Abstract
Berries possess structurally diverse polyphenols that may have different effects on their antioxidant properties; a combination of various berries may exhibit additive, synergistic, or antagonistic interactions between their different bioactive compounds. Even if this kind of interactions between the antioxidant properties of green tea polyphenols and other plant-derived foods has already been studied, in what concerns berries and their major polyphenols, such study has not yet been undertaken. Therefore, the goal of this work was to study the individual antioxidant activity of 19 selected major polyphenols from the most consumed berries as well as the activity of the mixtures resulting from the 171 possible combinations between them using 2 in vitro model systems. It was observed that 2.92% of the mixtures presenting synergistic effects in scavenging DPPH free radicals were composed by cyanidin, and 2.34% composed by cyanidin-3-O-glucoside. These results indicate that cyanidin, a common anthocyanin present in berries, is responsible for the enhancement of the radical scavenging properties of other berries’ polyphenols.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Members of the families Rosaceae (strawberry, raspberry, blackberry) and Ericaceae (blueberry, cranberry) are the most consumed types of berries, and are among the species with more bioactive compounds, allowing their classification as functional foods [1, 2]. The bioactive compounds found in berries are mainly polyphenols such as anthocyanins, which are plant pigments that can be either red or purple, and are often present in the form of glycosides [3]. The type and content of polyphenols differ among the varieties of berries, and depend on climatic and cultivation conditions, as well as the timing of the harvest [3]. The biological activities and health-related benefits of polyphenols are well known and documented, particularly their antioxidant properties [4, 5]. Moreover, the intake of berries has been associated with the decrease of blood pressure, inhibition of platelet aggregation, improvement of plasma lipid profile and endothelial function [6, 7].
Since berries possess structurally diverse polyphenols that may have analogous, overlapping, or diverse but complementary effects in their antioxidant properties, a combination of various berries may exhibit additive, synergistic, or antagonistic interactions between their different bioactive compounds. These effects may also occur when different polyphenol-rich foods are ingested simultaneously, for example, tea, wine, pomegranate and grapes [8]. An additive effect is observed when a combination of compounds provides the sum of the effects of the individual ones by themselves; a synergistic effect occurs when the obtained effect is greater than the sum of the individual compounds; an antagonistic effect happens when the sum of the effects is lesser than the mathematical sum predicted for the individual components in the mixture [8]. This kind of interactions in the antioxidant properties of green tea polyphenols and other plant-derived foods has already been widely studied [8,9,10]. For example, the antioxidant synergistic effects of Osmanthus fragrans flowers with green tea and their major contributed antioxidant compounds were undertaken, being observed that significant synergistic effect between O. fragrans flowers and green tea occurred [11]. Among the combinations, acteoside and gallic acid contributed most to the antioxidant synergy between O. fragrans flowers and green tea [11]. The combination of chemoprevention with grape polyphenols was also evaluated and recently reviewed [12]. More recently, the association between the antioxidant capacity of blackberry extracts with their different phytochemical compositions was studied [13], being concluded that diverse antioxidant capacities can result even when total polyphenols is similar [13].
The study of the effects on antioxidant activity resulting from the interactions between the major polyphenols of berries, to our knowledge, remains yet to be undertaken. For this reason, the general aim of this work is to study the individual antioxidant activity of 19 selected major polyphenols of the most consumed berries as well as that of the mixtures resulting from the 171 possible combinations between them using 2 in vitro model systems. The DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging assay and β-carotene bleaching test were employed to determine the additive, synergistic and antagonistic antioxidant interactions between berries’ major polyphenols. Furthermore, this work will allow to know which polyphenols are responsible for the antioxidant properties of berries and which combinations result in the enhancement of the observed antioxidant activity in berries. In addition, and to predict the effects on antioxidant activity resulting from the interactions between polyphenols in the mixtures, a mathematical model will be developed based on a multinomial logistic regression.
Materials and methods
Compounds
Naringin, pelargonidin-3-O-rutinoside chloride, cyanidin chloride and cyanidin-3-O-glucoside chloride were purchased from EXTRASYNTHESE (France). Pterostilbene and pinosylvin were obtained from Sequoia Research Products Ltd. (United Kingdom), and resveratrol and (−)-epicatechin were supplied from TCI Europe N.V. (Belgium), and Fluka (Portugal), respectively. Quercetin, rutin, gallic acid, vanillic acid, caffeic acid, ferulic acid, ellagic acid, chlorogenic acid, syringic acid, p-coumaric acid, and (+)-catechin were provided by Sigma-Aldrich (USA). Stock solutions of the individual compounds (0.5 and 1 mM) were prepared in methanol (Sigma-Aldrich, USA).
Mixtures of compounds
The mixtures of the individual compounds were prepared by mixing 600 µL of each stock solution at 1 mM, resulting in combinations of compounds in equimolecular proportions (1:1 v/v) at a final concentration of 0.5 mM for each compound in the mixture.
Antioxidant activity evaluation
DPPH free radical scavenging assay
The DPPH free radical scavenging assay was used to evaluate the antioxidant activity of the individual compounds as well as of the mixtures [14, 15]. Briefly, 100 µL of each individual compound (0.5 mM) was added to 3.9 mL of a 0.1 mM DPPH (Sigma-Aldrich, USA) methanolic solution. These samples were shaken and kept in the dark at room temperature for 15 min. After this time, the absorbances were measured at 517 nm, using a UV–Vis spectrophotometer (Helios–Omega, Thermo Scientific, USA). The assays were performed in triplicate, and the percentage of inhibition of DPPH free radical by the samples (% Inhibition) was determined. The same procedure was followed to determine the % Inhibition of the mixtures of compounds [9].
The Experimental Scavenging Capacity (ESC) of the individual compounds and mixtures was calculated using the following equation:
where A sample is the absorbance of the sample after the reaction with DPPH, A blank is the absorbance of the methanolic solutions of polyphenols or its mixtures, and A control is the absorbance of the control sample [9].
The Theoretical Scavenging Capacity (TSC) was only determined for the mixtures, and is defined as the sum of the individual scavenging capacities of each compound present in the mixtures, calculated using the following equation:
where ESC A and ESC B are the ESC values of the each individual polyphenol in the mixture [9, 16].
β-Carotene bleaching test
The β-carotene bleaching test was also employed to evaluate the antioxidant properties of the samples [17]. After the preparation of a β-carotene (Sigma-Aldrich, USA) solution (20 mg/mL in chloroform), 500 μL was added to 40 μL of linoleic acid (TCI Europe N.V., Belgium), 400 μL of Tween 40 (Riedel-de Häen, Germany) and 1 mL of chloroform (Scharlab, Spain). The chloroform was then removed under vacuum (45 °C) and 100 mL of oxygenated distilled water was added to the mixture to form an emulsion. Five mL of this emulsion was transferred into test tubes containing 300 μL of each individual compound (0.5 mM) or 300 μL of the mixtures. Finally, the tubes were shaken and placed at 50 °C in a water bath for 1 h. The absorbances of the samples were measured at 470 nm, using a UV–Vis spectrophotometer (Helios–Omega, Thermo Scientific, USA). The measurements were carried out in triplicate at initial time (t = 0 h) and at final time (t = 1 h). The antioxidant activity was measured in terms of percentage of inhibition of β-carotene’s oxidation (% Inhibition) [14, 18].
The calculation of ESC of the individual compounds and mixtures, and TSC of the mixtures was performed as described in the DPPH free radical scavenging assay (Eqs. 1 and 2).
Calculation of the effects on antioxidant activity between polyphenols in the mixtures
The effects of the combinations between polyphenols in the mixtures were calculated as Synergistic Effects (SE), defined as the ratio of the obtained antioxidant activity in the mixtures (ESCmixture) and the expected antioxidant activity (TSCmixture), as follows:
The occurrence of synergistic interactions was considered if the SE was higher than 1 (SE >1), antagonistic effect was considered when SE was lower than 1 (SE <1), and additive effect was confirmed when SE was approximately 1 [9, 16].
Statistical analysis
The data were analyzed using the statistical program SPSS version 24. The 95% confidence interval (CI) for the mean was calculated for all cases. One-way analysis of variance (ANOVA) was undertaken to test for significant differences among means (p < 0.05 was considered significant). Furthermore, synergistic, or antagonistic effects were only assigned if there were significant differences (p < 0.05) between the mean values of ESCmixture and TSCmixture.
Multinomial logistic regression
To predict the antioxidant activity between polyphenols in the mixtures (categorical dependent variable), a multinomial logistic regression was developed, considering that in each mixture the total number of hydroxyl groups, aromatic rings, carboxyl groups, carbonyl groups and double bonds in the linear chain, is independent variables that are known to contributing to the antioxidant properties. This mathematical model was developed using the SPSS version 24 software.
Quality assurance/quality control (QA/QC)
In this work, to ensure the quality assurance (QA), the methods employed (DPPH free radical scavenging assay and β-carotene bleaching test) were previously validated considering the Harmonized Guidelines for Internal Quality Control in Analytical Chemistry Laboratories from International Union of Pure and Applied Chemistry (IUPAC) [19], to evaluate the antioxidant properties of several types of matrixes, being extensively documented, and widely used by the international scientific community.
In what concerns the quality control (QC), it involves the practical steps undertaken to ensure that errors in analytical data are of a magnitude appropriate for the use to which the data will be put [19]. In the present work, the QC was performed, using strict statistical control of the data obtained, as described previously, being the measurements repeated at least three independent times. The use of reference materials was implicit, since the pure polyphenolic compounds under investigation were the ones used as reference compounds.
In general, the limit of detection (LOD) is taken as the lowest concentration of an analyte in a sample that can be detected, but not necessarily quantified, under the stated conditions of the test [20]. The limit of quantitation (LOQ) is the lowest concentration of an analyte in a sample that can be determined with acceptable precision and accuracy under the stated conditions of test [20]. As it was said previously, the methods now used were previously implemented according to these requirements.
Results and discussion
Polyphenols are the main bioactive compounds present in berries that could potentially lead to health benefits in humans [21]. In the present work, the antioxidant activity of 19 selected major polyphenols of the most consumed berries was evaluated. Additionally, the antioxidant properties resulting from the interactions between those polyphenols were also studied. Two different methods were employed to measure the antioxidant activities of the individual polyphenols as well as of the mixtures between them, because the specificity and sensitivity of a single method would not guarantee a reliable assessment of all types of antioxidant properties [8]. Finally, a multinomial logistic regression was developed to explain the relationship between the antioxidant properties of the mixtures and the independent variables, total number of hydroxyl groups, aromatic rings, carboxyl groups, carbonyl groups and double bonds in the linear chain.
Antioxidant properties of the individual polyphenols
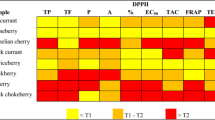
Table 1 summarizes the antioxidant properties, expressed as % Inhibition, of the 19 polyphenols measured by the 2 methods for testing the antioxidant activity.
Regarding the results of DPPH free radical scavenging assay, gallic and ellagic acids are the compounds with the best antioxidant activity, presenting 97.48 ± 0.11 and 94.95 ± 0.12%, respectively, of inhibition of DPPH free radical (Table 1). Otherwise, vanillic acid, p-coumaric acid and naringin presented residual activity, near 0%, in scavenging the DPPH free radicals (Table 1). Additionally, flavonols, like quercetin and rutin, resulted in 78% of DPPH free radical inhibition, a result that is similar to those obtained with syringic acid, (−)-epicatechin, and (+)-catechin, compounds that belong to the same statistical group (Table 1). The antioxidant properties of gallic acid, and particularly its ability to scavenge free radicals, have already been exhaustively studied and documented, since this compound is considered one of the major phenolic acids present in plant-derived products [1, 22]. This ability is mainly linked to the presence of 3 hydroxyl groups, which can easily and rapidly donate an hydrogen atom to the free radical, leading to the formation of a non-radical chemical structure. Moreover, the small and simple molecular structure of gallic acid means that it does not shield the hydrogen atoms of its hydroxyl groups, making easier its donation to the free radical. Ellagic acid is chemically characterized as a natural dimeric derivative of gallic acid and is found in fruits, namely berries and pomegranates [23]. It has 4 hydroxyl groups that are not blocked and can react with the free radicals, conferring this compound its relevant antioxidant properties. In what concerns the results of flavonols (quercetin and rutin), the presence of several hydroxyl groups on phenolic A and B rings, together with the carbonyl group on the heterocyclic C ring, gives these compounds the ability to react with free radicals, donating a hydrogen atom or even an electron, due to the delocalization of the electrons in those molecules. Quercetin has an identical number of hydroxyl groups in the same positions as (+)-catechin and contains in addition the 2,3-double bond in the C ring and the 4-oxo function, which explains the results of the antioxidant properties of these compounds [24]. Vanillic acid, p-coumaric acid and naringin showed residual capacity to scavenge DPPH free radicals, which is explained by the low number of hydroxyl groups present in vanillic and p-coumaric acids and by the complexity of the chemical structure of naringin, disabling them from donating a hydrogen atom to the free radicals.
Concerning the results of β-carotene bleaching test, cyanidin and quercetin showed the best antioxidant properties, opposing to what was observed to p-coumaric acid and naringin (Table 1). The β-carotene bleaching test is an indirect measure of the inhibition of lipid peroxidation by the compounds. This inhibition may occur in different moments: in the Initiation step of lipid peroxidation, inhibiting the oxidation of linoleic acid molecule, and thus the generation of highly reactive allyl and lipid peroxyl radicals; in the Propagation step, inhibiting the production of lipid hydroperoxides; in the Branching step, inhibiting the breakdown of lipid hydroperoxides; or, in the Termination step, inhibiting the combination of radicals [25]. This variety of inhibition pathways may explain the different results obtained in the 2 methods for testing the antioxidant properties of the berries’ polyphenols.
Cyanidin with the central anthocyanidin C ring, that allows conjugation, presented approximately the same antioxidant activity as quercetin, measured with the β-carotene bleaching test (Table 1). This demonstrates the importance of the unsaturation in the C ring and allows electron delocalization across the molecule for stabilization of the aryloxyl radical, as it was also previously described [24]. The glycosylation of flavonoids reduces their activity when compared to the corresponding aglycones [24]. Blocking the 3-hydroxyl group in the C ring of quercetin as a glycoside, as in rutin (or quercetin-3-O-rutinoside), decreases its antioxidant activity, measured by this method (Table 1).
The results obtained in the present work allow to conclude that the diverse antioxidant properties of berries are due to the presence of quercetin, rutin, gallic acid, ellagic acid, syringic acid, (−)-epicatechin, (+)-catechin and cyanidin, indicating the potential use of these fruits as functional foods for the prevention and/or treatment of oxidative stress-related diseases in humans.
Effects on antioxidant activity resulting from the interactions between polyphenols in the mixtures
Table 2 indicates the antioxidant activities resulting from the interactions between polyphenols in the mixtures.
DPPH free radical scavenging assay
It was observed that 2.92% of the mixtures presenting synergistic effects in scavenging DPPH free radicals were composed by cyanidin; and 2.34% composed by cyanidin-3-O-glucoside (Table 2). In contrast, 9.36% of mixtures with antagonistic effects possess pelargonidin-3-O-rutinoside (Table 2). These interesting results indicate that cyanidin, a common anthocyanin present in berries [26], is responsible for the enhancement of the radical scavenging properties of other berries’ polyphenols, contrariwise to the effects of pelargonidin-3-O-rutinoside. Pelargonidin-3-O-rutinoside confers to the mixtures antagonistic effects because of its large chemical structure that makes the interactions with the other polyphenols in the mixtures more difficult. In contrast, cyanidin and its glycosylated form are the compounds responsible for the synergistic interactions, which must be highlighted, since berries are known to possess high concentrations of these compounds, which also may explain the strong antioxidant properties of these fruits [27]. The antioxidant activity of the anthocyanins can be attributed to the reducing power of the O-dihydroxy structure in the B ring [24].
As it was mentioned previously, gallic acid was the compound with the highest ability to scavenge the DPPH free radicals; however, when it was mixed with other compounds, namely phenolic acids, stilbenes and catechins, the resulting antioxidant activity of the mixtures decreased significantly (Table 2). Similar results were found for the mixtures of other phenolic acids, namely caffeic and ferulic acids (Table 2). These findings show that the compounds in the mixtures are interacting, making difficult the donation of a hydrogen atom to the DPPH free radicals. The compounds in the mixtures may be linked by hydrogen bonds, blocking the ability of the hydrogen atoms of the hydroxyl groups to leave its original position in the molecule to stabilize the radical structure of DPPH.
β-Carotene bleaching test
In contrast to the results obtained in the DPPH free radical scavenging assay, in which cyanidin and cyanidin-3-O-glucoside contributed significantly to the occurrence of synergistic effects in the mixtures, in the β-carotene bleaching test, 18.72% of the mixtures with antagonistic effects were the ones composed by those 2 compounds (Table 2). Ellagic acid was the tested polyphenol that contributed most to the synergistic effects, being present in 7.02% of mixtures with synergism (Table 2). These apparent conflicting results are explained by the different antioxidant properties of the compounds, which reinforce the importance of using different methods to evaluate the antioxidant activity of the samples.
Another surprising result was the synergistic effects observed in the mixtures composed by naringin and quercetin, or rutin, or resveratrol, or (+)-catechin, or pelargonidin-3-O-rutinoside (Table 2). Naringin alone presented 3.93 ± 1.40% of inhibition of linoleic acid oxidation; however, when it was mixed with one of the above-mentioned compounds, it was able to produce synergistic effects (Tables 1, 2). This can possibly be explained by the molecular interactions on those mixtures, protecting the linoleic acid from the oxidation. Naringin is a flavanone glycoside; 2 rhamnose units are attached to its aglycone portion, naringenin, at the 7-carbon position [28]. Both these compounds are considered to be antioxidants; however, naringin is less potent compared with naringenin because the sugar moiety in the former causes steric hindrance of the scavenging group [28].
More mixtures with synergistic effects by this method were obtained (Table 2), probably due to the different antioxidant mechanisms exerted by the compounds. The lipid peroxidation is a more complex process than the chemical reaction between the DPPH free radicals and the antioxidant compounds.
The results clearly show that berries’ polyphenols could inhibit the lipid peroxidation and when in combination they interact favorably to protect the oxidation of linoleic acid molecules.
Mathematical model
To predict the effects on antioxidant activity resulting from the interactions between polyphenols in the mixtures, a mathematical model was developed based on a multinomial logistic regression, considering in each mixture the total number of hydroxyl groups, aromatic rings, carboxyl groups, carbonyl groups and double bonds in the linear chain. The obtained results can only be applied to the DPPH free radical scavenging assay, since for the β-carotene bleaching test the results of the multinomial logistic regression were not adjusted to the results obtained experimentally. The mathematical model allows to conclude that the factor that contributes significantly (p < 0.05) to the existence of synergistic effects in the mixtures is the total number of aromatic rings (Odds Ratio = 3.146; 95% Confidence Interval 1.304–7.593; p = 0.011); and the factors that contribute significantly (p < 0.05) to the existence of antagonistic effects in the mixtures are the total number of carbonyl and hydroxyl groups (Odds Ratio = 0.373; 95% Confidence Interval 0.195–0.714; p = 0.003 and Odds Ratio = 1.270; 95% Confidence Interval 1.085–1.486; p = 0.003, respectively), together with the total number of double bonds in the linear chain (Odds Ratio = 0.406; 95% Confidence Interval 0.200–0.824; p = 0.012).
The aromatic rings confer to the compounds their resonance structures, due to the delocalization of the electrons in the molecules. When different aromatic compounds are mixed, their electrons can be delocalized from one compound to another, and so can easily react with free radicals, leading to synergistic effects. Otherwise, carbonyl and hydroxyl groups result in antagonistic effects because in mixtures with compounds possessing those functional groups the presence of hydrogen bonds is favored, inhibiting the hydrogen atoms from leaving and reacting with free radicals.
The following equations describe the mathematical model obtained to predict the interactions in the mixtures:
where P is the probability of a given mixture of compounds which have additive, antagonistic, or synergistic effects; OH represents the total number of hydroxyl groups in the mixture; AR is the number of aromatic rings in the mixture; CN corresponds to the carbonyl groups in the mixture; and DB is the total number of double bonds in the linear chain present in mixtures.
Moreover, the obtained model has an acceptable discrimination. It correctly classifies 60.1% of the mixtures (Table 3); corresponding to 70.1% of mixtures with additive effects, and 66.7% of mixtures with antagonistic effects.
This work concludes that gallic acid, quercetin, ellagic acid, and cyanidin are compounds with remarkable antioxidant activity, in scavenging free radicals and inhibiting the lipid peroxidation. Additionally, concerning the results obtained by mathematical modeling, it was possible to conclude that the factor that contributes significantly to the existence of synergistic effects in the mixtures is the total number of aromatic rings. Moreover, it was also demonstrated that berries’ polyphenols can interact between them, enhancing their antioxidant properties, and resulting in numerous health benefits to humans. In conclusion, the results obtained in this work allow to predict which combinations have the best antioxidant activities in view of the future application of these compounds.
References
Nile S, Park S (2014) Edible berries: bioactive components and their effect on human health. Nutrition 30:134–144
Skrovankova S, Sumczynski D, Mlcek J, Jurikova T, Sochor J (2015) Bioactive compounds and antioxidant activity in different types of berries. Int J Mol Sci 16:24673–24706
Tsuda T (2016) Recent progress in anti-obesity and anti-diabetes effect of berries. Antioxidants. doi:10.3390/antiox5020013
Arts I, Hollman P (2005) Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr 81:317S–325S
Dai J, Mumper R (2010) Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 15:7313–7352
Erlund I, Koli R, Alfthan G, Marniemi J, Puukka P, Mustonen P, Mattila P, Jula A (2008) Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am J Clin Nutr 87:323–331
Yang B, Kortesniemi M (2015) Clinical evidence on potential health benefits of berries. Curr Opin Food Sci 2:36–42
Wang S, Meckling K, Marcone M, Kakuda Y, Tsao R (2011) Synergistic, additive, and antagonistic effects of food mixtures on total antioxidant capacities. J Agric Food Chem 59:960–968
Colon M, Nerín C (2016) Synergistic, antagonistic and additive interactions of green tea polyphenols. Eur Food Res Technol 242:211–220
Yin J, Becker E, Andersen M, Skibsted L (2012) Green tea extract as food antioxidant. Synergism and antagonism with α-tocopherol in vegetable oils and their colloidal systems. Food Chem 135:2195–2202
Mao S, Wang K, Lei Y, Yao S, Lu B, Huang W (2017) Antioxidant synergistic effects of Osmanthus fragrans flowers with green tea and their major contributed antioxidant compounds. Sci Rep 7:4651
Singh C, Siddiqui I, El-abd S, Mukhtar H, Ahmad N (2016) Combination chemoprevention with grape antioxidants. Mol Nutr Food Res 60:1406–1415
Figueiras A, Mendoza N, Valadez N, Escamilla E (2017) Antioxidant capacity analysis of blackberry extracts with different phytochemical compositions and optimization of their ultrasound assisted extraction. Plant Foods Hum Nutr. doi:10.1007/s11130-017-0616-3
Brand-Williams W, Cuvelier M, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol 30:25–30
Sánchez-Moreno C, Larrauri J, Saura-Calixto F (1998) A procedure to measure the antiradical efficiency of polyphenols. J Sci Food Agric 76:270–276
Fuhrman B, Volkova N, Rosenblat M, Aviram M (2000) Lycopene synergistically inhibits LDL oxidation in combination with vitamin E, glabridin, rosmarinic acid, carnosic acid, or garlic. Antioxid Redox Signal 2:491–506
Obied H, Bedgood D, Prenzler P, Robards K (2007) Bioscreening of Australian olive mill waste extracts: biophenol content, antioxidant, antimicrobial and molluscicidal activities. Food Chem Toxicol 45:1238–1248
Luís Â, Domingues F, Duarte AP (2011) Bioactive compounds, RP-HPLC analysis of phenolics, and antioxidant activity of some Portuguese shrub species extracts. Nat Prod Commun 6:1863–1872
Thompson M, Wood R (1995) Harmonized guidelines for internal quality control in analytical chemistry laboratories (Technical Report). Pure Appl Chem 67:649–666
Shrivastava A, Gupta V (2001) Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron Young Sci 2:21–25
Costa A, Garcia-Diaz D, Jimenez P, Silva P (2013) Bioactive compounds and health benefits of exotic tropical red–black berries. J Funct Foods 5:539–549
Fernandes F, Salgado H (2016) Gallic acid: review of the methods of determination and quantification. Crit Rev Anal Chem 46:257–265
Oliveira M (2016) The effects of ellagic acid upon brain cells: a mechanistic view and future directions. Neurochem Res 41:1219–1228
Rice-Evans C, Miller N, Paganga G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20:933–956
Antolovich M, Prenzler P, Patsalides E, McDonald S, Robards K (2002) Methods for testing antioxidant activity. Analyst 127:183–198
Serraino I, Dugo L, Dugo P, Mondello L, Mazzon E (2003) Protective effects of cyanidin-3-O-glucoside from blackberry extract against peroxynitrite-induced endothelial dysfunction and vascular failure. Life Sci 73:1097–1114
Ludwig I, Mena P, Calani L, Borges G, Pereira-Caro G, Bresciani L, Del Rio D, Lean M, Crozier A (2015) New insights into the bioavailability of red raspberry anthocyanins and ellagitannins. Free Radic Biol Med 89:758–769
Alam M, Subhan N, Rahman M, Uddin S, Reza H, Sarker S (2014) Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv Nutr 5:404–417
Acknowledgements
Ângelo Luís acknowledges the Post-Doc research fellowship within the scope of the protocol signed between Universidade da Beira Interior and bank Santander/Totta with the reference BIPD/ICI-FC-BST-UBI 2016. This work was supported by FEDER funds through the POCI-COMPETE 2020-Operational Program Competitiveness and Internationalization in Axis I-Strengthening research, technological development, and innovation (Project POCI-01-0145- FEDER-007491) and National Funds by FCT-Foundation for Science and Technology (Project UID/Multi/00709/2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Rights and permissions
About this article
Cite this article
Luís, Â., Duarte, A.P., Pereira, L. et al. Interactions between the major bioactive polyphenols of berries: effects on antioxidant properties. Eur Food Res Technol 244, 175–185 (2018). https://doi.org/10.1007/s00217-017-2948-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-017-2948-5