Abstract
A rapid and simple HS-SPME–GC–MS method was developed and applied to investigate the effect of the storage time on the release of not intentionally added substances (NIAS) in PET bottled mineral water. The method, validated in terms of linearity, precision, detection, and quantification limits, resulted highly reproducible with limits of detection ranging between 0.05 and 0.17 µg/L. Saturated and 2-unsaturated aliphatic aldehydes, ketones, aliphatic and aromatic hydrocarbon, terpenes, and phthalates were identified and quantified, most of them for the first time in PET bottled mineral water. The levels of the identified NIAS showed statistically significant increases during the shelf-life. Decanal and nonanal were the most abundant compounds identified with levels increasing from 1.42 to 5.07 µg/L and from 0.61 to 1.25 µg/L, respectively. Considering the identified substances, a migration not only from packaging materials but also from the closure caps and adhesive used for sticking the bottle labels may be plausible. Due to the growing popularity of bottled water consumption, the determination of NIAS in mineral water is becoming a top priority. In this context, the method, here developed, could be of great importance not only to assure the safety of bottled mineral water but also to guarantee the sensory quality during the shelf-life.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The worldwide consumption of bottled water has been steadily increasing. European countries have traditionally the highest bottled water consumption and 12 of the top 20 countries with the highest per capita consumption are still European [1]. Polyethylene terephthalate (PET) is a semi-crystalline plastic polymer belonging to the family of polyesters and universally used as packaging material for mineral water due to its strength, lightweight, flexibility, clarity, resistance to high temperature, and its negligible permeability to carbon dioxide [2, 3]. In case of plastic materials in contact with foodstuffs, a declaration of conformity according to the EU Regulation No. 10/2011 is required [4]. The regulation states that the risk assessment of a substance should cover the substance itself, relevant impurities, and foreseeable reaction and degradation products in the intended use; it also reports the list of the authorized monomers, other starting substances, additives, and polymer allowed in the production. Some substances are subject to restrictions and/or specifications according to their toxicological data; others, such as the non-intentionally added substances (NIAS) that may be present in the plastic materials, are not listed. NIAS could arise from starting substances, such as monomers and catalysts, used for the initial polymerization step or from additives and plasticizers added during manufacturing to achieve special material properties. These substances can undergo degradation and decomposition reactions during polymer manufacture and use originating products non-intentionally present in the plastic material that can leach to packaged food over time [5]. Furthermore, starting substances or additives can contain impurities, which again might leach from the packaging [6, 7]. Another possible source of NIAS can be the acrylic adhesives used in food packaging to form the geometric shape of the package as well as to stick labels on the packages [8]. In addition, PET is the most recycled plastic packaging material in Europe. The use of recycled PET in the manufacture of new bottles for mineral water is allowed in the European Union and currently its amount in the new bottles can be up to 50% [9]. The use of recycled PET could be a source of NIAS in packaged mineral water, too. Recently, some Authors suggested three key sources of contamination in recycled PET: (1) misuse of the package by the consumers, (2) food products (fermented and fortified alcoholic beverages), and (3) non-food products (petroleum products, detergents, and cleaning products, compounds containing ethers, and unknown products), apart from other compounds resulting from the deterioration of the original product and the storage in an inappropriate place [10].
Several authors have reported finding chemical mixtures with estrogenic activity in PET bottled water. The presence of NIAS has been suggested as the source of this toxicological effect [11]. To date, the research has been mainly focused on the levels of short-chain carbonyl compounds, namely formaldehydes and acetaldehyde, and phthalates because of their carcinogenic and estrogenic effects [12,13,14,15,16,17,18,19,20,21]. Acetaldehyde is generated during the polymerization reaction and the melt process during manufacturing of PET bottles. Formaldehyde is formed by an internal cleavage of the polymeric chain. It has also been demonstrated that the migration of formaldehyde and acetaldehyde from PET packaging to water is a thermally activated process [5].
In contrast, few publicacations are devoted to studying the presence of higher volatile carbonyl compounds, saturated and unsaturated, that are considered responsible of off-taste problems for bottled water [22] and toxic for humans [23, 24]. The analytical procedures proposed for aldehyde quantification in water employed both high-performance liquid chromatography (HPLC) [25] and gas-chromatography (GC) analysis after sample derivatization [26, 27]. Although these methods provide good sensibility, they involve an extensive work-up, consume materials, and solvents for the derivatization and isolation steps.
In this context, the present study aimed to develop a simple and solvent-free analytical method based on headspace solid-phase microextraction–gas chromatography–mass spectrometry (HS-SPME–GC–MS) technique for the quantification of medium-chain aldehydes and other possible volatile migrants in mineral water packaged in PET. Once developed, the method was applied to monitor migrant levels during the shelf-life. In Italy, a shelf-life of 18 or 24 months, depending on the producer, is established for natural mineral water. At the best of our knowledge, no paper is present in literature dealing with the migration levels of short- and medium-chain aldehydes and/or other volatile migrants during the entire shelf-life of bottled mineral water.
Materials and methods
Bottled water samples and storage conditions
Fifteen sample bottles (500 mL) of still mineral natural water belonging to the same batch were purchased from a local market. The bottles were of uncolored PET, sealed with plastic caps, and labeled with wax paper strips; no mention was made by bottle producers on the presence of r-PET in the purchased samples.
Water bottles were transferred to the laboratory and stored at room temperature for 6 months in a cool, clean, well-ventilated, odorless, and dry place, away from direct sunlight and heat sources. The chemical analyses were carried out immediately after the sample collection and at specific intervals during storage. Each time, three bottles were opened and three aliquots were taken from each bottle (No. 9 determination per each storage time).
NIAS volatile determination was carried out on the samples during the shelf-life under normal conditions at room temperature and under accelerate conditions according to the Commission regulation (EU) No. 10/2011 [4].
The bottle cap material as well as the adhesive used for sticking the bottle labels has been also investigated.
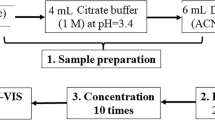
Headspace solid-phase microextraction (HS-SPME) procedure
For the extraction of the migrant volatiles, the headspace solid-phase microextraction technique was applied. In detail, a 40-mL vial, equipped with a “mininert” valve (Supelco, Bellefonte, PA, USA), was filled with 20 mL of each water sample. Extraction was performed in the headspace of the vial kept at 60 °C. A divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) fiber of 50/30 μm film thickness (Supelco, Bellefonte, PA, USA) housed in its manual holder (Supelco, Bellefonte, PA, USA) was used. The sample was equilibrated for 20 min and then extracted for 15 min under constant stirring. After the sampling, the SPME fiber was kept for 3 min at 260 °C into the splitless injector of the GC/MS for the desorption of the analytes. Distilled water was analyzed as blank after each sample.
Gas chromatograph/mass spectrometer (GC–MS) analysis
A Varian 450-GC gas chromatograph directly interfaced with a Varian 220-MS ion trap mass spectrometer (Varian Spa, Turin, Italy) was used. The conditions were as follows: injector temperature, 260 °C; injection mode, splitless; capillary column, CP-Wax 52 CB, 60 m, 0.25 mm i.d., 0.25 μm film thickness (Chrompack Italy, s.r.l. Turin, Italy); oven temperature, 60 °C held for 5 min, then increased to 240 °C at 5 °C/min, held at 240 °C for 20 min; carrier gas, helium at a constant pressure of 10 psi; transfer line temperature, 250 °C; acquisition range, 30 to 300 m/z; scan rate, 1 scan/s. Chromatogram acquisitions were in full scan mode. Each compound was identified using mass spectral data, NIST 14 library (NIST/EPA/NIH Mass Spectra Library, version 2.2g, USA), linear retention indices, literature data, and the injection of standards, where available. The linear retention indices (LRI) were calculated according to Van den Dool and Kratz [28] after injection of a mixture of C6–C23 homologue n-alkanes analyzed under the same GC conditions. Peaks were considered “identified” when their mass spectral fit values were at the default value of 700 or above; their LRI provided a low match window of ±10 index units respect to those from literature and their RT matched to that of standards.
Quantitative analysis
The volatile compounds were quantified using the method of standard addition [29]. Nonanal, tetradecanal, (E)-oct-2-enal, nonadecane, limonene, toluene, and dibutyl phthalate standards were purchased from Sigma-Aldrich s.r.l. (Milan, Italy) at the highest purity available. Stock solutions of individual standard were prepared by dissolving the appropriate amount of each compound in ethyl alcohol (95%) in order to obtain a final concentration of 1.2 mg/mL. The stock solutions were stored at −20 °C. A working solution was prepared daily mixing different volumes of the stock solutions and diluting in HPLC-grade water to a final volume of 10.0 mL. Furthermore, five different amounts of working solution were added to five aliquots, each one of 20 mL, of every water sample. The spiked and non-spiked samples were analyzed in triplicate. Quantification was based on a six-point calibration curve generated by plotting detector response versus the amount spiked of each standard. The calibration curve of nonanal was used to quantify aliphatic aldehydes from C8 to C11, that of tetradecanal to quantify aliphatic aldehydes from C12 to C16. Unsaturated aldehydes and ketones were quantified by (E)-oct-2-enal calibration curve. For the quantification of the aliphatic hydrocarbons, the calibration curve of nonadecane was used.
Method validation
Linearity, precision, limit of detection (LOD), and limit of quantification (LOQ) were determined for the validation of the used method. The linearity was evaluated using the calibration curve of each analyte. In detail, five different amounts of the working solution using for quantification were added to five 20 mL aliquots of a blank sample (HPLC-grade water), the obtained mixtures were analyzed in triplicate and the detector response plotted versus the amount spiked of each standard. Precision was calculated using a water sample that was analyzed four times consecutively and expressed as relative standard deviation (RSD %). Limits of quantification (LOQ) and detection (LOD) were calculated from the calibration curve using Eqs. (1) and (2), respectively:
where σ is the standard deviation of the intercept and m is the slope of the calibration curve.
ATR-FTIR analysis
IR spectra of bottle caps and adhesives removed from label were acquired using a Shimadzu IRAffinity-1S FTIR spectrophotometer (Shimadzu Italia S.r.l., Milan, Italy) equipped with a sealed and desiccated interferometer, a deuterated triglycine sulfate doped with l-alanine (DLATGS) detector, and a Specac Quest ATR accessory (Specac Ltd, London, England).
For measurement, a small piece of each sample was positioned in contact with attenuated total reflectance on a diamond crystal. All FTIR spectra were recorded in the range from 4000 to 350 cm−1 co-adding 45 interferograms at a resolution of 4 cm−1 with Happ–Genzel apodization.
ATR crystal was carefully cleaned before each analysis with acetone and dried with soft tissue paper. Spectra of the clean and dry diamond against air were recorded before each sample measurement and used as background. The identification of polymer and adhesive types has been achieved comparing the IR spectra of the samples with those of the LabSolutions IR spectral library (Shimadzu Italia S.r.l., Milan, Italy) and then confirmed by certified materials.
Statistical analysis
One-way ANOVA was performed on the data, using Statgraphics plus software 5.1, to investigate the effects of the storage time on the levels of the volatile NIAS.
Results and discussions
A rapid and simple procedure for sample preparation followed by GC–MS analysis was developed and applied to the mineral water samples packaged in PET bottles allowing the simultaneous determination of different volatile organic compounds. The method was validated in terms of linearity, precision, detection, and quantification limits (Table 1). The calibration curves generated by plotting detector response versus the amount spiked of each standard showed coefficients of correlation 0.9976 ≤ r 2 ≤ 0.9993 and linear ranges 0.38–6.06 µg/L for nonanal, 0.38–3.57 µg/L for tetradecanal, 0.40–3.03 µg/L for (E)-oct-2-enal, 0.17–2.03 µg/L for nonadecane, 0.20–2.14 µg/L for limonene, 0.33–3.45 µg/L for toluene, and 0.57–6.57 µg/L for dibutyl phthalate. LOD values ranged between 0.05 and 0.17 µg/L and LOQ ones between 0.17 and 0.57 µg/L. Considering the peak area obtained for each compound during the quadruplicate analyses of the same samples, the coefficient of variation (CV) resulted <12% for all the quantified compounds. Concerning LOD and LOQ values of volatile migrants in mineral water, only values for nonanal, dibutyl phthalate, and toluene are present in literature. However, these data are hardly comparable with those reported in this study since they were obtained with analytical methods requiring the derivatization of the analytes [17, 21, 26]. Just Bianchin et al. [30] reported a similar LOD value (0.1 µg/L) for toluene in water obtained by using a HS-SPME–GC–MS method.
Table 2 reports the volatile organic compounds identified and quantified in water samples at different storage times at room and stressed temperature. The developed method allowed the identification of a large number of compounds, including saturated and unsaturated aliphatic aldehydes, ketones, aliphatic and aromatic hydrocarbon, terpenes, and phthalates.
Decanal and nonanal were the most abundant aldehydes; their levels ranged between 1.42 and 5.07 µg/L and between 0.61 and 1.25 µg/L, respectively. Decanal has been previously identified in mineral water packaged in PET but no quantitative data were reported in literature [31]. An higher amount of nonanal (0.9–11.3 µg/L) has been reported by Nawrocki et al. [27] in mineral water bottled in PET, stored at room and stressed temperature, whereas lower (0.3–0.4 µg/L) by Kim et al. [26]. Higher aldehydes have never been detected previously in mineral water packaged in PET bottles. Among the unsaturated aldehydes, only (E)-ept-2-enal and (E)-non-2-enal showed levels above their LOQ just at the end of the shelf-life.
From the statistical analysis of the quantitative data, a significant increase in the amounts of all the saturated aldehydes and the unsaturated (E)-ept-2-enal and (E)-non-2-enal occurred during the shelf-life of the mineral water samples. Mono- and di-unsaturated aldehydes have been already identified in mineral water packaged in PET by Strube et al. [31].
Medium-chain aldehydes, both saturated and unsaturated, having odor thresholds in water of a few tens of ppb or inferior, are able to affect the sensory quality of mineral water giving the “sunlight” off-flavor of bottled mineral water [31]. In our study, only decanal was present at level higher than the odor threshold (2 µg/L orthonasal and 7 µg/L retronasal) [31] starting from 6 months. These data were confirmed by an informal sensory test conducted on these samples; in fact, the panel was able to identify only the plastic odor descriptor starting from 12 months (data not reported).
Another carbonyl compound has been identified in the analyzed water samples, namely 1-octen-3-one. It was detected in all the samples regardless the storage time, at trace levels. Other Authors reported the presence of 1-octen-3-one both in PET bottled mineral water and in PET polymer [31].
Preliminarily, the bottle cap material and the adhesive used for sticking the bottle labels have been investigated by ATR-FTIR, as above reported; the screw caps resulted made of high-density polyethylene (HDPE), whereas the adhesive was a vinyl adhesives based on vinyl acetate ethylene (VAE).
Some authors affirmed that saturated and unsaturated aldehydes and ketones could be related to the presence of lubrificants, and their impurities, used for the manufacture of polyolefins and polyolefin closures. Lubricants such as erucamide and also oleamide are authorized in Europe for the manufacture of plastic materials intended to come in contact with food [4]. No SMLs have been prescribed for these substances. These oleamides have double bonds which are susceptible to attack by oxygen; the result of this attack is that compounds such as aldehydes and ketones may be produced. To our knowledge, erucamide and oleamide are not used in the manufacture of PET bottles but erucamide could be used in the manufacture of bottle closures to facilitate their removal from the container on opening [32]. Moreover, saturated and mono-unsaturated aldehydes and ketones were reported as byproducts resulting from the thermal oxidation of polyethylene that takes place during processing [33].
The lubricants of HDPE bottle caps and the caps themselves could be the sources of the carbonyl compounds identified in this study.
In the volatile fraction of mineral water samples, they were detected also limonene, aliphatic hydrocarbons, from C11 to C19, and toluene. Among these, only limonene, undecane, tetradecane, and nonadecane exceeded their LOQ; their amount statistically increased till 6 months and then decreased. n-Alkanes and aromatic hydrocarbons have been identified in potable water [34] but never reported before in bottled mineral water. Also limonene was identified here for the first time in mineral water packaged in PET bottles. Nevertheless, Bayer et al. [35], found 121 contaminants in post-consumer recycled PET, including limonene, n-alkanes, toluene and other aromatic hydrocarbons, acids, aliphatic and aromatic aldehydes, alcohols, ketones, and terpenes, as residual after the commercial wash operations. Similar compounds were reported by other Authors in independent studies on recycled PET flakes [36, 37].
As regards phthalates, only dibutyl phthalate has been detected in the mineral water samples analyzed in this study. It was present at trace level in 3-month water samples, then its level significantly increased reaching the amount of 6.01 µg/L at the end of the shelf-life. Some studies reported the presence of phthalates in mineral water packaged in PET with different levels for dibutyl phthalate which were included between 0.043 and 50 µg/L [17,18,19,20,21, 38]. Phthalates, mainly bis-2-ethylhexyl phthalate, di-n-butyl phthalate, and butylbenzyl phthalate, are used as plasticisers to improve the mechanical properties of polymers. Since they are not chemically but only physically bound to the polymer chains, they may be leached into food and beverages from the packaging.
Due to their adverse effects on human health [39, 40], phthalates in food contact materials are strictly regulated. Although they are not thought to be used in the manufacture of PET bottles (ILSI, 2000), they have been found in PET material and in PET bottled water. Till now, convincing explanations have never been offered.
Phthalates, namely di-n-butyl phthalate and bis-2-ethylhexyl phthalate, have been identified in adhesive based on VAE, that could thus be considered the source of phthalates in our water samples [8].
Conclusions
The presence of NIAS volatiles into mineral water bottled in PET was investigated during shelf-life. A simple and automatic analytical method based on headspace solid-phase microextraction–gas chromatography–mass spectrometry (HS-SPME–GC–MS) technique has been developed and validated. The method resulted to be highly reproducible with limits of detection ranging between 0.05 and 0.17 µg/L. Many volatiles, mainly aliphatic aldehydes, were identified and quantified and some of these for the first time in PET packaged mineral water. The levels of the identified NIAS showed statistically differences during the shelf-life and most of them increased as the storage time increased. Considering the identified substances, a migration not only from packaging materials but also from the closure caps and adhesive may be plausible. Whatever the origin of migrants, the determination of NIAS in mineral water is a top priority due to the growing popularity of bottled water consumption. Although the Regulation on Food Contact Materials [4] recognizes that during the manufacture and use of plastic materials and articles NIAS can be formed, the same Regulation affirmed that it is not possible to list and consider all of these. As regards aldehydes, only formaldehyde and acetaldehyde limits are reported in the Regulation, otherwise, a total limit of migration for aliphatic aldehydes could be included since a synergic toxic effect could be expected. This could be of great importance not only to assure the safety of bottled mineral water but also to guarantee the sensory quality during the shelf-life.
References
Rodwan Jr JG (2010) Bottled water 2009 International Bottled Water Association. http://www.bottledwater.org/files/2009BWstats.pdf. Accessed 28 March 2011
Begley TH, Dennison JL, Hollifield HC (1990) Migration into food of polyethylene terephthalate (PET) cyclic oligomers from PET microwave susceptor packaging. Food Addit Contam 7:797–803
Ashby R (1988) Migration from polyethylene terephthalate under all conditions of use. Food Addit Contam 5:485–492
European Commission (2011) Commission Regulation (EU) N 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. Off J Eur Comm L12:1–89
Bach C, Dauchy X, Severin I, Munoz JF, Etienne S, Chagnon MC (2013) Effect of temperature on the release of intentionally and non-intentionally added substances from polyethylene terephthalate (PET) bottles into water: chemical analysis and potential toxicity. Food Chem 139:672–680
Muncke J (2009) Exposure to endocrine disrupting compounds via the food chain: is packaging a relevant source? Sci Total Environ 407:4549–4559
Yang CZ, Yaniger SI, Jordan VC, Klein DJ, Bittner GD (2011) Most plastic products release estrogenic chemicals: a potential health problem that can be solved. Environ Health Persp 119:989
Aznar M, Vera P, Canellas E, Nerín C, Mercea P, Störmer A (2011) Composition of the adhesives used in food packaging multilayer materials and migration studies from packaging to food. J Mater Chem 21:4358–4370
Welle F (2011) Twenty years of PET bottle to bottle recycling—an overview. Resour Conserv Recycl 55:865–875
Widén H, Leufvén A, Nielsen T (2005) Identification of chemicals, possibly originating from misuse of refillable PET bottles, responsible for consumer complaints about off-odours in water and soft drinks. Food Addit Contam 22:681–692
Evandri MG, Tucci P, Bolle P (2000) Toxicological evaluation of commercial mineral water bottled in polyethylene terephthalate: a cytogenetic approach with Allium cepa. Food Addit Contam 17:1037–1045
Wegelin M, Canonica S, Alder C, Marazuela D, Suter MF, Bucheli TD, Ibrahim P (2001) Does sunlight change the material and content of polyethylene terephthalate (PET) bottles? J Water Supply Res Technol AQUA 50:125–135
Sugaya N, Nakagawa T, Sakurai K, Morita M, Onodera S (2001) Analysis of aldehydes in water by head space-GC/MS. J Health Sci 47:21–27
Dąrowska A, Borcz A, Nawrocki J (2003) Aldehyde contamination of mineral water stored in PET bottles. Food Addit Contam 20:1170–1177
Mutsuga M, Kawamura Y, Sugita-Konishi Y, Hara-Kudo Y, Takatori K, Tanamoto K (2006) Migration of formaldehyde and acetaldehyde into mineral water in polyethylene terephthalate (PET) bottles. Food Addit Contam 23:212–218
Ioannidou MD, Samouris G, Achilias DS (2016) Acetaldehyde contamination of water, alcoholic, and non-alcoholic beverages stored in glass or plastic bottles. Toxicol Environ Chem 98:1183–1190
Psillakis E, Kalogerakis N (2003) Hollow-fibre liquid-phase microextraction of phthalate esters from water. J Chromatogr A 999:145–153
Bošnir J, Puntarić D, Galić A, Škes I, Dijanić T, Klarić M, Šmit Z (2007) Migration of phthalates from plastic containers into soft drinks and mineral water. Food Technol Biotechnol 45:91–95
Amiridou D, Voutsa D (2011) Alkylphenols and phthalates in bottled waters. J Hazard Mater 185:281–286
Keresztes S, Tatár E, Czégény Z, Záray G, Mihucz VG (2013) Study on the leaching of phthalates from polyethylene terephthalate bottles into mineral water. Sci Total Environ 458:451–458
Jeddi MZ, Rastkari N, Ahmadkhaniha R, Yunesian M (2015) Concentrations of phthalates in bottled water under common storage conditions: do they pose a health risk to children? Food Res Int 69:256–265
Song YS, Al-Taher F, Sadler G (2003) Migration of volatile degradation products into ozonated water from plastic packaging materials. Food Addit Contam 20:985–994
LoPachin RM, Gavin T (2014) Molecular mechanisms of aldehyde toxicity: a chemical perspective. Chem Res Toxicol 27:1081–1091
O’Brien PJ, Siraki AG, Shangari N (2005) Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit Rev Toxicol 35:609–662
Takeda K, Katoh S, Nakatani N, Sakugawa H (2006) Rapid and highly sensitive determination of low-molecular-weight carbonyl compounds in drinking water and natural water by preconcentration HPLC with 2, 4-dinitrophenylhydrazine. Anal Sci 22:1509–1514
Kim HJ, Shin HS (2011) Simple and automatic determination of aldehydes and acetone in water by headspace solid-phase microextraction and gas chromatography–mass spectrometry. J Sep Sci 34:693–699
Nawrocki J, Dąbrowska A, Borcz A (2002) Investigation of carbonyl compounds in bottled waters from Poland. Water Res 36:4893–4901
Van den Dool H, Kratz PD (1963) A generalization of the retention index system including linear temperature programmed gas–liquid partition chromatography. J Chromatogr A 11:463–471
Verzera A, Condurso C, Romeo V, Tripodi G, Ziino M (2010) Solid-phase microextraction coupled to fast gas chromatography for the determination of migrants from polystyrene-packaging materials into yoghurt. Food Anal Methods 3:80–84
Bianchin JN, Nardini G, Merib J, Dias AN, Martendal E, Carasek E (2012) Simultaneous determination of polycyclic aromatic hydrocarbons and benzene, toluene, ethylbenzene and xylene in water samples using a new sampling strategy combining different extraction modes and temperatures in a single extraction solid-phase microextraction–gas chromatography–mass spectrometry procedure. J Chromatogr A 1233:22–29
Strube A, Buettner A, Groetzinger C (2009) Characterization and identification of a plastic-like off-odor in mineral water. Water Sci Technol 9:299–309
Shi Y, Liu H, Rule M (2003). US Patent Application No. 10/393,857
Bravo A, Hotchkiss JH, Acree TE (1992) Identification of odor-active compounds resulting from thermal oxidation of polyethylene. J Agric Food Chem 40:1881–1885
Grob K, Grob G (1975) Organic substances in potable water and in its precursor: III. The closed-loop stripping procedure compared with rapid liquid extraction. J Chromatogr A 106:299–315
Bayer FL (2002) Polyethylene terephthalate recycling for food-contact applications: testing, safety and technologies: a global perspective. Food Addit Contam 19:111–134
Nerin C, Albinana J, Philo MR, Castle L, Raffael B, Simoneau C (2003) Evaluation of some screening methods for the analysis of contaminants in recycled polyethylene terephthalate flakes. Food Addit Contam 20:668–677
Dimitrov N, Krehula LK, Siročić AP, Hrnjak-Murgić Z (2013) Analysis of recycled PET bottles products by pyrolysis-gas chromatography. Polym Degrad Stab 98:972–979
Casajuana N, Lacorte S (2003) Presence and release of phthalic esters and other endocrine disrupting compounds in drinking water. Chromatographia 57:649–655
Arcadi FA, Costa C, Imperatore C, Marchese A, Rapisarda A, Salemi M, Costa G (1998) Oral toxicity of bis (2-ethylhexyl) phthalate during pregnancy and suckling in the Long–Evans rat. Food Chem Toxicol 36:963–970
Petrović M, Eljarrat E, de Alda MJL, Barceló D (2001) Analysis and environmental levels of endocrine-disrupting compounds in freshwater sediments. TrAC Trends Anal Chem 20:637–648
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Fabrizio Cincotta, Antonella Verzera, Gianluca Tripodi, and Concetta Condurso declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Not applicable.
Rights and permissions
About this article
Cite this article
Cincotta, F., Verzera, A., Tripodi, G. et al. Non-intentionally added substances in PET bottled mineral water during the shelf-life. Eur Food Res Technol 244, 433–439 (2018). https://doi.org/10.1007/s00217-017-2971-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-017-2971-6