen-3-ol has a higher aroma intensity than the other two with the same OAV, which is supported by Zhao’s experiment [77]. This may be the main reason for SDBT lacking a floral fragrance. SDBT has a stronger oily fragrance than SFBT. This is mainly caused by nonanal (OAV = 525.81). However, the opposite was observed for trans,trans-2,4-heptadienal, which has a high OAV but does not show a characteristic aroma. This is caused by high volume of 1-octen-3-ol that enhances the intensity of the oily fragrance.
Among all the odour-active compounds, only epoxydihydrolinalool I and epoxydihydrolinalool II are described as having creamy aromas. Additionally, an olfactory experiment shows that only a slight creamy aroma was detected in SFBT. However, no such aroma was detected in SDBT, even though such odour-active compounds are present. This result might be caused by the difference in the odour-active rates, which can lead to changes in or the masking of the fragrance profile.
The experiments on mixtures of two single aroma substances showed that benzaldehyde, 1-octen-3-ol, methyl salicylate, trans,trans-2,4-heptadienal and nonanal showed stronger aromas that can easily be recognized (Fig. 6). The above five aroma components’ intensities are 0.94, 0.97, 0.91, 0.90 and 0.89. This result indicates that those substances mentioned above provide greater contributions to the tea aroma.
Conclusions
There were 20 odour-active compounds among the volatile compounds of SFBT, while the number in SDBT was 21. 20 substances were the same in both teas, including β-ionone; linalool; benzyl acetate; β-cyclocitral; cis-3-hexenyl acetate; geranylacetone; hexyl hexanoate; benzaldehyde; (2,6,6-trimethyl-2-hydroxycyclohexylidene)acetic acid lactone; epoxydihydrolinalool I; epoxydihydrolinalool II; methyl salicylate; 1-octen-3-ol; 6-methyl-5-hepten-2-one; trans,trans-2,4-heptadienal; nonanal; α-terpineol; nerolidol and indole. These substances constituted the basic fragrance of dark tea.
What caused the ‘fungi flower aroma’ and ‘aged fragrance’ is the volume and OAV differences of those above odour-active compounds. ‘Fungi flower aroma’ showed a floral fragrance represented by β-ionone, a sweet fragrance represented by epoxydihydrolinalool II and methyl salicylate, and a fruity fragrance represented by geranylacetone. ‘Aged fragrance’ showed a fragrance of wood that is represented by nerolidol, a fruity fragrance represented by benzaldehyde and benzyl acetate, an fatty fragrance that is represented by nonanal and trans,trans-2,4-heptadienal, and a soil fragrance that is represented by 1-octen-3-ol.
Abbreviations
- OAV:
-
Odour activity value
- GC–MS:
-
Gas chromatography–mass spectrometry
- SDBT:
-
Sichuan Dark brick tea
- SFBT:
-
Sichuan Fuzhuan brick tea
- HS-SPME:
-
Headspace solid-phase microextraction
- OT:
-
Odour threshold
- PCA:
-
Principal component analysis
References
Adams TB, Cohen SM, Doull J, Feron VJ, Goodman JI, Marnett LJ et al (2004) The FEMA GRAS assessment of cinnamyl derivatives used as flavor ingredients. Food Chem Toxicol 42(2):157–185
Afsharypuor S, Suleimany M (2002) Volatile oil constituents of Brassica oleracea var. gongylodes seeds. J Essent Oil Res 14(1):18–19
And CS, Schieberle P (2006) Characterization of the key aroma compounds in the beverage prepared from darjeeling black tea: quantitative differences between tea leaves and infusion. J Agric Food Chem 54(3):916–924
Angioni A, Barra A, Coroneo V, Dessi S, Cabras P (2006) Chemical composition, seasonal variability, and antifungal activity of Lavandula stoechas L. ssp. stoechas essential oils from stem/leaves and flowers. J Agric Food Chem 54(12):4364–4370
Ansorena D, Gimeno O, Astiasarán I, Bello J (2001) Analysis of volatile compounds by GC–MS of a dry fermented sausage: chorizo de Pamplona. Food Res Int 34(1):67–75
Asuming WA, Beauchamp PS, Descalzo JT, Dev BC, Dev V, Frost S, Ma CW (2005) Essential oil composition of four Lomatium Raf. species and their chemotaxonomy. Biochem Syst Ecol 33(1):17–26
Baranauskiene R, Venskutonis PR, Viskelis P, Dambrauskiene E (2003) Influence of nitrogen fertilizers on the yield and composition of thyme (Thymus vulgaris). J Agric Food Chem 51(26):7751–7758
Boulanger R, Crouzet J (2000) Free and bound flavour components of Amazonian fruits: 2. cupuacu volatile compounds. Flavour Fragr J 15(4):251–257
Cajka T, Hajslova J, Cochran J, Holadova K, Klimankova E (2007) Solid phase microextraction—comprehensive two dimensional gas chromatography—time-of-flight mass spectrometry for the analysis of honey volatiles. J Sep Sci 30(4):534–546
Chaturvedula PSV, Prakash I (2011) The aroma, taste, color and bioactive constituents of tea. J Med Plants Res 5(11):2110–2124
Chen G, Song H, Ma C (2010) Aroma-active compounds of Beijing roast duck. Flavour Fragr J 24(4):186–191
Chen B, Liu XY, Pu HJ, Chen YH, Jiang DH, Gao XL et al (2015) Study on variation of main chemical components during pu’er tea fermentation process of different raw materials. J Food Saf Qual 6(4):1279–1286
Cho IH, Namgung H-J, Choi H-K, Kim Y-S (2008) Volatiles and key odorants in the pileus and stipe of pine-mushroom (Tricholoma matsutake Sing.). Food Chem 106(1):71–76
Chun WX (2005) Tea biochemistry, 3rd edn. China Agricultural Press, BeiJing
Cui J, Yang X, Dong A-J, Cheng D-Y, Wang J, Zhao H-T, Xu R-B, Wang P, Li W-J (2011) Chemical composition and antioxidant activity of Euphorbia fischeriana essential oil from China. J Med Plants Res 5(19):4798–4894
Fanaro GB, Duarte RC, Santillo AG, Pinto-e-Silva MEM, Purgatto E, Villavicento ALCH (2012) Evaluation of γ-radiation on oolong tea odor volatiles. Radiat Phys Chem 81(8):1152–1156
Forero DP, Orrego CE, Peterson DG, Osorio C (2015) Chemical and sensory comparison of fresh and dried lulo (Solanum quitoense Lam.) fruit aroma. Food Chem 169:85–91
Gassenmeier K, Schieberle P (1994) Comparison of important odorants in puff-pastries prepared with butter or margarine. LWT Food Sci Technol 27(3):282–288
Grosch W (2010) Determination of potent odourants in foods by aroma extract dilution analysis (AEDA) and calculation of odour activity values (OAVs). Flavour Fragr J 9(4):147–158
Han W-W, LI Z-S, Zheng Q-C, Sun C-C (2006) Toward a blueprint for β-primeverosidase from tea leaves structure/function properties: Homology modeling study. J Theor Comput Chem 05 (spec01):433–446
Hazzit M, Baaliouamer A, Faleiro ML, Miguel MG (2006) Composition of the essential oils of Thymus and Origanum species from Algeria and their antioxidant and antimicrobial activities. J Agric Food Chem 54(17):6314–6321
Huang H, Liu ZH, Huang JA, Li S, Li J, Wu Y et al (2010) Isolation and identification of “Jinhua” fungi from the loose tea with “fungus growing”. J Tea Sci 30(5):350–354
Ibdah M, Azulay Y, Portnoy V, Wasserman B, Bar E, Meir A et al (2006) Functional characterization of cmccd1, a carotenoid cleavage dioxygenase from melon. Phytochemistry 67(15):1579–1589
Isleten MH (2017) Aroma characterization of five microalgae species using solid-phase microextraction and gas chromatography-mass spectrometry/olfactometry. Food Chem 240:1210–1218
Jalali-Heravi M, Zekavat B, Sereshti H (2006) Characterization of essential oil components of Iranian geranium oil using gas chromatography-mass spectrometry combined with chemometric resolution techniques. J Chromatogr A 1114(1):154–163
Joshi R, Gulati A (2015) Fractionation and identification of minor and aroma-active constituents in kangra orthodox black tea. Food Chem 167:290–298
Karagül-Yüceer Y, Vlahovich KN, Drake MA, Cadwallader KR (2003) Characteristic aroma components of rennet casein. J Agric Food Chem 51(23):6797–6801
Kartal N, Sokmen M, Tepe B, Daferera D, Polissiou M, Sokmen A (2007) Investigation of the antioxidant properties of Ferula orientalis L. using a suitable extraction procedure. Food Chem 100(2):584–589
Kukic J, Petrovic S, Pavlovic M, Couladis M, Tzakou O, Niketic M (2006) Composition of essential oil of Stachys alpina L. ssp dinarica Murb. Flavour Fragr J 21(3):539–542
Kundakovic T, Fokialakis N, Kovacevic N, Chinou I (2007) Essential oil composition of Achillea lingulata and A. umbellata. Flavour Fragr J 22(3):184–187
Liu D (2017) Effect of Fuzhuan brick-tea addition on the quality and antioxidant activity of skimmed set-type yoghurt. Int J Dairy Technol 71:22–23
Liu Y, He C, Song H (2018) Comparison of fresh watermelon juice aroma characteristics of five varieties based on gas chromatography-olfactometry-mass spectrometry. Food Res Int 107:119–129
Liu F, Wang Y, Zhang T, Tang X, Wang X, Chunhua LI (2018) Review on aroma change during black tea processing. J Tea Sci 38(1):9–19
Lozano PR, Drake M, Benitez D, Cadwallader KR (2007) Instrumental and sensory characterization of heat-induced odorants in aseptically packaged soy milk. J Agric Food Chem 55(8):3018–3026
Maia JGS, Andrade EHA, Zoghbi MGB (2000) Volatile constituents of the leaves, fruits and flowers of cashew (Anacardium occidentale L.). J Food Comp Anal 13(3):227–232
Maia JGS, Taveira FSN, Andrade EHA, da Silva MHL, Zoghbi MGB (2003) Essential oils of Lippia grandis Schau. Flavour Fragr J. 18(5):417–420
Maia JGS, Andrade EHA, da Silva ACM, Oliveira J, Carreira LMM, Araújo JS (2005) Leaf volatile oils from four Brazilian Xylopia species. Flavour Fragr. J 20(5):474–477
Mallard WG, Andriamaharavo NR, Mirokhin YA, Halket JM, Stein SE (2014) Creation of libraries of recurring mass spectra from large data sets assisted by a dual-column workflow. Anal Chem 86(20):10231–10238
Marongiu B, Porcedda S, Piras A, Sanna G, Murreddu M, Loddo R (2006) Extraction of Juniperus communis L. ssp. nana Willd. essential oil by supercritical carbon dioxide. Flavour Fragr J 21(1):148–154
Mesa-Arango AC, Betancur-Galvis L, Montiel J, Bueno JG, Baena A, Duran DC, Martinez JR, Stashenko EE (2010) Antifungal activity and chemical composition of the essential oils of Lippia alba (Miller) N.E. Brown grown in different regions of Colombia. J Essent Oil Res. 22(6):568–574
Miettinen SM, Hyvönen LA, Tuorila H (2003) Timing of intensity perception of a polar vs nonpolar aroma compound in the presence of added vegetable fat in milk. J Agric Food Chem 51(18):5437–5443
Mo H, Zhang H, Li Y, Zhu Y (2008) Antimicrobial activity of the indigenously microbial fermented Fuzhuan brick-tea. J Biotechnol 136(4):S722
Morawicki RO, Beelman RB (2008) Study of the biosynthesis of 1-octen-3-ol using a crude homogenate of Agaricus bisporus in a bioreactor. J Food Sci 73(3):C135–C139
Morawicki RO, Beelman RB, Petreson D, Demirci A (2005) Biosynthesis of 1-octen-3-ol and 10-oxo-trans-8-decenoic acid using a crude homogenate of Agaricus bisporus: reaction scale up. Process Biochem 40(1):131–137
Noudogbessi J-P, Yedomonhan P, Sohounhloue DCK, Chalchat J-C, Figueredo G (2008) Chemical composition of essential oil of Syzygium guineense (Willd.) DC .var. guineense (Myrtaceae) from Benin. Rec Nat Prod, 33–38
Ogawa K, Moon JH, Guo W, Yagi A, Watanabe N, Sakata K (1995) A study on tea aroma formation mechanism: alcoholic aroma precursor amounts and glycosidase activity in parts of the tea plant. Z Naturforsch C 50(7–8):493–498
Ortega-Heras M, Gonzalez-Sanjose ML, Beltran S (2002) Aroma composition of wine studied by different extraction methods. Anal Chim Acta 458(1):85–93
Palmeira SF Jr, Moura FS, Alves VL, de Oliveira FM, Bento ES, Conserva LM, Andrade EHA (2004) Neutral components from hexane extracts of Croton sellowii. Flavour Fragr J 19(1):69–71
Pavlovic M, Kovacevic N, Tzakou O, Couladis M (2006) Essential oil composition of Anthemis triumfetti (L.) DC. Flavour Fragr J. 21(2):297–299
Pino JA, Mesa J, Muñoz Y, Martí MP, Marbot R (2005) Volatile components from mango (Mangifera indica L.) cultivars. J Agric Food Chem 53(6):2213–2223
Radulescu V, Chiliment S, Oprea E (2004) Capillary gas chromatography-mass spectrometry of volatile and semi-volatile compounds of Salvia officinalis. J Chromatogr A 1027(1–2):121–126
Roussis V, Tsoukatou M, Petrakis PV, Chinou I, Skoula M, Harborne JB (2000) Volatile constituents of four Helichrysum species growing in Greece. Biochem Syst Ecol 28(2):163–175
Saroglou V, Arfan M, Shabir A, Hadjipavlou-Litina D, Skaltsa H (2007) Composition and antioxidant activity of the essential oil of Teucrium royleanum Wall. ex Benth growing in Pakistan. Flavour Fragr J 22(2):154–157
Selli S, Rannou C, Prost C, Robin J, Serot T (2006) Characterization of aroma-active compounds in rainbow trout (Oncorhynchus mykiss) eliciting an off-odor. J Agric Food Chem 54(25):9496–9502
Seo WH, Baek HH (2005) Identification of characteristic aroma-active compounds from water dropword (Oenanthe javanica DC.). J Agric Food Chem 53(17):6766–6770
Siani AC, Ramos MFS, Menezes-de-Lima O Jr., Ribeiro-dos-Santos R, Fernadez-Ferreira E, Soares ROA, Rosas EC, Susunaga GS, Guimarães AC, Zoghbi MGB, Henriques MGMO (1999) Evaluation of anti-inflammatory-related activity of essential oils from the leaves and resin of species of Protium. J. Ethnopharmacol. 66(1):57–69
Skaltsa HD, Mavrommati A, Constantinidis T (2001) A chemotaxonomic investigation of volatile constituents in Stachys subsect Swainsonianeae (Labiatae). Phytochemistry 57(2):235–244
Song H, Cadwallader KR (2008) Aroma components of American country ham. J Food Sci 73(1):C29–C35
Song H, Xia L (2010) Aroma extract dilution analysis of a beef flavouring prepared from flavour precursors and enzymatically hydrolysed beef. Flavour Fragr J 23(3):185–193
Song LB, Huang JA, Liu ZH, Huang H, Wang KB (2009) Study on the activity of dark tea extracts to fxr and lxr model. J Tea Sci 29(2):131–135
Stojanovic IZ, Radulovic NS, Mitrovic YLJ, Stamenkovic SM, Stojanovic GS (2011) Volatile constituents of selected Parmeliaceae lichens. J Serb Chem Soc 76(7):987–994
Su Y, Wang C, Yinlong G (2009) Analysis of volatile compounds from Mentha hapioealyx Briq by GC–MS based on accurate mass measurements and retention indices. Acta Chem Sinica 67(6):546–554
Tuberoso CIG, Kowalczyk A, Coroneo V, Russo MT, Dessì S, Cabras P (2005) Chemical composition and antioxidant, antimicrobial, and antifungal activities of the essential oil of Achillea ligustica all. J Agric Food Chem 53(26):10148–10153
van Gemert LJ (2011) Compilations of odour threshold values in air, water and other media and compilations of flavour threshold values in water & other media. http://www.thresholdcompilation.com/
Varlet V, Knockaert C, Prost C, Serot T (2006) Comparison of odor-active volatile compounds of fresh and smoked salmon. J Agric Food Chem 54(9):3391–3401
Waché Y, Bosser-DeRatuld A, Lhuguenot JC, Belin JM (2003) Effect of cis/trans isomerism of β-carotene on the ratios of volatile compounds produced during oxidative degradation. J Agric Food Chem 51(7):1984–1987
Wang D, Ando K, Morita K, Kubota K, Kobayashi A (1994) Optical isomers of linalool and linalool oxides in tea aroma. J Agric Chem Soc Jpn 58(11):2050–2053
Xiao-Ming JI (2006) The trade and dissemination of China dark tea. J Tea Sci 49(8):159–165
Xu A, Wang Y, Wen J, Liu P, Liu Z, Li Z (2011) Fungal community associated with fermentation and storage of Fuzhuan brick-tea. Int J Food Microbiol 146(1):14–22
Xue JB (2016) Microbial community in Chinese rice wine inoculated raw wheat Qu and analysis of enzyme and flavour produced by isolated microbes. Doctoral dissertation, Jiangnan University
Yan M, Wei BY, Teng JW, Li H, Ning X (2017) Analyses of fungal community by illumina miseq platforms and characterization of Eurotium species on Liupao tea, a distinctive post–fermented tea from China. Food Res Int 99(1):641–649
Yang Z, Baldermann S, Watanabe N (2013) Recent studies of the volatile compounds in tea. Food Res Int 53(2):585–599
Yao Y, Wu M, Huang Y, Li C, Pan X, Zhu W et al (2017) Appropriately raising fermentation temperature beneficial to the increase of antioxidant activity and gallic acid content in Eurotium cristatum fermented loose tea. LWT Food Sci Technol 82:248–254
Zeng Y-X, Zhao C-X, Liang Y-Z, Yang H, Fang H-Z, Yi L-Z, Zeng Z-D (2007) Comparative analysis of volatile components from Clematis species growing in China. Anal Chim Acta 595(1–2):328–339
Zeng L, Zhou Y, Fu X, Mei X, Cheng S, Gui J et al (2017) Does oolong tea (Camellia sinensis) made from a combination of leaf and stem smell more aromatic than leaf-only tea? contribution of the stem to oolong tea aroma. Food Chem 237:488–498
Zhang L, Zhang ZZ, Zhou YB, Ling TJ, Wan XC (2013) Chinese dark teas: postfermentation, chemistry and biological activities. Food Res Int 53(2):600–607
Zhao MM, Cao Y, Cai Y, Guo-Wan SU, Feng YZ (2016) Identification of aroma-active compounds from Yang Jiang Douchi by sde and hs-spme combined with GC–MS/O. Mod Food Sci Technol (5):264–275
Zheng CH, Kim TH, Kim KH, Leem YH, Lee HJ (2004) Characterization of potent aroma compounds in Chrysanthemum coronarium L. (Garland) using aroma extract dilution analysis. Flavour Fragr J 19(5):401–405
Zhu M, Li E, He H (2008) Determination of volatile chemical constitutes in tea by simultaneous distillation extraction, vacuum hydrodistillation and thermal desorption. Chromatographia 68(7/8):603–610
Zhu J, Wang L, Xiao Z, Niu Y (2018) Characterization of the key aroma compounds in mulberry fruits by application of gas chromatography-olfactometry (GC-O), odor activity value (OAV), gas chromatography-mass spectrometry (GC–MS) and flame photometric detection (FPD). Food Chem 245:775–785
Acknowledgements
First, I would like to thank my wife Li Hui for her encouragement and assistance during my writing. Second, I would like to thank Professor Xu Jing-yi and Lecturer Zhou Yao for their valuable opinions which were put forward in the manuscript, in addition, I would like to thank Master Zhou Li-he and Master Luo Wei-chen for their selfless help in language.
Author information
Authors and Affiliations
Contributions
CN was responsible for the sorting of the experimental data and proofreading and wrote the manuscript. XZ was responsible for the design, implementation of the tests and collection of raw data. LH revised the manuscript critically for important intellectual content. YG, XZ, CW were responsible for some experiments. XD provided design ideas, ensured the smooth progress of the tests and revised the outline and the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest in this manuscript, which has been approved by all authors for publication. On behalf of my co-authors, I declare that this study is an unpublished original study and that it is not considered to be published in whole or in part elsewhere. All the authors listed approved the declaration.
Compliance with ethics requirements
The study was approved by the local ethics committee in China.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Practical application: The results of this study can be used to guide the development of new flavours and provide technical support for the process improvement of dark tea after fermentation.
Rights and permissions
About this article
Cite this article
Nie, Cn., Zhong, Xx., He, L. et al. Comparison of different aroma-active compounds of Sichuan Dark brick tea (Camellia sinensis) and Sichuan Fuzhuan brick tea using gas chromatography–mass spectrometry (GC–MS) and aroma descriptive profile tests. Eur Food Res Technol 245, 1963–1979 (2019). https://doi.org/10.1007/s00217-019-03304-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-019-03304-1