Abstract
An experiment was conducted to study the dietary effect that the inclusion (40 g kg−1) of grape seed (GS), grape skin (SS), grape pomace (GP), and (0.2 g kg−1) of vitamin E (E) had on the composition and microbiological quality of chicken breast meat and on the physico-chemical parameters (TBARS, pH, color, Kramer shear force), sensorial characteristics, and microbiological quality of chicken breast meat patties during chilled storage (0, 3, 6, and 9 days) at 2 °C. In general, proximate composition and microbial counts of the raw chicken breast meat and the patties were not affected. Lower TBARS values were detected in patties formulated with breast meat obtained from birds fed E, SS, and GP diets. No clear effect was observed on the color or textural characteristics of the different patties. The addition of SS and GP in chicken diets reduced TBARS values showing some improvement in the oxidative stability of breast patties without affecting its technological properties, sensorial attributes, or microbial quality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the last few decades, chicken meat and chicken products have grown in popularity and are massively consumed at global level [1] due to a series of factors including their relatively low cost of production, low fat content, and high nutritional value [2]. However, chicken meat can spoil very quickly even under refrigerated conditions mainly due to microbial growth and chemical deterioration such as oxidation. These latter processes are especially relevant when chickens are given feed to improve the fatty acid composition and nutritional value of these meats. High polyunsaturation levels accelerate oxidative processes which have a negative impact on the flavor and nutritional value of the meat [3]. As a result, nutritional and technological strategies have been devised to help preserve meat and control the oxidation process to extend the shelf life of meat products [4]. Nutritional approaches to improve meat stability could be more effective than using additives as food ingredients. Moreover, diet is often the only strategy available to improve the stability of muscle foods where the use of exogenous additives is difficult if not impossible [5]. Furthermore, modification of meat composition by improving animal diets would be more readily accepted by consumers who are becoming increasingly reluctant to consume food additives. Diet is used as one way to incorporate different natural ingredients with antimicrobial and antioxidant properties in animal feed to improve animal health and the quality of the meat they produce [6]. Vitamin E is the antioxidant most frequently added to animal feed to maintain optimal health and production and enhance reproduction, and is also very effective in preventing the development of undesirable off-flavors during meat storage [7]. Thus, dietary vitamin E requirements increase in diets containing high amounts of polyunsaturated fatty acids [3].
Therefore, there is great interest in the introduction of natural ingredients such as herbs, fruit, (especially grapes and grape by-products), and other products rich in polyphenols in animal feed with a view to improving the health qualities and stability of meat. The antioxidant and antimicrobial capacity of the polyphenols present in grape by-products has been clearly demonstrated [8]. Several authors have shown the positive effect of grape by-products such as grape pomace and grape seed extract incorporated into feed in decreasing lipid oxidation in chicken meat [9,10,11,12]. Brenes et al. [13] and Goni et al. [14] indicated that the intake of grape pomace increases the antioxidant capacity of the breast and thigh meat of broiler chickens in the same way as vitamin E in experimental diets.
Other studies have also shown that the direct addition of grape by-products or other plants and fruits rich in polyphenols to meat products has various effects on lipid oxidation, color, and microbial and sensorial properties [15,16,17]. However, there is little information available on the effect that chicken feed enriched with grape by-products (grape pomace, skin, and seed) rich in polyphenols has on chicken meat. In this regard, Sáyago-Ayerdi et al. [11] studied the antioxidant effect that feed enriched with grape pomace concentrate had on the lipid oxidation of chilled chicken patties and those frozen over long periods. Positive effects were observed on the inhibition of lipid oxidation. However, these authors did not study the separate effects that the main components of grape pomace (skin and seed) incorporated into feed had on chicken meat and meat products. Numerous in vitro studies have shown [18,19,20] that the polyphenols found in grape by-products inhibit the growth of certain pathogens such as Staphylococcus aureus, Escherichia coli, Campylobacter, Salmonella, and Helicobacter pylori. The antimicrobial effectiveness of directly adding grape, plant extracts, and fruit to reformulated meat products has been reported in a few studies [16] using meat purchased at the market, but the results reported are not definitive. However, we were unable to find any studies addressing the antimicrobial effect that polyphenols from grape by-products in chicken feed have on chicken breast meat or their impact on the patties during typical commercial storage. Our intention here is to conduct a complete ‘farm-to-fork’ study of the animal-based food-production chain to examine the effect that a diet supplemented with polyphenols has on animals and the meat they produce for human consumption.
The aim of this paper is to examine the effect that adding grape by-products (grape skin, seed, and pomace) to chicken feed has on the physico-chemical, microbiological, and sensorial attributes of chicken patties during chilled storage (at 2 °C for 9 days), simulating the real processing and storage conditions of these products. The composition and technological and microbial characteristics of chicken breast meat used for these formulations were also evaluated.
Materials and methods
Grape pomace (GP), grape skin (SS), and grape seed (GS)
Grape Pomace (GP, consisting mainly of skin, stems, and seeds), Grape Skin (SS), and Grape Seed (GS) from red grapes (Vitisvinifera var. Cencibel) were obtained from the Explotaciones Hermanos Delgado winery (EHD, Socuéllamos, Ciudad Real, Spain) from a vinification tank after 15 days of alcoholic fermentation. These winery by-products were dried (indirect air at below 80 °C). Seed and skin were mechanically separated from GP during this process and ground to 0.5 mm. Seeds were subjected to a cool press oil extraction process before grinding. The total extractable polyphenol content of GS, SS, and GP was 79.3, 62.4, and 32.4 g GAE (gallic acid equivalents) kg−1 of dry matter, respectively. In the same samples, the protein content was 178.6, 109.6, and 113.7 g kg−1, while crude fiber was 252.8, 144.0, and 333.9 g kg−1, respectively.
Birds and diets
A total of 125 1-day-old male broilers Cobb chicks were obtained from a commercial hatchery. The birds were housed in electrically heated starter battery brooders in an environmentally controlled room with 23 h of constant overhead fluorescent lighting during 3 weeks. The chicks were distributed in 25 pens, each containing five randomly assigned chicks that received 5 dietary treatments during 21 days with five replicates per treatment. Diets in mash form and water were provided ad libitum. Diets were stored in a cool, dark dry location during the experimental period. All diets were formulated to meet or exceed the minimum National Research Council (NRC) (1994) [21] requirements for broiler chickens. Experimental procedures were approved by the Universidad Complutense de Madrid (UCM) Animal Care and Ethics Committee in compliance with Ministry of Agriculture, Fishery and Food requirements for the Care and Use of Animals for Scientific Purposes. Experimental diets were: (1) control diet (C); (2) C + 0.2 g kg−1 vitamin E (E); (3) C + 40 g kg−1 grape seed (GS); (4) C + 40 g kg−1 grape skin (SS); (5) C + 40 g kg−1 grape pomace (GP). The ingredients and nutritional composition of diets are shown in Table 1. Vitamin E was purchased from DSM Nutritional Products Iberia S.A. Diets were formulated to contain the same energy, protein, and fiber content, and consequently, straw was incorporated into the formula at different doses.
Collection of chicken meat samples
At 21 days, ten birds per treatment (body weight, 0.824 ± 0.02 kg) were slaughtered and the breast meat was immediately trimmed and ground (4 mm plate) using a grinder (Mainca, Granollers, Spain). Four representative samples of meat per treatment were taken for proximate composition analysis and another three samples per treatment were used for microbial analysis. The remaining meat was packed in plastic bags, stored at −20 °C, and used to formulate the patties (no more than 4 days). After being thawed in the refrigerator (2 °C) for ~12 h, meat samples were used to make patties.
Chicken patty preparation
Patties were prepared using the breast meat from the chickens fed with the different experimental diets. The formulation consisted of 854 g kg−1 of meat, 68 g kg−1 whole egg, 68 g kg−1 breadcrumbs, and 10 g kg−1 salt. The ground meat was first blended (Hobart, Model N50, USA) for 60 s and the salt was added to the meat and mixed for an additional 30 s. The eggs were then beaten and added to the mixture by blending for 20 s. The breadcrumbs were then placed into the mixer and mixed for another 60 s. This blend yielded a total of 26 patties (~60 g per patty) per treatment using a conventional burger maker (Ministeak burger maker, O.L. Smith Co. Ltd., Italy). The patties were packed in high oxygen barrier vacuum bags (nylon/polyethylene, 9.3 ml O2/m2/24 h at 0 °C, Koch Kansas City, MO). Each bag contained 2 patties which were stored in a refrigerator at 2 ± 1 °C. A total of 16 replicates per treatment were used to determine lipid oxidation (TBARS), Kramer shear force (KSF), pH, and microbiology after 0, 3, 6, and 9 days of storage (four replicates per day). A further ten replicates per treatment were used for sensory evaluation (six replicates) and proximate analysis (four replicates).
Proximate composition and total extractable polyphenols
The protein content of grape by-products, diets, meat, and patties samples was evaluated using a nitrogen determinator LECO FP-2000 (Leco Corporation, St Joseph, MI, USA). Moisture and ash in meat and patties were analyzed according to the methods of the AOAC [22] and fat content according to the method described by Bligh and Dyer [23]. Determination of total extractable polyphenols in the diets was performed following the procedure described by Chamorro et al. [24] using gallic acid (Sigma-Aldrich, St. Louis, MO) as the standard and expressed as gallic acid equivalents (GAE) (g GAE kg−1 of sample).
pH
pH was determined using a pH meter (827 pH Lab Methrom, Herisau, Switzerland) on 10 g of sample blended with 100 ml of distilled water.
Lipid oxidation
Lipid oxidation was determined by measuring the thiobarbituric acid-reactive substances (TBARS) in the raw patties [25]. A calibration curve was plotted with 1,1,3,3-tetraethoxypropane (Sigma Chemical Co., St. Louis, MO, USA) to measure malonaldehyde (MDA). Values were expressed as mg of malonaldehyde per kilogram of sample.
Color measurement
Color, CIE-LAB tristimulus values, lightness (L*), redness (a*), and yellowness (b*) were measured on the surface of raw patties using a CM-3500d Chroma Meter (Konica Minolta Business Technologies, Tokyo, Japan). Before use, the colorimeter was calibrated on the Hunterlab color space system using a white tile.
Kramer shear force (KSF)
Kramer shear force (KSF) was performed using a miniature Kramer (HDP/MK05) cell. A mini 5-bladed head was used to perform a shearing test. Kramer shear tests were performed on 2 × 2 cm sections of previously weighed patty formulation at room temperature. A 5 kg load cell was used. The force was exerted at a compression distance of 20 mm at 8 mm/s crosshead speed using a TA-XT plus Texture Analyzer (Texture Technologies Corp. Scarsdale, NY). KSF values were calculated as the maximum force per g of sample (N g−1).
Microbiological analysis
Ten grams of sample were placed in a sterile plastic bag (Sterilin, Stone, Staffordshire, UK) with 90 ml of peptone (Panreac Química, S.A. Barcelona, Spain). After 1 min in a stomacher blender (Colworth 400, Seward, London, UK), appropriate decimal dilutions were prepared and pour-plated on the following media: Plate Count Agar (PCA, Oxoid) for the total viable count (TVC) (30 °C, 72 h); De Man, Rogosa, Sharpe Agar (MRS) (Oxoid) for lactic acid bacteria (LAB) (30 °C, 72 h); Violet Red Bile Glucose Agar (VRBG) (Oxoid) for Enterobacteriaceae (37 °C, 24 h); and Coli ID agar (Biomerieux, Marcy l’Etoile, France) to count positive β-glucuronidase coliforms (37 °C, 48 h). Microbial counts were expressed as logarithms of colony-forming units per gram (Log cfu g−1).
Sensory evaluation
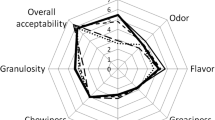
Patties were assessed by a 13-member panel of people who regularly consume this type of product. The panel was selected after preliminary training (two sessions) on the products and terminology. Samples were cooked in an electric pan (Plactronic, Selecta, J.P. Selecta, S.A. Barcelona, Spain) for 1.5 min per side at 210 ± 4 °C. Patties were cut into pieces of uniform size (2 × 2 cm) and were immediately presented to the panel of judges. Judges were instructed to evaluate color, flavor, hardness, and juiciness on a non-structured descriptive scale (0–10), and general acceptability (0 = dislike very much, 10 = like very much) and on a hedonic scale rating test with fixed extremes. Each point was later converted to a numerical scale. Sensory analysis was performed 2 days after preparation of the patties.
Statistical analysis
One-way analysis of variance (ANOVA) was performed to study the effect of the dietary treatments on the microbial and proximate composition analysis of breast meat and for sensory and proximate composition analysis of breast patties. A two-way ANOVA analysis was performed to study the effect of the dietary treatments and the storage time on TBARS, pH, KSF, color, and microbial analysis of the breast patty formulations. The general linear model (GLM) procedure of the software SPSS (v.22, IBM SPSS Inc.; Chicago, IL, USA) was used. Tukey’s HSD test was used to compare means and significant differences were declared at P < 0.05.
Results and discussion
Proximate composition of chicken meat
Table 2 shows the effect that dietary supplementation with grape by-products and vitamin E had on the proximate composition of chicken breast meat which, in general, was hardly affected at all. Fat and protein (15.4–19.7 and 228.3–218.6 g kg−1, respectively) were unaffected by the treatment (Table 2). The effect (P < 0.05) on moisture and ash content (753.5–743.8 and 12.3–13.6 g kg−1, respectively) was of little quantitative relevance. Meat from chickens fed on diets supplemented with vitamin E, grape skin, and grape pomace showed statistically (P < 0.05) lower moisture values compared to the control. Meat from diets containing grape by-products exhibited lower ash values (P < 0.05). Shirzadegan and Falahpour [26] found a linear decrease in the ash content of chicken thigh meat fed with a diet containing a medicinal herbal extract mixture derived from green tea, cinnamon, garlic, and chicory which are sources of natural antioxidants or functional materials.
Microbiological counts in chicken meat
The microbiological counts in breast chicken meat are shown in Table 2. The level of TVC ranged between 4.68 and 5.37 Log cfu g−1. In general, there were no significant differences in the TCV or LAB count except for the meat from chickens fed the diet supplemented with grape seed which exhibited a higher (P < 0.05) level than those fed the control diet.
The results of Enterobacteriaceae tests showed no statistical differences among groups and ranged between 2.51 and 3.23 Log cfu g−1 which is considered hygienically acceptable for raw meat. A higher (P < 0.05) level of coliforms was found in the meat of birds fed diets supplemented with GS and vitamin E.
The antimicrobial effect of grape extract against some microorganisms has been demonstrated in numerous in vitro studies [18,19,20]. A little information is available regarding dietary strategies to reduce microbial proliferation in meat. To the best of our knowledge, there is no information regarding the effect that adding grape to animal diets has on the microbiological count of meat. No antimicrobial effect on chicken thigh meat was reported by Lee et al. [27] when birds were fed diets supplemented with gallic acid, a phenolic compound also present in grape. In contrast, Jung et al. [10] reported that dietary gallic acid had a mild antimicrobial effect on chicken breast meat during storage.
Proximate composition of chicken patties
The proximate composition of the chicken patties is reported in Table 3. These results are related to the components used in the preparation. Results were similar to those found in other chicken patty studies [11] using similar ingredients. However, protein content was higher in this study (212.7–203.6 g kg−1) attributable to the higher proportion of protein from chicken breast meat used in these patties (Table 2). Despite the statistical significance observed in the composition of the patties, these differences were generally not quantitatively relevant.
Lipid oxidation of chicken patties
The effect that grape by-products in the diet had on the TBARS value of chicken breast patties is presented in Table 4. The initial TBARS values were low in all the samples (0.23–0.56 mg MDA Kg−1 sample) as expected considering the low fat content and early evaluation of samples and coincided with those previously reported by Sáyago-Ayerdi et al. [11]. Initially, lower (P < 0.05) TBARS values were detected for chicken patties obtained from birds fed vitamin E, SS, and GP compared to the control group, while the patties obtained from the GS group showed similar values to the control patties. These low TBARS levels could be due to greater pre slaughter (stress during capture and transport) and postslaughter control (deboning, handling, packaging, storage, and others) which are important factors for determining the rate and extent of meat product lipid oxidation [28]. These birds were not subjected to transport stress; and meat was carefully manipulated and vacuum packaged immediately after slaughter and was, therefore, less exposed to oxygen than commercial meat products.
In general, TBARS values for treatments E, SS, and GP were similar (P > 0.05) during storage and even decreased in the case of treatments C and GS (P < 0.05) compared with the initial values. Decreases in TBARS levels in meat have also been described at different stages of storage [29,30,31], presumably due to intermolecular reactions in the malonaldehyde that is formed (polymerization) and reactions with other constituents, especially amino acids/proteins [32, 33]. In addition, in this study, a decline in TBARS levels is easier to observe due to low oxidation levels resulting from the conservation conditions (high oxygen barrier vacuum bags) used in this experiment.
The addition of antioxidants such as vitamin E in chicken diets has been extensively demonstrated as an effective strategy to protect fatty acids and decrease lipid oxidation in both raw and cooked poultry meat [9, 13, 34, 35]. This high efficiency has been attributed to the radical scavenger α-tocopherol which is incorporated into cell membranes where oxidation is initiated [35]. Vitamin E requirements increase when the diet contains polyunsaturated fat sources such as sunflower oil. Thus, in this study, we assessed whether grape polyphenol afforded the same degree of protection obtained with high doses of vitamin E (0.2 g kg−1) in diets with high levels of sunflower oil. Our results corroborate the previous findings, indicating that diets containing grape pomace delayed meat lipid oxidation by protecting PUFA meat content from oxidation processes, exhibiting a protective effect similar to that observed with vitamin E supplement [36]. The antioxidant effect of dietary grape by-products on raw chicken meat has also been demonstrated by several authors [9, 13, 14, 37, 38], and has been attributed to the ability of their phenolic compounds to scavenge free radicals, to form metal ions complexes, and to prevent or reduce the development of singlet oxygen [6]. These studies suggest that the polyphenols present in grape by-products are absorbed, distributed, and remained active modulating antioxidant activity in muscle tissue. In this connection, Sáyago-Ayerdi et al. [11] reported the anti-oxidative effect of dietary grape pomace (3 and 6%) on lipid oxidation in chilled and long-term frozen chicken patties. Similarly, although to a lesser extent, our results showed a reduction in TBARS value with dietary skin and seed when added separately and combined (pomace). Our results corroborated the antioxidant effect of dietary grape pomace previously reported and also suggest that the skin contributed more than seed to this antioxidant effect. Differences among phenolic compounds present in skin and seed [39] might account for these biological differences. However, the previous studies [40, 41] have demonstrated that grape seed has a higher polyphenol content and antioxidant capacity than grape skin. Differences in the nature of the phenolic compounds present in both of these fractions have also been reported [39, 40]. It has been noted that seeds contain higher amounts of monomeric and dimeric proanthocyanidins but lower amounts of anthocyanins and phenolic acids than skin. However, differences in the intestinal use, metabolism, and bioavailability of the different phenolic compounds present in skin and seed [39], and the importance of intestinal microbiota in the metabolism and biological function of grape polyphenols could account for such discrepancies. In contrast, other authors [38, 42] failed to observe decreased meat lipid peroxidation when chickens were fed grape by-products. As already mentioned, differences in experimental conditions (diet, environmental stress factors, etc.) and the phenolic composition of grapes among different experiments could account for these conflicting results. In fact, the polyphenolic composition of grape by-products varies depending on the part of the grape and the grape variety, and is also influenced by growing conditions, climate, maturity, fermentation time, degree of ripeness, etc. [43]. Moreover, the antioxidant capacities of phenolic compounds were affected by other factors such as kind and concentration of polyphenols, other compounds presents in the matrix such as fiber and the polyphenols, the synergic effect among several compounds, etc. [42, 44,45,46]. Furthermore, it is also known that the chemical structure of polyphenols rather than the concentration is what determines the rate and extent of absorption and the nature of the metabolites circulating in the plasma [47]. Moreover, an important fraction of the ingested grape polyphenols is digested (disappear) and metabolized by the intestinal microbiota generating microbial-derived bioactive phenolic metabolites [13, 36, 47, 48]. Gut microbiota are responsible for the extensive breakdown of the original polyphenolic structures into a series of low-molecular-weight phenolic metabolites making them absorbable. These metabolites could be responsible for the biological activity resulting from polyphenol-rich food consumption rather than the original compounds found in foods. Until now, research on the digestibility of polyphenols from grape by-products in domestic animals has been scarce and studies have focused on their effect on the digestibility of other nutrients [6].
Microbiological count in chicken patties
The microbiological study of chicken patties is shown in Fig. 1. The initial levels of TVC and LAB were approximately 4 and 3.5 Log cfu g−1, respectively, and no significant difference among treatments was observed. The level of Enterobacteriaceae and Coliforms was very low (<3 Log cfu g−1), the lowest level being detected in the control patties at the beginning of storage. A higher bacterial count in these groups was observed for SS and GP patties, and remained very similar throughout the storage period.
Microbiological counts (Log cfu g−1): a total viable count, b lactic acid bacteria, c Enterobacteriaceae, and d coliforms in chicken patties formulated with breast meat obtained from birds fed dietary treatments (C control, E control + vitamin E, GS control + grape seed 40 g kg−1), SS control + grape skin 40 g kg−1, GP control + grape pomace 40 g kg−1 during refrigerated storage (2 °C)
In general, TVC and LAB levels increased slightly during chilled storage, the highest value being observed at the end of storage, but no significant differences among samples were observed (Fig. 1). TVC and LAB levels were associated with the anaerobic storage conditions and the pH of these samples (Table 4). In this study, no clear effect from the addition of grape by-products and vitamin E in chicken diets was observed on the growth of microorganisms in patties. The antimicrobial effect of grape products against several microorganisms has been demonstrated in in vitro studies [18,19,20]. Other authors [16] report a decrease in total viable counts (TVC), lactic acid bacteria (LAB), and other microorganisms in raw porcine patties during chilled storage when grape was incorporated into the formulation. However, under industrial meat processing conditions that we simulated in our study, many factors influence the microbial population in meat and the antimicrobial effect of grape by-products does not appear to be very clear. As was discussed above, there is very little information on the use of dietary strategies to reduce microbial proliferation in raw meat and no studies on the effect that adding grape by-products to animal diets has on patty formulation. This study suggests that the new compounds generated after being metabolized have different antimicrobial effects than those present in the original by-product, this effect being more clear when grape products were added directly to the meat rather than when they were used as animal diet supplements.
pH of chicken patties
The initial pH value of patties ranged from 6.00 to 6.14 (Table 4). Similar levels were also found by other authors in pork patties reformulated with natural extracts [16, 49].
The patties formulated with meat obtained from chickens fed grape by-products and vitamin E showed lower (P < 0.05) pH values than those obtained from chickens fed the control diet during the experiment. This lower pH could be a consequence of the properties and the nature of the active compounds present in the meat of chickens fed with a diet rich in grape by-products. Lower pH values were also observed in the thigh muscle of broilers fed diets supplemented with a medicinal herbal extract mixture consisting of green tea, cinnamon, garlic, and chicory [26] and dietary Chinese medicine by-products [50] possibly due to the effect of its metabolites in the muscle following its digestibility as mentioned in the lipid section. This phenomenon was also observed by other authors in the context of pork patties made with natural extracts such as tea, grape, chestnut, and seaweed [16]. A slight decrease in pH values in all patties was observed during chilled storage (Table 4). This decrease was mostly due to the growth of lactic acid bacteria and the lactic acid produced as observed in our study and also reported by other authors [25].
Color parameters in chicken patties
The effect of grape by-product diet supplements on color stability (Lightness L*, redness a*, and yellowness b*) of chicken patties is shown in Fig. 2. The initial higher (P < 0.05) levels of Lightness (L*) were found in C and GS patties (49.60 and 49.82, respectively). In this study, L* values were lower than those observed by other authors in chicken patties formulated with fruit extract rich in polyphenols and BHT [49], but similar to those of other authors [37] who tested grape products. A slight increase in some parameters was observed during storage. The initial yellowness values (12.86–12.41) did not differ among patties (Fig. 2). Barely, any changes were observed in the levels of b* during storage. Hence, storage and dietary treatment had virtually no effect on the lightness and yellowness (Fig. 2) values of the chicken patties.
Lightness (L*), redness (a*), and yellowness (b*) in chicken patties formulated with breast meat obtained from birds fed dietary treatments (C control, E control + vitamin E, GS control + grape seed 40 g kg−1, SS control + grape skin 40 g kg−1, GP control + grape pomace 40 g kg−1) during refrigerated storage (2 °C)
The initially higher redness values (a*) (Fig. 2) observed in the patties obtained from the vitamin E group (2.12) were maintained through to the end of storage. An increase in a* was observed in all the samples during storage. Dietary alpha-tocopherol is widely used to reduce lipid oxidation and drip loss and to maintain color stability of meat [51]. Our study corroborates these results insofar as the patties made with meat from chickens fed vitamin E also exhibited lower oxidation levels (Table 4). Other studies on dietary supplementation with oregano essential oil showed modification in meat color, probably by modifying pigment distribution in animal tissue [52]. It is also important to note that the color of poultry meat is affected by numerous factors such as age, sex, strain, diet, intramuscular fat, meat moisture content, preslaughter conditions, and processing variables [53].
Other authors have observed differences in color parameters in patties containing grape extracts or grape powder when these products were added as ingredients [16, 37, 49]. In this study, the new bioactive compounds generated during digestion and metabolism processes of grape by-products in the animal and retained in the meat could account for the differences with the previous studies as mentioned above. It is well known that the biological activity of polyphenols depends on their availability. In this regard, while monomeric and oligomeric polyphenols might be directly absorbed, polymeric polyphenols must be metabolized by intestinal microbiota [54], generating new phenolic compounds with different biological activity and, therefore, exerting a different effect on technological properties.
Texture of chicken patties
The effect of grape by-products as a dietary supplement on the Kramer shear force (KSF) of chicken patties is shown in Table 4. The initial KSF levels were between 2.13 and 2.66 N g−1 with no significant differences among samples. These results indicate that grape by-products and vitamin E supplements in animals do not significantly affect the textural characteristics of processed meat products. Similarly, the inclusion of 50 g kg−1 of grape seed in chicken diets had no effect on organoleptic texture attributes [55]. While no changes (P > 0.05) in KSF were observed during the first 6 days of storage, there was a decrease (P < 0.05) at the end of storage (9 days) for all samples except for the GP patties (Table 4). In pork patties formulated with a combination of phyto-extracts (sea buckthorn and grape seed extracts), hardness did not follow any particular pattern during storage [56].
Sensory evaluation of chicken patties
No particular differences were observed in sensorial parameters among patties. The score was very high for all the parameters studied except for hardness (Fig. 3). Moreover, the overall acceptability parameter scored very high for all the patties with no significant differences among them. Consequently, the panel judges considered all the products acceptable. These results coincide with the other parameters studied in this experiment with few differences between the control and the other samples. A slightly lower score on the juiciness parameter was obtained for patties obtained from birds fed diets containing vitamin E and SS (Fig. 3). Other authors have also failed to find any differences in the color, flavor, and overall acceptability of goat patties formulated with fruit extract [15] and cooked chicken patties [49].
However, other authors did observe effects from the direct addition of vitamin E and grape by-products as ingredients on sensorial attributes (mainly color and flavor) of patties and from the addition of extracts from different plants or fruit [37, 57]. However, effects on color and other sensorial parameters disappeared when chicken diets were supplemented with these products.
Conclusions
In general, the addition of grape by-products, mainly skin and grape pomace, to chicken diets reduced the TBARS content of breast meat patties without affecting sensorial properties (texture and color) or microbial quality. All patties were well evaluated for their sensory attributes with the highest score being awarded for general acceptability. The incorporation of grape by-products into chicken feed could be as effective against secondary oxidation products (TBARS test) as vitamin E. Use in chicken feed also reduces the environmental impact of these by-products.
References
Magdelaine P, Spiess MP, Valceschini E (2008) Poultry meat consumption trends in Europe. Worlds Poult Sci J 64:53–63
Karpińska-Tymoszczyk M (2014) The effect of antioxidants, packaging type and frozen storage time on the quality of cooked turkey meatballs. Food Chem 148:276–283
Barroeta AC (2007) Nutritive value of poultry meat: relationship between vitamin E and PUFA. Worlds Poultry Sci 63:277–284
Jiménez-Colmenero F, Herrero AM, Cofrades S, Ruiz-Capillas C (2015) Meat: eating quality and preservation. In: Caballero B, Finglas P, Toldra F (eds) Encyclopedia of food and health. Elsevier Science, Oxford
Decker EA, Faustman C, López-Bote CJ (2000) Antioxidants in muscle foods: nutritional strategies to improve quality. Wiley, New Jersey
Brenes A, Viveros A, Chamorro S, Arija I (2016) Use of polyphenol-rich grape by-products in monogastric nutrition. A review. Anim Feed Sci Tech 211:1–17
Mielnik MB, Olsen E, Vogt G, Adeline D, Skrede G (2006) Grape seed extract as antioxidant in cooked, cold stored turkey meat. LWT Food Sci Tech 39:191–198
Georgiev V, Ananga A, Tsolova V (2014) Recent advances and uses of grape flavonoids as nutraceuticals. Nutrients 6:391–415
Brenes A, Viveros A, Goni I, Centeno C, Saura-Calixto F, Arija I (2010) Effect of grape seed extract on growth performance, protein and polyphenol digestibilities, and antioxidant activity in chickens. Span J Agric Res 8:326–333
Jung S, Choe JH, Kim B, Yun H, Kruk ZA, Jo C (2010) Effect of dietary mixture of gallic acid and linoleic acid on antioxidative potential and quality of breast meat from broilers. Meat Sci 86:520–526
Sáyago-Ayerdi SG, Brenes A, Viveros A, Goñi I (2009) Antioxidative effect of dietary grape pomace concentrate on lipid oxidation of chilled and long-term frozen stored chicken patties. Meat Sci 83:528–533
Selani MM, Contreras-Castillo CJ, Shirahigue LD, Gallo CR, Plata-Oviedo M, Montes-Villanueva ND (2011) Wine industry residues extracts as natural antioxidants in raw and cooked chicken meat during frozen storage. Meat Sci 88:397–403
Brenes A, Viveros A, Goni I, Centeno C, Sayago-Ayerdy SG, Arija I, Saura-Calixto F (2008) Effect of grape pomace concentrate and vitamin E on digestibility of polyphenols and antioxidant activity in chickens. Poultry Sci 87:307–316
Goni I, Brenes A, Centeno C, Viveros A, Saura-Calixto F, Rebole A, Arija I, Estévez R (2007) Effect of dietary grape pomace and vitamin E on growth performance, nutrient digestibility, and susceptibility to meat lipid oxidation in chickens. Poultry Sci 86:508–516
Devatkal SK, Narsaiah K, Borah A (2010) Anti-oxidant effect of extracts of kinnow rind, pomegranate rind and seed powders in cooked goat meat patties. Meat Sci 85:155–159
Lorenzo JM, Sineiro J, Amado IR, Franco D (2014) Influence of natural extracts on the shelf life of modified atmosphere-packaged pork patties. Meat Sci 96:526–534
Rodríguez-Carpena JG, Morcuende D, Estévez M (2011) Avocado by-products as inhibitors of color deterioration and lipid and protein oxidation in raw porcine patties subjected to chilled storage. Meat Sci 89:166–173
Ganan M, Martínez-Rodríguez AJ, Carrascosa AV (2009) Antimicrobial activity of phenolic compounds of wine against Campylobacter jejuni. Food Control 20:739–742
Mingo E, Carrascosa AV, de Pascual-Teresa M, Martínez-Rodríguez AJ (2014) Grape phenolic extract potentially useful in the control of antibiotic resistant strains of Campylobacter. Adv Microbiol 4:73–80
Papadopoulou C, Soulti K, Roussis IG (2005) Potential antimicrobial activity of red and white wine phenolic extracts against strains of Staphylococcus aureus, Escherichia coli and Candida albicans. Food Technol Biotech 43:41–46
NRC (1994) Nutrient Requirements of poultry. Academic Press, Washington, DC
AOAC (2005) Official method of analysis of AOAC international (18th ed.), Association of official analytical chemistry, Maryland, USA
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Phys 37:911–917
Chamorro S, Goni I, Viveros A, Hervert-Hernández D, Brenes A (2012) Changes in polyphenolic content and antioxidant activity after thermal treatments of grape seed extract and grape pomace. Eur Food Res Tech 234:147–155
Triki M, Herrero AM, Jiménez-Colmenero F, Ruiz-Capillas C (2013) Effect of preformed konjac gels, with and without olive oil, on the technological attributes and storage stability of merguez sausage. Meat Sci 93:351–360
Shirzadegan K, Falahpour P (2014) The physicochemical properties and antioxidative potential of raw thigh meat from broilers fed a dietary medicinal herb extract mixture. Open Vet J 4:69–77
Lee KH, Jung S, Kim HJ, Kim IS, Lee JH, Jo C (2012) Effect of dietary supplementation of the combination of gallic and linoleic acid in thigh meat of broilers. Asian Aust J Anim Sci 25:1641–1648
Min B, Ahn DU (2005) Mechanism of lipid peroxidation in meat and meat products—a review. Food Sci Biotechnol 14:152–163
Bhattacharya M, Hanna MA, Mandigo RW (1988) Lipid oxidation in ground-beef patties as affected by time-temperature and product packaging parameters. J Food Sci 53:714–717
Delgado-Pando G, Cofrades S, Ruiz-Capillas C, Solas MT, Triki M, Jiménez-Colmenero F (2011) Low-fat frankfurters formulated with a healthier lipid combination as functional ingredient: microstructure, lipid oxidation, nitrite content, microbiological changes and biogenic amine formation. Meat Sci 89:65–71
Salcedo-Sandoval L, Cofrades S, Ruiz-Capillas C, Matalanis A, McClements DJ, Decker EA, Jiménez-Colmenero F (2015) Oxidative stability of n-3 fatty acids encapsulated in filled hydrogel particles and of pork meat systems containing them. Food Chem 184:207–213
Caprioli I, O’Sullivan M, Monahan FJ (2011) Interference of sodium caseinate in the TBARS assay. Food Chem 124:1284–1287
Jamora JJ, Rhee KS (2002) Storage stability of extruded products from blends of meat and nonmeat ingredients: evaluation methods and antioxidative effects of onion, carrot, and oat ingredients. J Food Sci 67:1654–1659
Botsoglou NA, Grigoropoulou SH, Botsoglou E, Govaris A, Papageorgiou G (2003) The effects of dietary oregano essential oil and α-tocopheryl acetate on lipid oxidation in raw and cooked turkey during refrigerated storage. Meat Sci 65:1193–1200
Jensen C, Lauridsen C, Bertelsen G (1998) Dietary vitamin E: quality and storage stability of pork and poultry. Trends Food Sci Tech 9:62–72
Chamorro S, Viveros A, Rebole A, Rica BD, Arija I, Brenes A (2015) Influence of dietary enzyme addition on polyphenol utilization and meat lipid oxidation of chicks fed grape pomace. Food Res Int 73:197–203
Sáyago-Ayerdi SG, Brenes A, Goñi I (2009) Effect of grape antioxidant dietary fiber on the lipid oxidation of raw and cooked chicken hamburgers. LWT Food Sci Technol 42:971–976
Smet K, Raes K, Huyghebaert G, Haak L, Arnouts S, De Smet S (2008) Lipid and protein oxidation of broiler meat as influenced by dietary natural antioxidant supplementation. Poultry Sci 87:1682–1688
Ky I, Lorrain B, Kolbas N, Crozier A, Teissedre P-L (2014) Wine by-products: phenolic characterization and antioxidant activity evaluation of grapes and grape pomaces from six different french grape varieties. Molecules 19:482–506
Pinelo M, Arnous A, Meyer AS (2006) Upgrading of grape skins: significance of plant cell-wall structural components and extraction techniques for phenol release. Trends Food Sci Tech 17:579–590
Yilmaz Y, Goksel Z, Erdogan SS, Ozturk A, Atak A, Ozer C (2015) Antioxidant activity and phenolic content of seed, skin and pulp parts of 22 grape (Vitis vinifera L.) cultivars (4 common and 18 registered or candidate for registration). J Food Process Preserv 39:1682–1691
Di Majo D, La Guardia M, Giammanco S, La Neve L, Giammanco M (2008) The antioxidant capacity of red wine in relationship with its polyphenolic constituents. Food Chem 111:45–49
Rodríguez-Montealegre R, Romero-Peces R, Chacon-Vozmediano JL, Martínez-Gascuena J, Garcia-Romero E (2006) Phenolic compounds in skins and seeds of ten grape Vitis vinifera varieties grown in a warm climate. J Food Compos Anal 19:687–693
Maier T, Schieber A, Kammerer DR, Carle R (2009) Residues of grape (Vitis vinifera L.) seed oil production as a valuable source of phenolic antioxidants. Food Chem 112:551–559
Monagas M, Bartolome B, Gomez-Cordoves C (2005) Updated knowledge about the presence of phenolic compounds in wine. Crit Rev Food Sci 45:85–118
Radovanovic AN, Jovancicevic BS, Radovanovic BC, Mihajilov-Krstev T, Zvezdanovic JB (2012) Antioxidant and antimicrobial potentials of Serbian red wines produced from international Vitis vinifera grape varieties. J Sci Food Agric 92:2154–2161
Monagas M, Urpi-Sarda M, Sánchez-Patan F, Llorach R, Garrido I, Gómez-Cordoves C, Andres-Lacueva C, Bartolome B (2010) Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct 1:233–253
Selma MV, Espin JC, Tomas-Barberan FA (2009) Interaction between phenolics and gut microbiota: role in human health. J Agric Food Chem 57:6485–6501
Naveena BM, Sen AR, Vaithiyanathan S, Babji Y, Kondaiah N (2008) Comparative efficacy of pomegranate juice, pomegranate rind powder extract and BHT as antioxidants in cooked chicken patties. Meat Sci 80:1304–1308
Park SJ, Yoo SO (1999) Effects of supplementation of Chinese medicine refuse on performance and physiology in broiler chicks. Korean J Poultry Sci 26:195–201
López-Bote CJ, Daza A, Soares M, Berges E (2001) Dose-response effect of dietary vitamin E concentration on meat quality characteristics in light-weight lambs. Animal Sci 73:451–457
Simitzis PE, Deligeorgis SG, Bizelis JA, Dardamani A, Theodosiou I, Fegeros K (2008) Effect of dietary oregano oil supplementation on lamb meat characteristics. Meat Sci 79:217–223
Santiago HL, Denbow DM, Emmerson DA, Denbow C, Graham P, Hohenboken W (2005) Effects of strain, plane of nutrition and age at slaughter on performance and meat quality traits of broilers. Poultry Sci 84:128–129
Gonthier MP, Cheynier V, Donovan JL, Manach C, Morand C, Mila I, Lapierre C, Remesy C, Scalbert A (2003) Microbial aromatic acid metabolites formed in the gut account for a major fraction of the polyphenols excreted in urine of rats fed red wine polyphenols. J Nutr 133:461–467
Francesch A, Cartana M (2015) The effects of grape seed in the diet of the Penedes chicken, on growth and on the chemical composition and sensory profile of meat. Br Poultry Sci 56:477–485
Kumar V, Chatli MK, Wagh RV, Mehta N, Kumar P (2015) Effect of the combination of natural antioxidants and packaging methods on quality of pork patties during storage. J Food Sci Tech Mys 52:6230–6241
Nardoia M, Ruiz-Capillas C, Herrero AM, Jiménez-Colmenero F, Chamorro S, Brenes A (2017) Effect of added grape seed and skin on chicken thigh patties during chilled storage. Int J Food Sci Nutr (in press)
FEDNA (2010) Tablas FEDNA de composición y valor nutritivo de alimentos para la fabricación de piensos compuestos (3ª edición). Ed. Fundación Española para el Desarrollo de la Nutrición Animal. Madrid. Spain
Acknowledgements
The authors thank the MINECO and CSIC for financial support of Projects AGL2012-31355/GAN, AGL2014-53207-C2-1-R, and the Intramural 2014470E073. In addition, we are grateful to CAM and ESI Funds for financially supporting project MEDGAN-CM S2013/ABI2913). We would also like to thank MIUR and UNIMOL for the Ph.D. fellowship of Maria Nardoia.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human. The experimental procedures with animals were approved by the University Complutense of Madrid Animal Care and Ethics Committee in compliance with the Ministry of Agriculture, Fishery and Food for the Care and Use of Animals for Scientific Purposes.
Rights and permissions
About this article
Cite this article
Nardoia, M., Ruiz-Capillas, C., Casamassima, D. et al. Effect of polyphenols dietary grape by-products on chicken patties. Eur Food Res Technol 244, 367–377 (2018). https://doi.org/10.1007/s00217-017-2962-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-017-2962-7